
Rice Science ›› 2025, Vol. 32 ›› Issue (5): 673-684.DOI: 10.1016/j.rsci.2025.04.011
收稿日期:2025-02-02
接受日期:2025-03-16
出版日期:2025-09-28
发布日期:2025-10-11
. [J]. Rice Science, 2025, 32(5): 673-684.
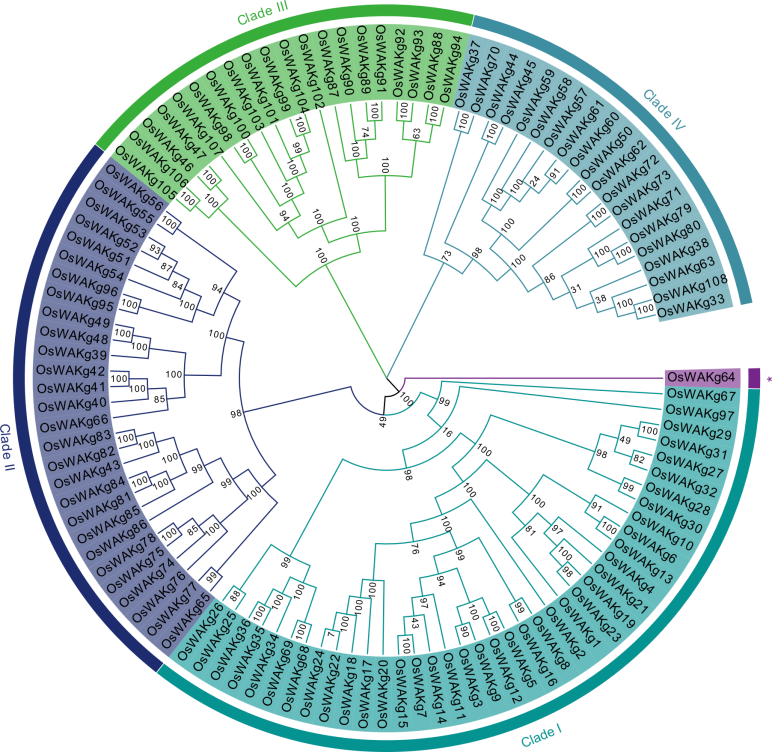
Fig. 2. Phylogenetic tree of OsWAKg genes based on protein sequences identified in rice. Different color represents different clade. * represents unassigned proteins.
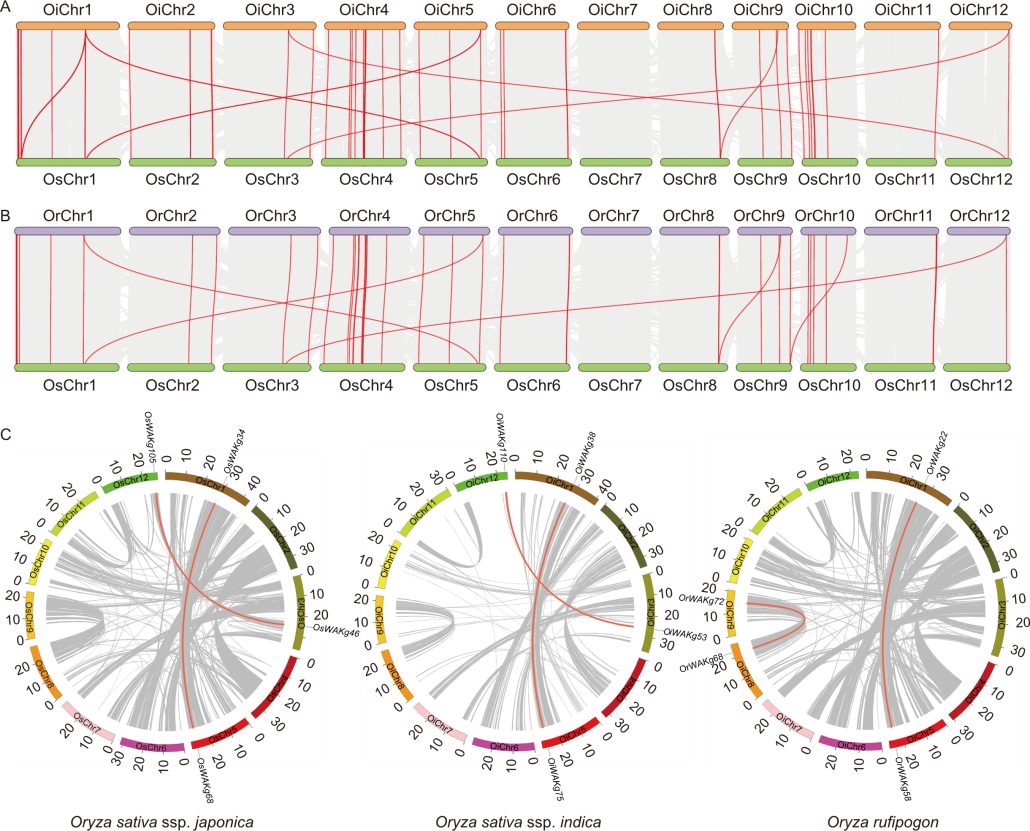
Fig. 3. Collinearity relationships and gene segmental duplication analysis of WAKg family genes in Oryza species. A and B, Collinearity relationships of WAKg genes between Oryza sativa ssp. japonica and O. sativa ssp. indica (A) as well as O. rufipogon (B). The gray lines show collinear blocks, and the red lines indicate syntenic WAKg gene pairs. C, Gene segmental duplication analysis of WAKg family genes in Oryza species. Gray lines indicate all synteny blocks in the rice genome, and red lines indicate duplicated WAKg gene pairs. The scale at the periphery of the chromosome represents the physical location (Mb). Os, Oryza sativa ssp. japonica; Or, Oryza rufipogon; Oi, Oryza sativa ssp. indica; Chr, Chromosome.
