
Rice Science ›› 2025, Vol. 32 ›› Issue (1): 81-93.DOI: 10.1016/j.rsci.2024.10.003
收稿日期:2024-07-02
接受日期:2024-10-10
出版日期:2025-01-28
发布日期:2025-03-25
. [J]. Rice Science, 2025, 32(1): 81-93.
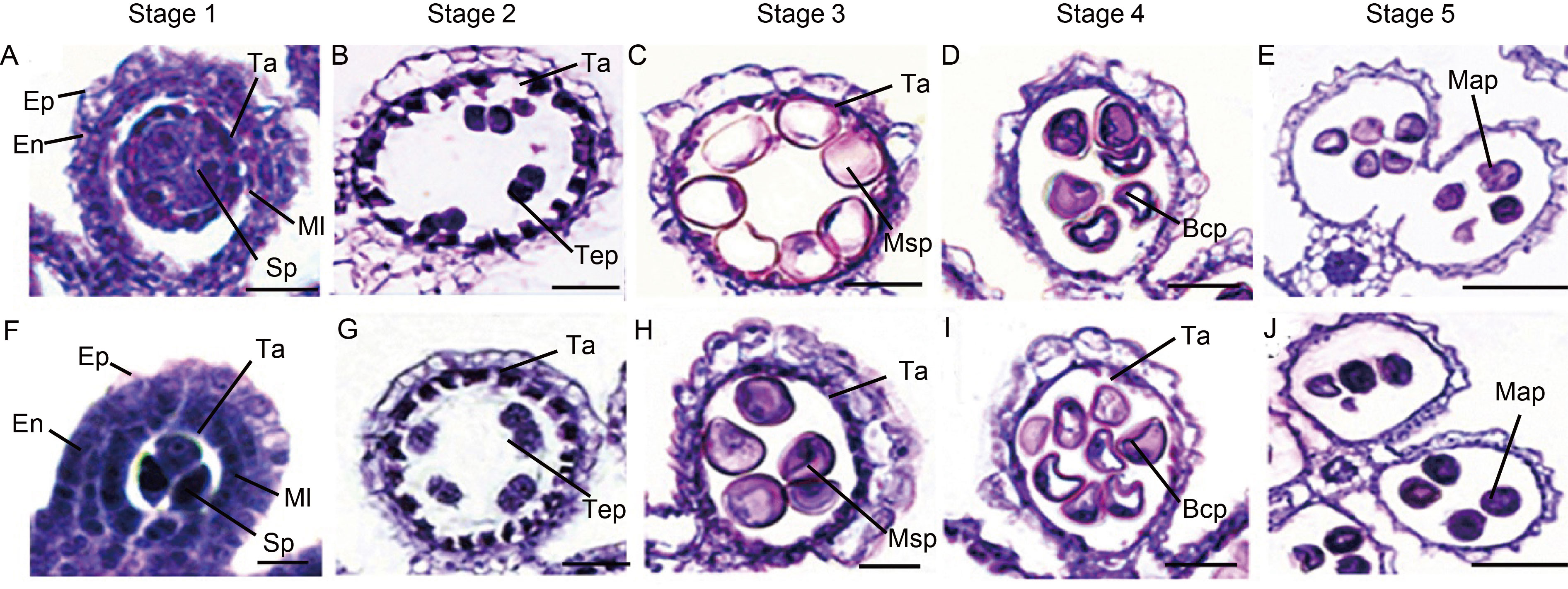
Fig. 1. Semi-thin section comparison of anther development in YA and NIL-Rf6 rice lines. A‒E, Pollen development of YA at Stages 1 to 5. Scale bars, 50 μm. F‒J, Pollen development of NIL-Rf6 lines at Stages 1 to 5. Scale bars, 50 μm. Stage 1, Microspore mother cell stage; Stage 2, Meiosis stage; Stage 3, Microspore stage; Stage 4, Binucleate stage; Stage 5, Mature stage. Ep, Epidermis; En, Inner wall layer; Ml, Middle layer; Ta, Tapetum; Sp, Sporogenous cell; Tep, Tetrad pollen; Msp, Microspore pollen; Bcp, Bicellular pollen; Map, Mature pollen.
