
Rice Science ›› 2025, Vol. 32 ›› Issue (4): 561-574.DOI: 10.1016/j.rsci.2025.03.005
收稿日期:2024-12-03
接受日期:2025-02-18
出版日期:2025-07-28
发布日期:2025-08-06
. [J]. Rice Science, 2025, 32(4): 561-574.

Fig. 1. Leaf (A) and root (B) dry weights of rice plants. Data were collected after 7 d of hydroponic cultivation with combinations of cadmium (Cd, 5 and 20 μmol/L, Cd5 and Cd20, respectively), nickel (Ni, 20 μmol/L), and selenium (Se, as selenate Se6+ or selenite Se4+, each at 5 μmol/L). CK, Control. Data are mean ± SE (n = 9). Different lowercase letters above bars identify significant differences among treatments after analysis of variance followed by Tukey’s HSD test (P < 0.05).

Fig. 2. Thiobarbituric acid reactive substances (TBARS) as an indictor of lipid peroxidation in hydroponic cultivation rice leaves. Data were collected after 7 d of hydroponic cultivation with combinations of cadmium (Cd, 5 and 20 μmol/L, Cd5 and Cd20, respectively), nickel (Ni, 20 μmol/L), and selenium (Se, as selenate Se6+ or selenite Se4+, each at 5 μmol/L). CK, Control. Data are mean ± SE (n = 3). Different lowercase letters above bars identify significant differences among treatments after analysis of variance followed by Tukey’s HSD test (P < 0.05).

Fig. 3. Cadmium (Cd), nickel (Ni), and selenium (Se) concentrations in leaves and roots of rice plants. A‒C, Cd (A), Ni (B), and Se (C) concentrations in leaves of rice plants. D‒F, Cd (D), Ni (E), and Se (F) concentrations in roots of rice plants. Data were collected after 7 d of hydroponic cultivation with combinations of cadmium (Cd, 5 and 20 μmol/L, Cd5 and Cd20, respectively), nickel (Ni, 20 μmol/L), and selenium (Se, as selenate Se6+ or selenite Se4+, each at 5 μmol/L). CK, Control.

Fig. 4. Translocation factors (TF) of cadmium (Cd, A), nickel (Ni, B), and selenium (Se, C) of rice plants. Data were collected after 7 d of hydroponic cultivation with combinations of cadmium (Cd, 5 and 20 μmol/L, Cd5 and Cd20, respectively), nickel (Ni, 20 μmol/L), and selenium (Se, as selenate Se6+ or selenite Se4+, each at 5 μmol/L). CK, Control.