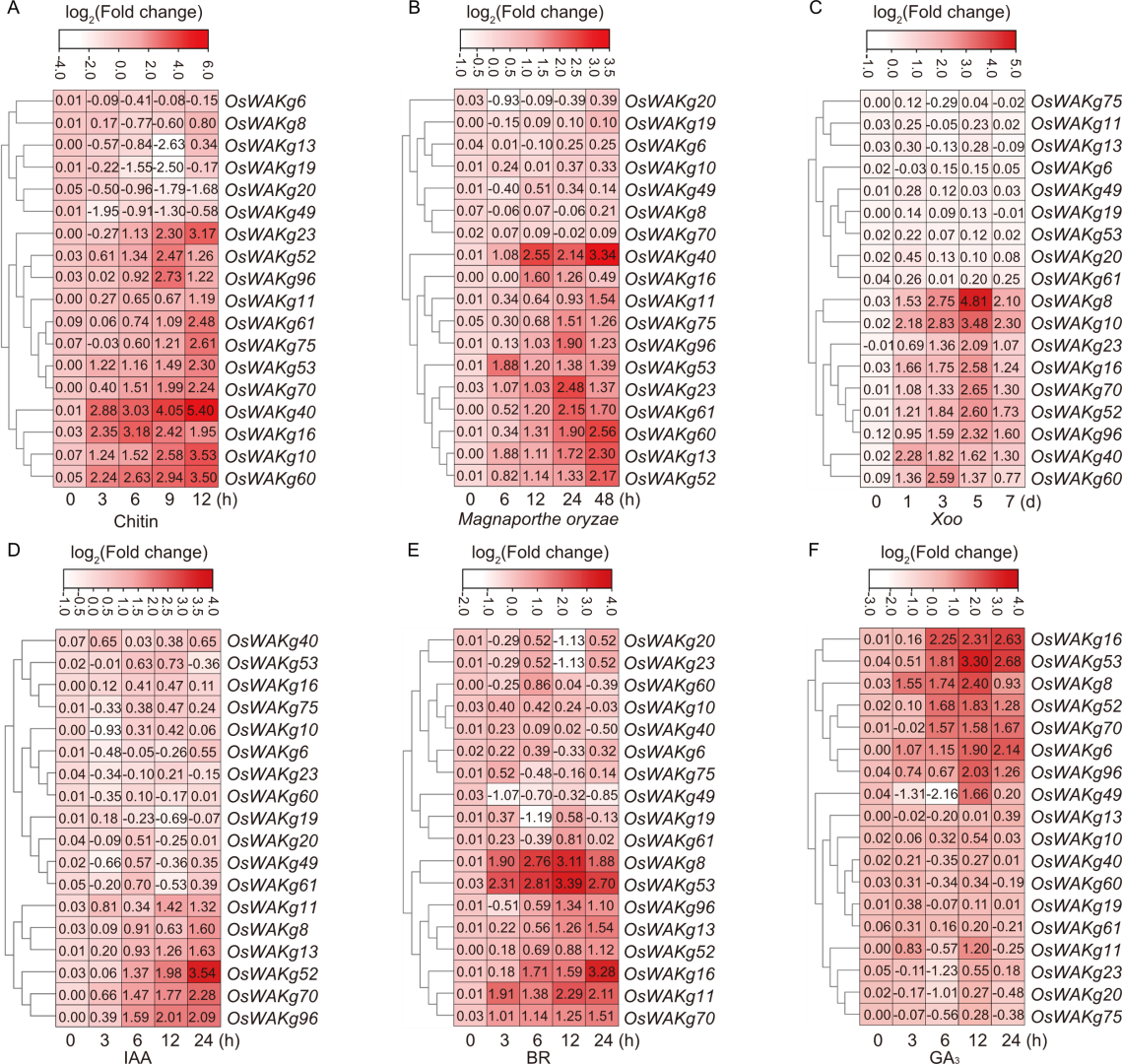
Fig. 4. qRT-PCR analysis of OsWAKgs expression induced by chitin (A), Magnaporthe oryzae (B), Xanthomonas oryzae pv. oryzae (Xoo) (C), indole-3-acetic acid (IAA) (D), brassinosteroid (BR) (E), and glibberellic acid (GA3) (F) in wild type rice Zhonghua 11. Eighteen OsWAKgs from the subgroup with the highest expression in leaves and roots (subgroup 6) were analyzed. Ten-day-old seedlings were used for chitin induction, 12-day-old seedlings for hormone (IAA, BR, and GA3) induction, and leaves of 30-day-old seedlings for induction with M. oryzae (strain GY1173) and Xoo (strain PXO99). Data are mean ± SD (n = 3).
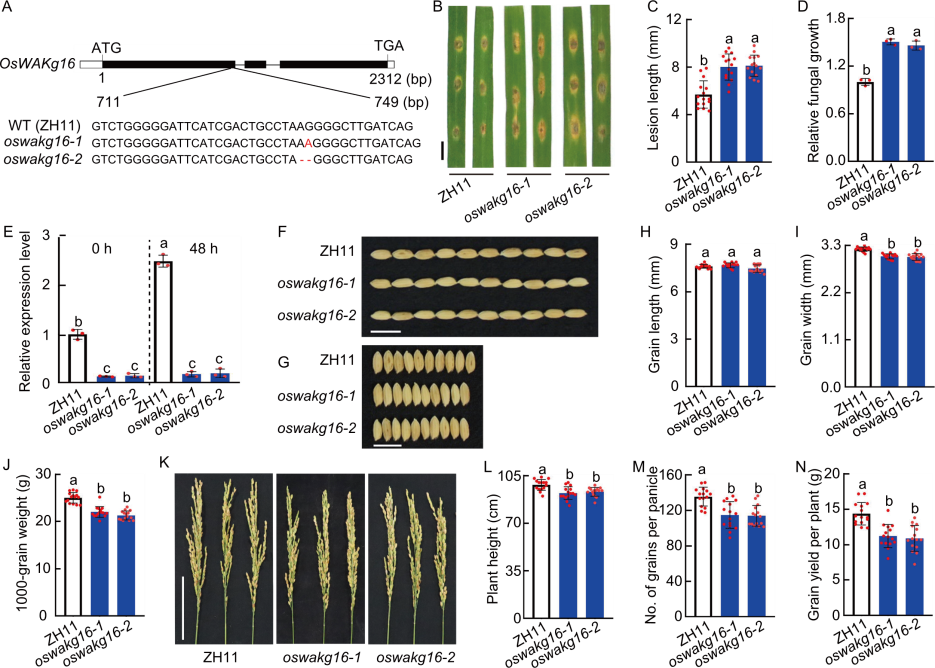
Fig. 5. Functional analysis of OsWAKg16. A, Diagram of CRISPR-Cas9 mediated mutation of OsWAKg16 in OsWAKg16-knockout lines (oswakg16-1 and oswakg16-2). Zhonghua 11 (ZH11) was used as the wild type (WT). ATG represents start codon and TGA represents stop codon. B, Blast resistance of ZH11 and OsWAKg16-knockout lines. Leaves of 30-day-old seedlings were punch inoculated with the Magnaporthe oryzae virulent isolate GY1173. Leaves were photographed at 6 d post-inoculation (dpi). Scale bar, 1 cm. C and D, Lesion legnth (C) and relative fungal growth (D) were measured at 6 dpi. Fungal growth was assessed by the fungal MoPot2 gene using qRT-PCR and normalized to the rice Ubiquitin gene. E, Analysis of OsWAKg16 expression in WT ZH11 and OsWAKg16-knockout lines before and after induction by M. oryzae. Leaves of 30-day-old seedlings were used for analysis, with Ubiquitin serving as the internal reference. F and G, Phenotypes of grain length (F) and width (G) of ZH11 and OsWAKg16-knockout lines. Scale bars, 1 cm. H‒J, Grain length (H), grain width (I), and 1000-grain weight (J) of ZH11 and OsWAKg16-knockout lines in field tests. K, Panicles of ZH11 and OsWAKg16-knockout lines. Scale bar, 10 cm. L‒N, Plant height (L), grain number per panicle (M), and grain yield per plant (N) of ZH11 and OsWAKg16-knockout lines in field tests. Data are mean ± SD (n = 15 in C, H‒J, and L‒N; 3 in D and E). Different lowercase letters indicate significant differences (P < 0.05) determined by Duncan’s multiple range test.