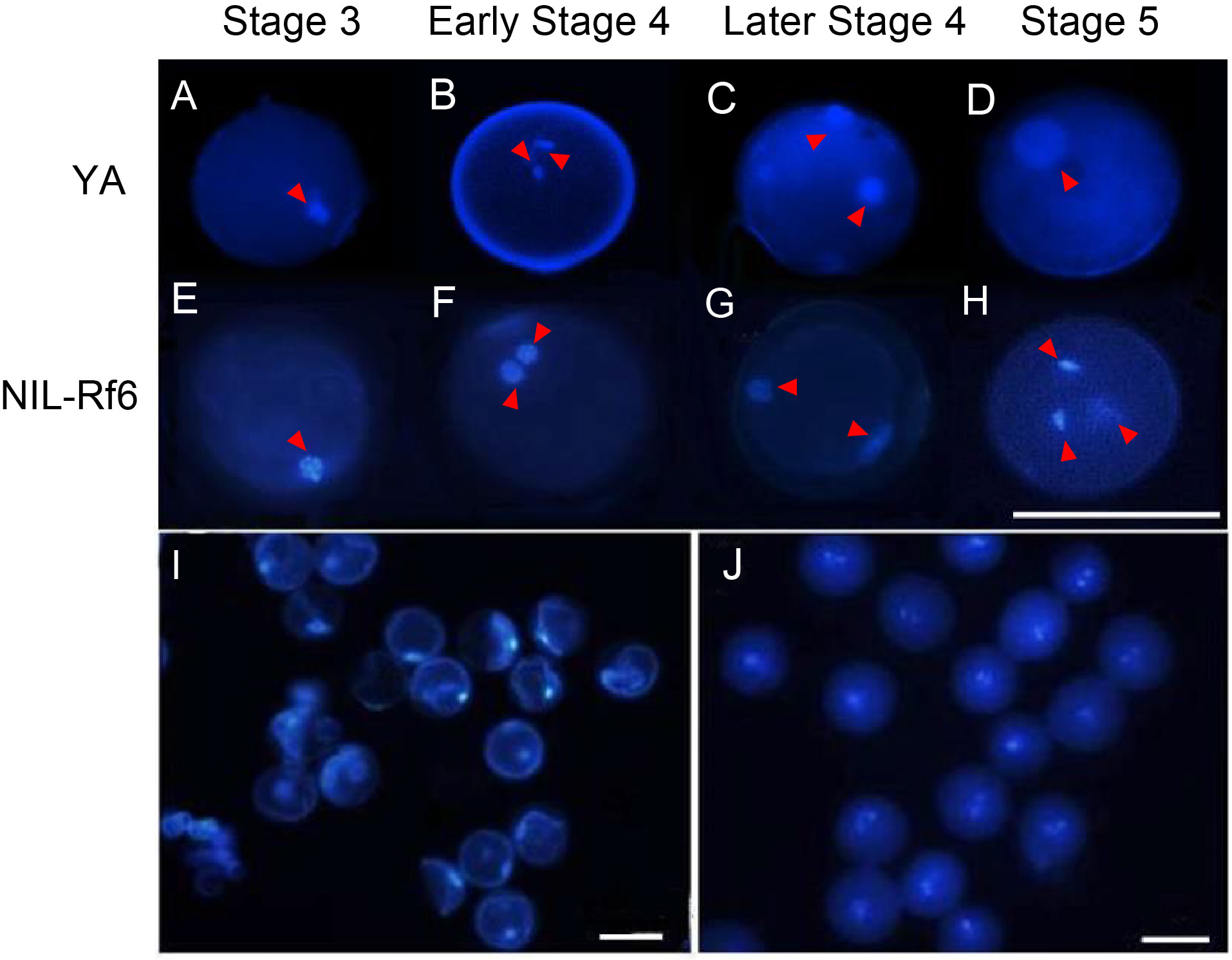
Fig. 2. 4ʹ,6-Diamidino-2-phenylindole (DAPI) staining illustrating microspore development. A‒H, Development of pollen grains in YA (A‒D) and NIL-Rf6 (E‒H) at Stages 3 to 5. Red arrows point to cell nuclei. Scale bar, 100 μm. I and J, DAPI stained pollen grains in YA (I) and NIL-Rf6 (J) at Stage 5. Scale bars, 100 μm.
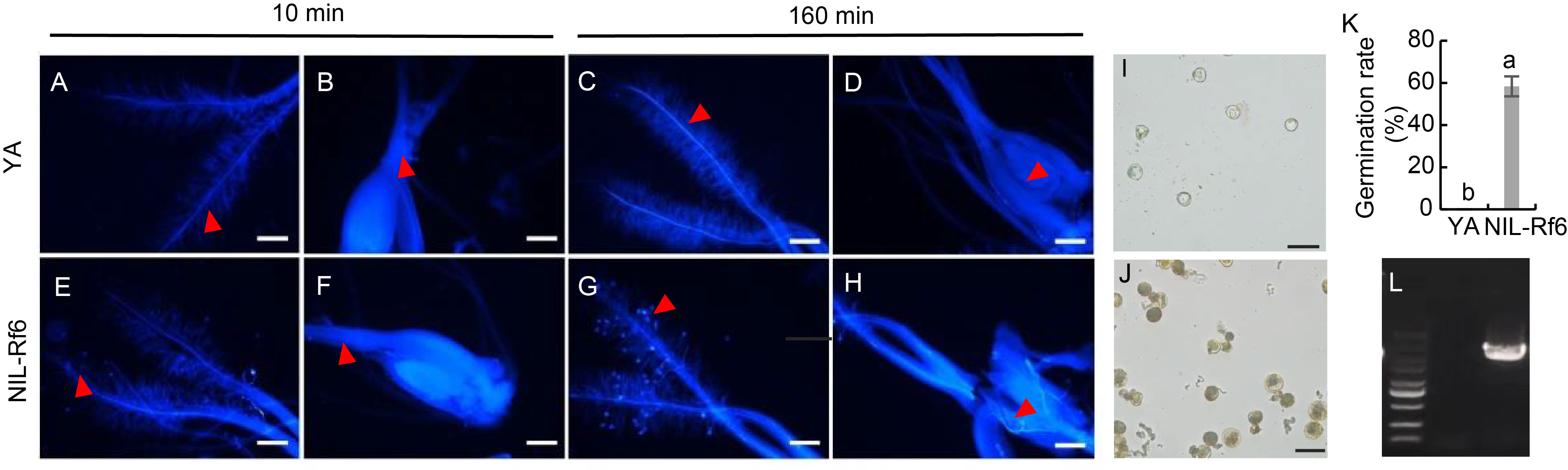
Fig. 3. Pollen germination in vivo and in vitro. A‒H, Observation of antherine blue staining of the stigma and ovary of YA and NIL-Rf6 rice lines. Scale bars, 200 μm. I and J, Observation of pollen germination of YA (I) and NIL-Rf6 (J) rice lines in liquid culture medium. Scale bars, 100 μm. K, Pollen germination rates for YA and NIL-Rf6. The data are Mean ± SD (n = 6). Lowercase letters above the bars indicate significant differences at P < 0.05. L, Identification of NIL-Rf6 molecular markers. The strips are marker (5 000 bp), YA, and NIL-Rf6 (2 682 bp) from left to right.
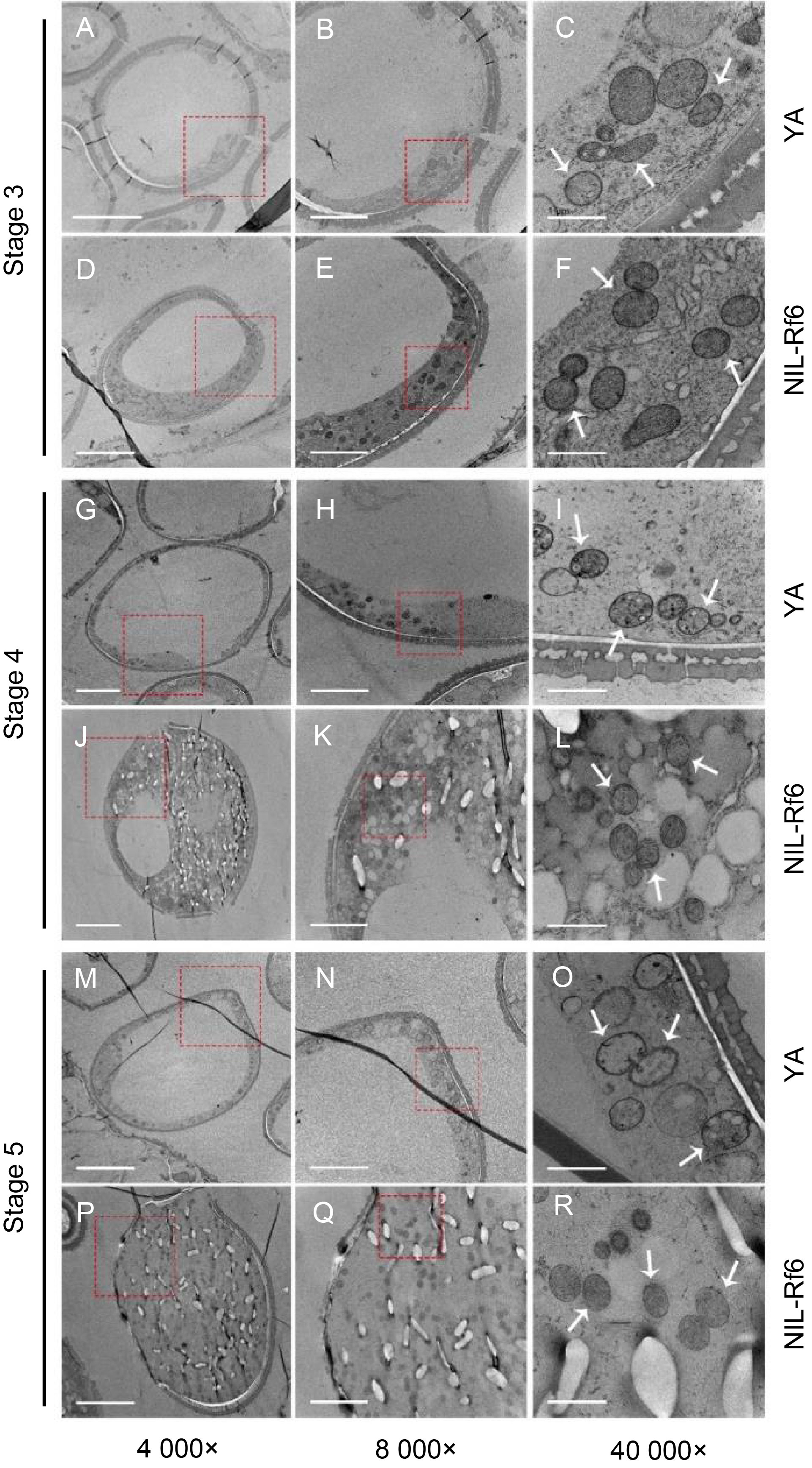
Fig. 4. Transmission electron microscopy (TEM) of pollen mitochondria. A‒F, TEM observations of internal structure of pollen for YA and NIL-Rf6 at Stage 3 (microspore stage). G‒L, TEM observations of internal structure of pollen for YA and NIL-Rf6 at Stage 4 (binucleate stage). M‒R, TEM observations of internal structure of pollen for YA and NIL-Rf6 at Stage 5 (mature stage). The red squares indicate the area of magnification, with the images magnified by 4 000×, 8 000×, and 40 000× from left to right. The white arrow points to the mitochondria. Scale bars are 10 μm in panels A, D, G, J, M, and P), 5 μm in panels B, E, H, K, N, and Q, and 1 μm in panels C, F, I, L, O, and R.