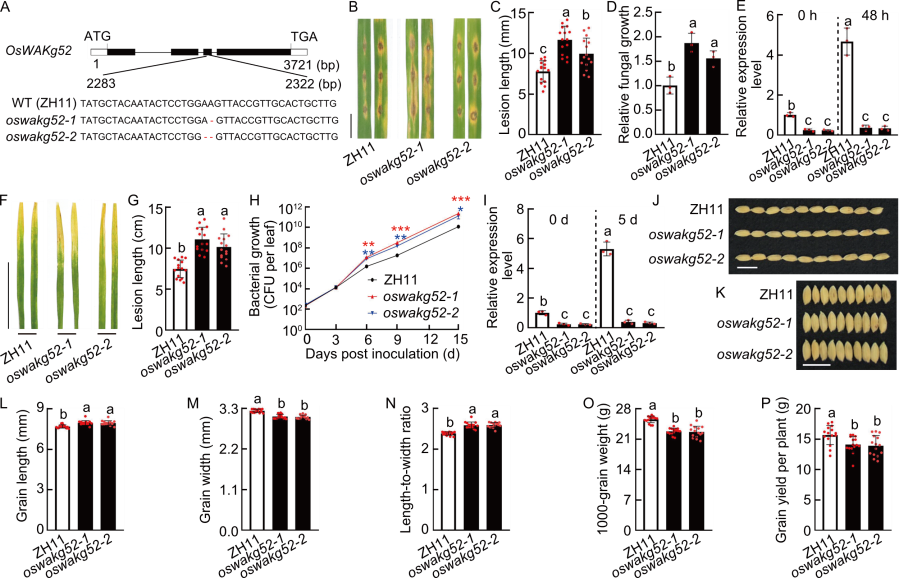
Fig. 6. Functional analysis of OsWAKg52. A, Diagram of CRISPR-Cas9 mediated mutation of OsWAKg52 in OsWAKg52-knockout lines (oswakg52-1 and oswakg52-2). Zhonghua 11 (ZH11) was used as the wild type (WT). ATG represents start codon and TGA represents stop codon. B, Blast resistance of ZH11 and OsWAKg52-knockout lines. Leaves of 30-day-old seedlings were punch inoculated with the Magnaporthe oryzae virulent isolate GY1173. Leaves were photographed at 6 d post-inoculation (dpi). Scale bar, 1 cm. C and D, Lesion length (C) and relative fungal growth (D) were measured at 6 dpi. Fungal growth was assessed by the fungal MoPot2 gene using qRT-PCR and normalized to the rice Ubiquitin gene. E, Analysis of OsWAKg52 expression in ZH11 and OsWAKg52-knockout lines before and after induction by M. oryzae. Leaves of 30-day-old seedlings were used for analysis, with Ubiquitin serving as the internal reference. F, Resistance analysis of ZH11 and OsWAKg52-knockout lines to bacterial blight. Leaves of 30-day-old seedlings were inoculated with Xanthomonas oryzae pv. oryzae (Xoo) strain PXO99. Leaves were photographed at 14 dpi. Scale bar, 10 cm. G, Lesion length was measured at 14 dpi. H, Growth of Xoo indicated by the number of colony-forming units (CFU) per leaf for ZH11 and OsWAKg52-knockout lines. *, P < 0.05; **, P < 0.01; ***, P < 0.001. I, Analysis of OsWAKg52 expression in ZH11 and OsWAKg52-knockout lines before and after induction by Xoo. Leaves of 30-day-old seedlings were used for analysis, with Ubiquitin serving as the internal reference. J and K, Grain size of ZH11 and OsWAKg52-knockout lines. Scale bars, 1 cm. L‒P, Grain length (L), grain width (M), length-to-width ratio (N), 1000-grain weight (O), and grain yield per plant (P) of ZH11 and OsWAKg52-knockout lines in field tests. Data are mean ± SD (n = 15 in C, G, and L‒P; 3 in D, E, and I; 5 in H). Different lowercase letters indicate significant differences (P < 0.05) determined by Duncan’s multiple range test.