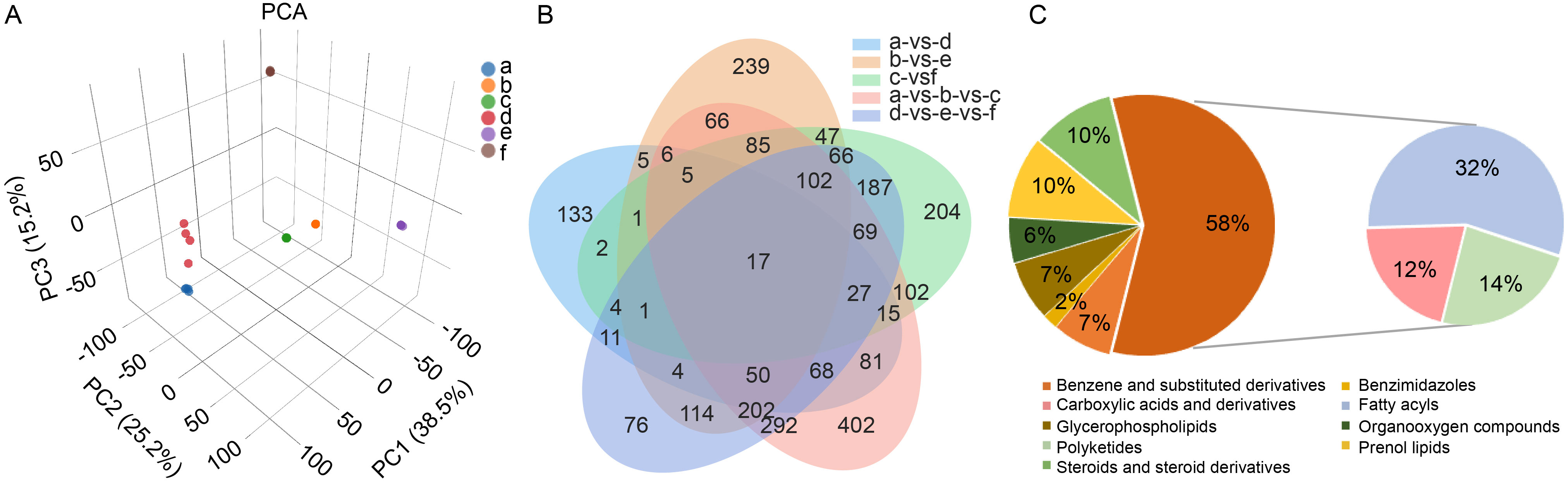
Fig. 5. Changes of pollen metabolomic in YA and NIL-Rf6 at different stages. A, Principal component analysis (PCA) of metabolites (n = 4). a‒c, Stages 3 (microspore stage), 4 (binucleate stage), and 5 (mature stage) of YA pollen; d‒f, Stages 3, 4, and 5 of NIL-Rf6 pollen. B, Venn diagram comparing different groups. C, Pie chart analysis of unique differentially abundant metabolites at Stage 5 of pollen development.
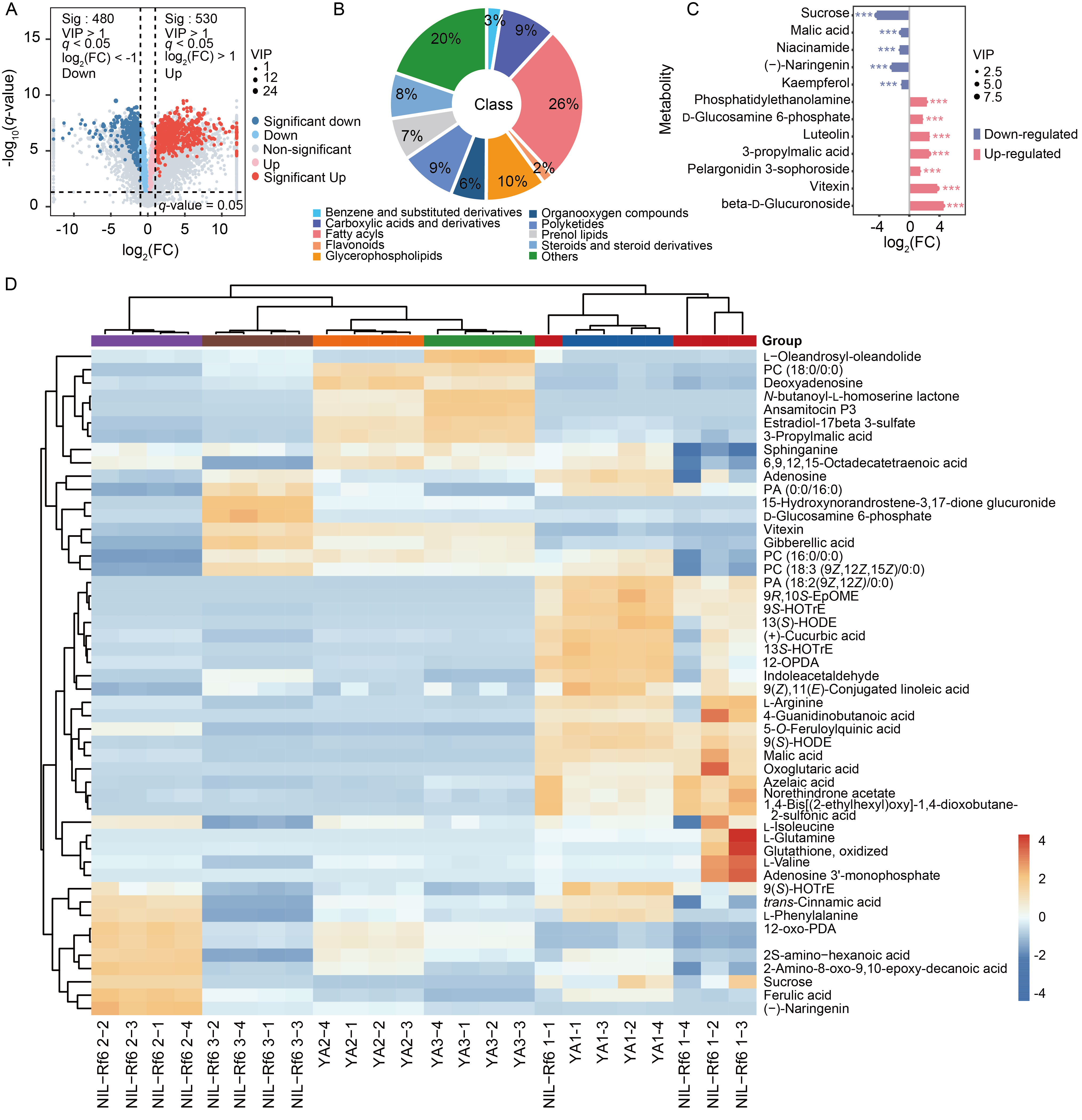
Fig. 6. Changes of pollen metabolites in YA and NIL-Rf6 at different stages. A, Volcano plot of metabolites at Stage 4 (binucleate stage). Red and blue represent significantly up-regulated and down-regulated differentially abundant metabolites, respectively. FC, Fold change; VIP, Variable importance in the projection. B, Pie chart of unique differentially abundant metabolites at Stage 4 (binucleate stage). C, Lollipop chart of differentially abundant metabolites related to oxidative stress and carbohydrate metabolic pathways. *** indicates significant differences at P < 0.001. D, Correlation heatmap of differential metabolites of YA and NIL-Rf6 pollen at different stages. The numbers 1, 2, and 3 following YA and NIL-Rf6 represent Stages 3 (microspore stage), 4 (binucleate stage), and 5 (mature stage), respectively, whereas the numbers -1, -2, -3, and -4 represent the four biological replicates. A redder color indicates a higher metabolite content, and a bluer color indicates a lower content. PC, Phosphatidylcholine; PA, Phosphatidic acid; EpOME, Epoxyoctadecadienoic acid; HOTrE, Hydroxyoctadecatrienoic acid; HODE, Hydroxyoctadecadienoic acid; OPDA, Oxophytodienoic acid; oxo-PDA, Oxophytodienoic acid; HpOTrE, Hydroxy-8,10,12-octadecatrienoic acid.
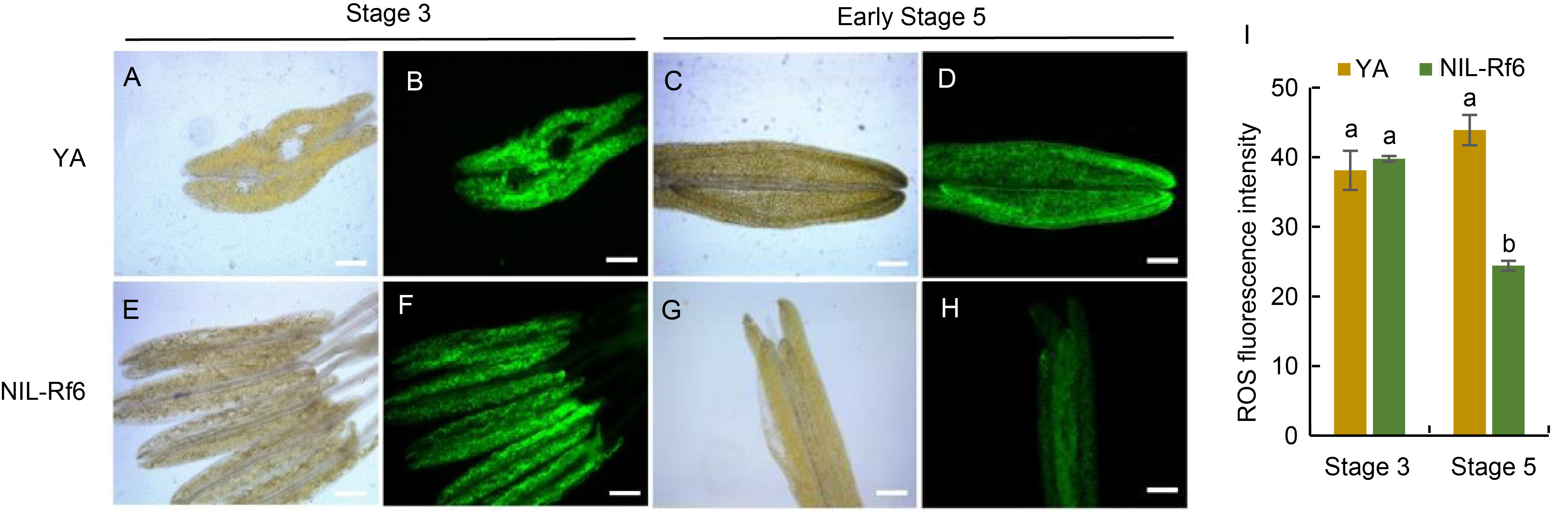
Fig. 7. Reactive oxygen species (ROS) fluorescence staining analysis at microspore (Stage 3) and mature stages (Stage 5). A‒H, 2ʹ,7ʹ-Dichlorodihydrofluorescein diacetate (H2DCF-DA) staining of YA (A‒D) and NIL-Rf6 (E‒H) pollen. Scale bars, 200 μm. I, Quantitative analysis of ROS staining at Stages 3 and 5. The data are presented as Mean ± SD (n = 3). Lowercase letters above bars indicate significant differences at P < 0.05.