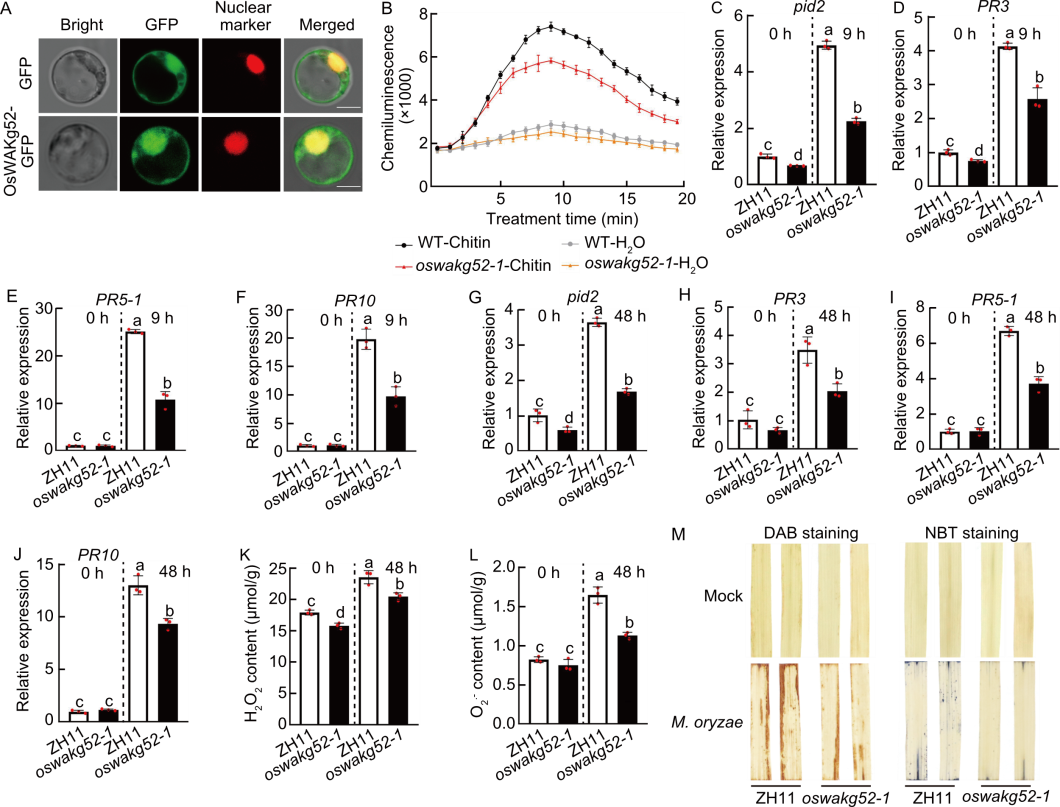
Fig. 7. OsWAKg52 enhanced resistance to rice blast and bacterial blight by positively regulating pattern-triggered immunity. A, Subcellular localization of OsWAKg52-GFP fusion protein in rice protoplasts. D53-mCherry was used as a nuclear marker. Scale bars, 10 μm. B, Chitin-induced reactive oxygen species (ROS) accumulation in Zhonghua 11 (ZH11, wild type, WT) and OsWAKg52-knockout lines (oswakg52-1). ROS was determined using a luminol-based chemiluminescence assay with H2O treatment as the negative control. Leaves of 30-day-old seedlings were used. C‒F, Relative expression levels of Pid2 (C), PR3 (D), PR5-1 (E), and PR10 (F) in ZH11 and oswakg52-1 before and after induction by chitin. Leaves of 10-day-old seedlings were used. Ubiquitin BQ serving as the internal reference. G‒J, Relative expression levels of Pid2 (G), PR3 (H), PR5-1 (I), and PR10 (J) in ZH11 and oswakg52-1 before and after induction with Magnaporthe oryzae. Leaves of 30-day-old seedlings were used. Ubiquitin serving as the internal reference. K and L, Measurements of H2O2 (K) and O2·̄ (L) contents in ZH11 and oswakg52-1 before and after induction of M. oryzae for 48 h. Leaves of 30-day-old seedlings were used. M, DAB (3,3′-diaminobenzidine) and NBT (nitro blue tetrazolium) staining of leaves from ZH11 and oswakg52-1 infected with M. oryzae for 48 h. Leaves of 30-day-old seedlings were used. Data are mean ± SD (n = 3). Different lowercase letters indicate significant differences (P < 0.05) determined by Duncan’s multiple range test.