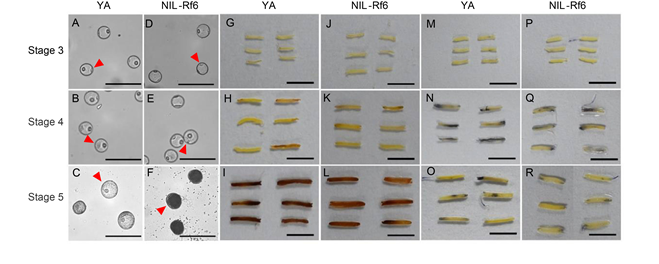
Fig. 8. Nitrotetrazolium blue chloride (NBT) and 3,3ʹ-diaminobenzidine (DAB) staining analysis in different stages of microspores development. A‒F, Observation of nuclear stage of YA and NIL-Rf6 pollen. The red arrows point to the pollen grains at the corresponding stage. Scale bars, 200 μm. G‒L, DAB staining of anthers at Stages 3 to 5 (microspore, binucleate, and mature stages). Scale bars, 1 cm. M‒R, NBT staining of anthers at Stages 3 to 5. Scale bars, 1 cm.
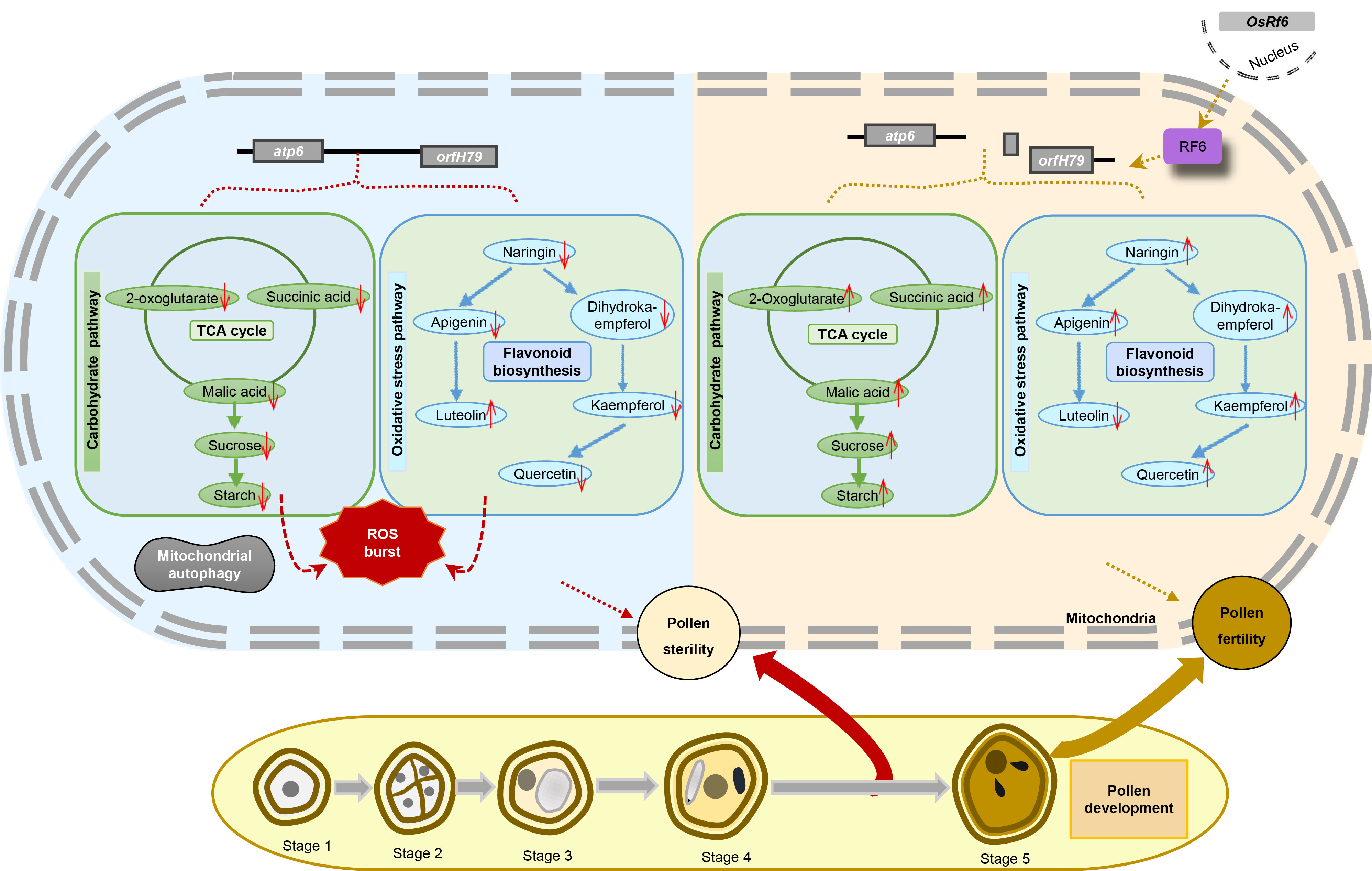
Fig. 9. Working model of pollen abortion in Honglian type-cytoplasmic male sterility (HL-CMS). The blue region on the left side of the figure illustrates the metabolic changes occurring within the YA pollen after the normal expression of atp6-orfH79. In YA pollen, the levels of key metabolites in the tricarboxylic acid (TCA) cycle, including 2-oxoglutarate, succinic acid, and malic acid, are significantly reduced. This alteration leads to the disruption of sucrose synthesis, which, in turn, triggers abnormal starch accumulation. Concurrently, the flavonoid biosynthetic pathway in YA pollen is also affected, with a decrease in the levels of metabolites such as naringin, apigenin, dihydrokaempferol, kaempferol, and quercetin. These changes collectively disrupt the homeostasis of reactive oxygen species (ROS) in the pollen, ultimately leading to mitochondrial autophagy and pollen sterility. The orange-yellow region on the right side of the figure shows the change in metabolite content of NIL-Rf6 pollen compared with YA pollen after atp6-orfH79 was spliced by the RF6 protein complex, which is expressed by OsRf6 in the nucleus. These changes restored the fertility of NIL-Rf6 pollen. The yellow region at the bottom of the figure shows the different stages of pollen development.
| [1] | Annesley S J, Fisher P R. 2019. Mitochondria in health and disease. Cells, 8(7): 680. |
| [2] | Barrera-Paez J D, Moraes C T. 2022. Mitochondrial genome engineering coming-of-age. Trends Genet, 38(8): 869-880. |
| [3] | Bian X H, Li W, Niu C F, et al. 2020. A class B heat shock factor selected for during soybean domestication contributes to salt tolerance by promoting flavonoid biosynthesis. New Phytol, 225(1): 268-283. |
| [4] | Charlesworth D. 2017. Origins of rice cytoplasmic male sterility genes. Cell Res, 27(1): 3-4. |
| [5] | Chase C D. 2007. Cytoplasmic male sterility: A window to the world of plant mitochondrial-nuclear interactions. Trends Genet, 23(2): 81-90. |
| [6] | Che R H, Hu B, Wang W, et al. 2022. POLLEN STERILITY, a novel suppressor of cell division, is required for timely tapetal programmed cell death in rice. Sci China Life Sci, 65(6): 1235-1247. |
| [7] | Ding X L, Wang X, Li Q, et al. 2019. Metabolomics studies on cytoplasmic male sterility during flower bud development in soybean. Int J Mol Sci, 20(12): 2869. |
| [8] | Ding X L, Guo J F, Lv M L, et al. 2023. The miR156b-GmSPL2b module mediates male fertility regulation of cytoplasmic male sterility-based restorer line under high-temperature stress in soybean. Plant Biotechnol J, 21(8): 1542-1559. |
| [9] | Farooq M A, Niazi A K, Akhtar J, et al. 2019. Acquiring control: The evolution of ROS-induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol Biochem, 141: 353-369. |
| [10] | Feng J H, Lu Y G, Liu X D, et al. 2001. Pollen development and its stages in rice (Oryza sativa L.). Chin J Rice Sci, 15(1): 21-28. (in Chinese with English abstract) |
| [11] | Fujii S, Toriyama K. 2009. Suppressed expression of RETROGRADE- REGULATED MALE STERILITY restores pollen fertility in cytoplasmic male sterile rice plants. Proc Natl Acad Sci USA, 106(23): 9513-9518. |
| [12] | Gass N, Glagotskaia T, Mellema S, et al. 2005. Pyruvate decarboxylase provides growing pollen tubes with a competitive advantage in Petunia. Plant Cell, 17(8): 2355-2368. |
| [13] | Hanson A D, Henry C S, Fiehn O, et al. 2016. Metabolite damage and metabolite damage control in plants. Annu Rev Plant Biol, 67: 131-152. |
| [14] | Hao T S, Yu J L, Wu Z D, et al. 2023. Hypoxia-reprogramed megamitochondrion contacts and engulfs lysosome to mediate mitochondrial self-digestion. Nat Commun, 14(1): 4105. |
| [15] | Huang W C, Hu J, Yu C C, et al. 2012. Two non-allelic nuclear genes restore fertility in a gametophytic pattern and enhance abiotic stress tolerance in the hybrid rice plant. Theor Appl Genet, 124(5): 799-807. |
| [16] | Huang W C, Yu C C, Hu J, et al. 2015. Pentatricopeptide-repeat family protein RF6 functions with hexokinase 6 to rescue rice cytoplasmic male sterility. Petroc Natl Acad Sci USA, 112(48): 14984-14989. |
| [17] | Kazama T, Itabashi E, Fujii S, et al. 2016. Mitochondrial ORF79 levels determine pollen abortion in cytoplasmic male sterile rice. Plant J, 85(6): 707-716. |
| [18] | Li S Q, Wan C X, Kong J, et al. 2004. Programmed cell death during microgenesis in a Honglian CMS line of rice is correlated with oxidative stress in mitochondria. Funct Plant Biol, 31(4): 369-376. |
| [19] | Muhlemann J K, Younts T L B, Muday G K. 2018. Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc Natl Acad Sci USA, 115(47): E11188-E11197. |
| [20] | Raimundo N. 2014. Mitochondrial pathology: Stress signals from the energy factory. Trends Mol Med, 20(5): 282-292. |
| [21] | Rejón J D, Delalande F, Schaeffer-Reiss C, et al. 2016. The pollen coat proteome: At the cutting edge of plant reproduction. Proteomes, 4(1): 5. |
| [22] | Santiago J P, Sharkey T D. 2019. Pollen development at high temperature and role of carbon and nitrogen metabolites. Plant Cell Environ, 42(10): 2759-2775. |