| [1] | Bailey T L, Boden M, Buske F A, et al. 2009. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res, 37: W202-W208. |
| [2] | Bolser D M, Staines D M, Perry E, et al. 2017. Ensembl plants: Integrating tools for visualizing, mining, and analyzing plant genomic data. Methods Mol Biol, 1533: 1-31. |
| [3] | Cai W G, Hong J, Liu Z Y, et al. 2023. A receptor-like kinase controls the amplitude of secondary cell wall synthesis in rice. Curr Biol, 33(3): 498-506.e6. |
| [4] | Camacho C, Coulouris G, Avagyan V, et al. 2009. BLAST+: Architecture and applications. BMC Bioinformatics, 10: 421. |
| [5] | Chen C J, Chen H, Zhang Y, et al. 2020. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol Plant, 13(8): 1194-1202. |
| [6] | Chen R Z, Deng Y W, Ding Y L, et al. 2022. Rice functional genomics: Decades’ efforts and roads ahead. Sci China: Life Sci, 65(1): 33-92. |
| [7] | Chen Y P, Dan Z W, Li S Q. 2024. GROWTH REGULATING FACTOR 7-mediated arbutin metabolism enhances rice salt tolerance. Plant Cell, 36(8): 2834-2850. |
| [8] | Cheng M X, Yuan H R, Wang R H, et al. 2023. Identification and characterization of BES1 genes involved in grain size development of Oryza sativa L. Int J Biol Macromol, 253(Pt 6): 127327. |
| [9] | Dai Z Y, Tan J, Zhou C, et al. 2019. The OsmiR396-OsGRF8-OsF3H-flavonoid pathway mediates resistance to the brown planthopper in rice (Oryza sativa). Plant Biotechnol J, 17(8): 1657-1669. |
| [10] | de Oliveira L F V, Christoff A P, de Lima J C, et al. 2014. The wall-associated kinase gene family in rice genomes. Plant Sci, 229: 181-192. |
| [11] | Delteil A, Gobbato E, Cayrol B, et al. 2016. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol, 16: 17. |
| [12] | Deng Y W, Zhai K R, Xie Z, et al. 2017. Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science, 355: 962-965. |
| [13] | Fan F F, Liu M M, Li N N, et al. 2023. Gain-of-function allele of HPY1 coordinates source and sink to increase grain yield in rice. Sci Bull, 68(19): 2155-2159. |
| [14] | Finn R D, Clements J, Eddy S R. 2011. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res, 39: W29-W37. |
| [15] | Gao F, Wang K, Liu Y, et al. 2016. Blocking miR396 increases rice yield by shaping inflorescence architecture. Nat Plants, 2: 15196. |
| [16] | Goodstein D M, Shu S Q, Howson R, et al. 2012. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res, 40: D1178-D1186. |
| [17] | Guo J P, Wang H Y, Guan W, et al. 2023. A tripartite rheostat controls self-regulated host plant resistance to insects. Nature, 618: 799-807. |
| [18] | Harkenrider M, Sharma R, de Vleesschauwer D, et al. 2016. Overexpression of rice wall-associated kinase 25 (OsWAK25) alters resistance to bacterial and fungal pathogens. PLoS One, 11(1): e0147310. |
| [19] | Huluka W, Kumsa L. 2022. Analysis of rice (Oryza sativa L. ssp. japonica) wall associated receptor-like protein kinase gene’s promoter region and regulatory elements. Curr Plant Biol, 31: 100254. |
| [20] | Krzywinski M, Schein J, Birol I, et al. 2009. Circos: An information aesthetic for comparative genomics. Genome Res, 19(9): 1639-1645. |
| [21] | Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol, 33(7): 1870-1874. |
| [22] | Li H, Zhou S Y, Zhao W S, et al. 2009. A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol Biol, 69(3): 337-346. |
| [23] | Li W T, Zhu Z W, Chern M, et al. 2017. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell, 170(1): 114-126.e15. |
| [24] | Liu Y, Zhang X, Yuan G X, et al. 2021. A designer rice NLR immune receptor confers resistance to the rice blast fungus carrying noncorresponding avirulence effectors. Proc Natl Acad Sci USA, 118: e2110751118. |
| [25] | Long S P, Marshall-Colon A, Zhu X G. 2015. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell, 161(1): 56-66. |
| [26] | Long W X, Li N W, Jin J, et al. 2023. Resequencing-based QTL mapping for yield and resistance traits reveals great potential of Oryza longistaminata in rice breeding. Crop J, 11(5): 1541-1549. |
| [27] | Rao Y C, Li Y Y, Qian Q. 2014. Recent progress on molecular breeding of rice in China. Plant Cell Rep, 33(4): 551-564. |
| [28] | Sipahi H, Whyte T D, Ma G, et al. 2022. Genome-wide identification and expression analysis of wall-associated kinase (WAK) gene family in Cannabis sativa L. Plants, 11(20): 2703. |
| [29] | Subramanian B, Gao S H, Lercher M J, et al. 2019. Evolview v3: A webserver for visualization, annotation, and management of phylogenetic trees. Nucleic Acids Res, 47(W1): W270-W275. |
| [30] | Verica J A, Chae L, Tong H Y, et al. 2003. Tissue-specific and developmentally regulated expression of a cluster of tandemly arrayed cell wall-associated kinase-like kinase genes in Arabidopsis. Plant Physiol, 133(4): 1732-1746. |
| [31] | Wang J, Zhou L, Shi H, et al. 2018. A single transcription factor promotes both yield and immunity in rice. Science, 361: 1026-1028. |
| [32] | Wang Y, Yue J L, Yang N, et al. 2023. An ERAD-related ubiquitin-conjugating enzyme boosts broad-spectrum disease resistance and yield in rice. Nat Food, 4(9): 774-787. |
| [33] | Wang Y H, Xue Y B, Li J Y. 2005. Towards molecular breeding and improvement of rice in China. Trends Plant Sci, 10(12): 610-614. |
| [34] | Wang Y P, Tang H B, Debarry J D, et al. 2012. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res, 40(7): e49. |
| [35] | Yang J, Zhao X Y, Sun J, et al. 2010. A novel protein Com1 is required for normal conidium morphology and full virulence in Magnaporthe oryzae. Mol Plant Microbe Interact, 23(1): 112-123. |
| [36] | Yuan H R, Cheng M X, Fan F F, et al. 2024a. OsGRF6-OsYUCCA1/ OsWRKY82 signaling cascade upgrade grain yield and bacterial blight resistance in rice. Adv Sci, 11: e2407733. |
| [37] | Yuan H R, Cheng M X, Wang R H, et al. 2024b. miR396b/GRF6 module contributes to salt tolerance in rice. Plant Biotechnol J, 22(8): 2079-2092. |
| [38] | Yue Z L, Liu N, Deng Z P, et al. 2022. The receptor kinase OsWAK11 monitors cell wall pectin changes to fine-tune brassinosteroid signaling and regulate cell elongation in rice. Curr Biol, 32(11): 2454-2466.e7. |
| [39] | Zhang F, Fang H, Wang M, et al. 2022. APIP5 functions as a transcription factor and an RNA-binding protein to modulate cell death and immunity in rice. Nucleic Acids Res, 50(9): 5064-5079. |
| [40] | Zhang S B, Chen C, Li L, et al. 2005. Evolutionary expansion, gene structure, and expression of the rice wall-associated kinase gene family. Plant Physiol, 139(3): 1107-1124. |
| [41] | Zhang Z Q, Ma W Y, Ren Z Y, et al. 2021. Characterization and expression analysis of wall-associated kinase (WAK) and WAK-like family in cotton. Int J Biol Macromol, 187: 867-879. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||