| [23] | Tang H L, Song Y L, Guo J L, et al. 2018. Physiological and metabolome changes during anther development in wheat (Triticum aestivum L.). Plant Physiol Biochem, 132: 18-32. |
| [24] | Tang M Q, Li Z Q, Luo D J, et al. 2021. A comprehensive integrated transcriptome and metabolome analyses to reveal key genes and essential metabolic pathways involved in CMS in kenaf. Plant Cell Rep, 40(1): 223-236. |
| [25] | Wang K, Gao F, Ji Y X, et al. 2013. ORFH79 impairs mitochondrial function via interaction with a subunit of electron transport chain complex III in Honglian cytoplasmic male sterile rice. New Phytol, 198(2): 408-418. |
| [26] | Wang L X, Ying Lam L P, Lui A C W, et al. 2020. Flavonoids are indispensable for complete male fertility in rice. J Exp Bot, 71(16): 4715-4728. |
| [27] | Wu H M, Xie D J, Jia P F, et al. 2023. Homeostasis of flavonoids and triterpenoids most likely modulates starch metabolism for pollen tube penetration in rice. Plant Biotechnol J, 21(9): 1757-1772. |
| [28] | Xiao S L, Song W, Xing J F, et al. 2023. ORF355 confers enhanced salinity stress adaptability to S-type cytoplasmic male sterility maize by modulating the mitochondrial metabolic homeostasis. J Integr Plant Biol, 65(3): 656-673. |
| [29] | Yamasaki H, Heshiki R, Ikehara N. 1995. Leaf-goldenning induced by high light in Ficus microcarpa L. f., a tropical fig. J Plant Res, 108(2): 171-180. |
| [30] | Yan J J, Tian H, Wang S Z, et al. 2014. Pollen developmental defects in ZD-CMS rice line explored by cytological, molecular and proteomic approaches. J Proteomics, 108: 110-123. |
| [31] | Yu L, McPhee C K, Zheng L X, et al. 2010. Termination of autophagy and reformation of lysosomes regulated by mTOR. Nature, 465: 942-946. |
| [32] | Yu X W, Zhao Z G, Zheng X M, et al. 2018. A selfish genetic element confers non-Mendelian inheritance in rice. Science, 360: 1130-1132. |
| [33] | Zang R, Shahzad K, Zhang X X, et al. 2023. Dose effects of restorer gene modulate pollen fertility in cotton CMS-D2 restorer lines via auxin signaling and flavonoid biosynthesis. Plant Cell Rep, 42(11): 1705-1719. |
| [34] | Zhang D B, Luo X, Zhu L. 2011. Cytological analysis and genetic control of rice anther development. J Genet Genomics, 38(9): 379-390. |
| [35] | Zhao W, Hou Q C, Qi Y C, et al. 2023. Structural and molecular basis of pollen germination. Plant Physiol Biochem, 203: 108042. |
| [36] | Zheng X M, Wei F, Cheng C, et al. 2024. A historical review of hybrid rice breeding. J Integr Plant Biol, 66(3): 532-545. |
| [37] | Zu X F, Luo L L, Wang Z, et al. 2023. A mitochondrial pentatricopeptide repeat protein enhances cold tolerance by modulating mitochondrial superoxide in rice. Nat Commun, 14(1): 6789. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||