Fig. 5. Distribution of selenium (Se), nickel (Ni), and iron (Fe) in roots of rice plants treated with Se and Ni. A‒C, Distribution of Se (A), Ni (B), and Fe (C) in roots of rice plants treated with 5 μmol/L Se6+ and 20 μmol/L Ni. D, Tricolor map showing simultaneous distribution of Se (red), Ni (green), and Fe (blue). Scale bars, 0.5 mm.
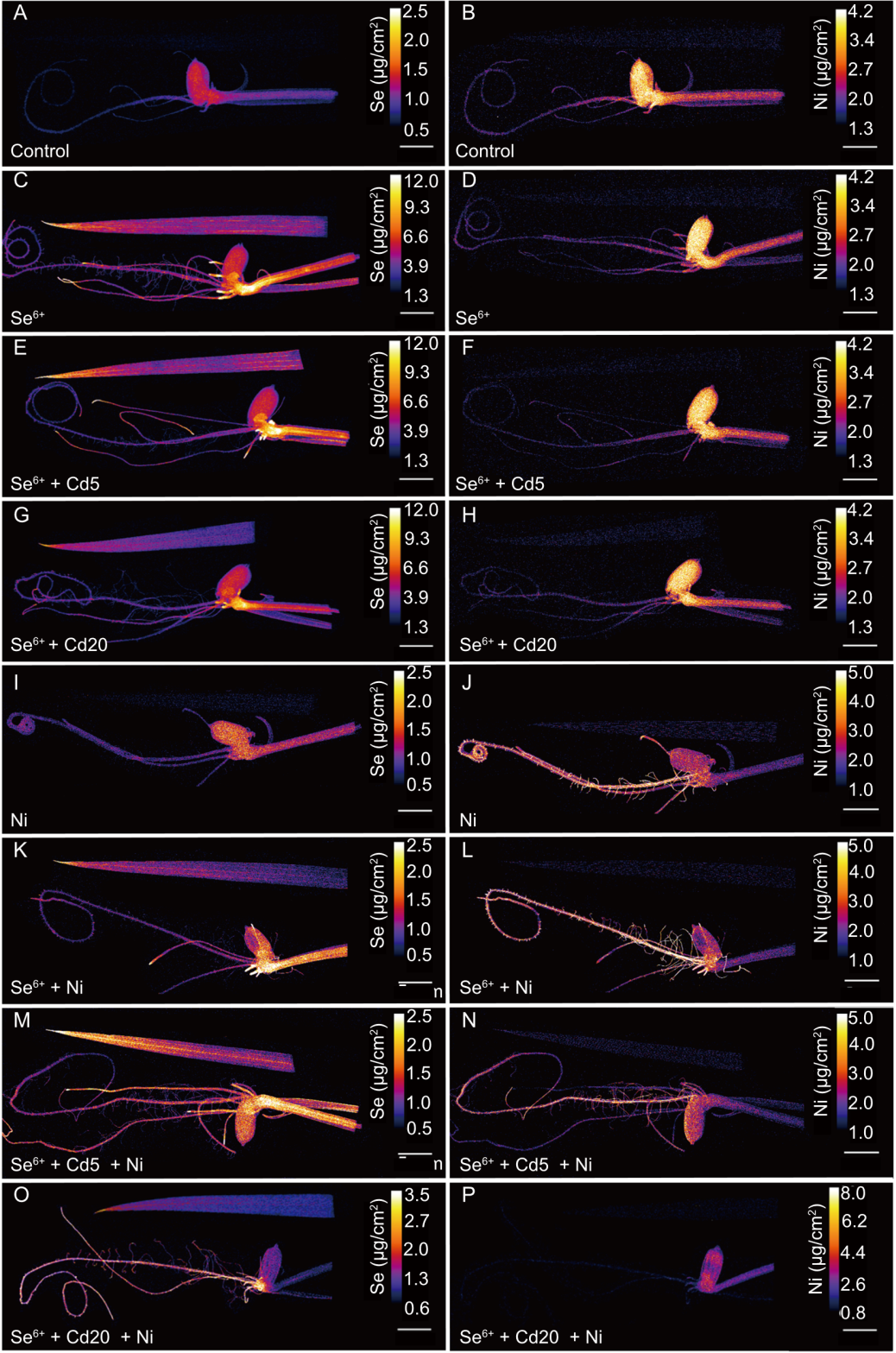
Fig. 6. Distribution of selenium (Se) and nickel (Ni) in leaves and roots of rice plants treated with 5 μmol/L Se6+ and different concentrations of cadmium (Cd) and Ni. A, C, E, G, I, K, M, and O, Distribution of Se in leaves and roots of rice plants treated with control (A), Se6+ (C), Se6+ and 5 μmol/L Cd (E), Se6+ and 20 μmol/L Cd (G), Ni (I), Se6+ and Ni (K), Se6+, 5 μmol/L Cd, and Ni (M), as well as Se6+, 20 μmol/L Cd, and Ni (O). B, D, F, H, J, L, N, and P, Distribution of Ni in leaves and roots of rice plants treated with control (B), Se6+ (D), Se6+ and 5 μmol/L Cd (F), Se6+ and 20 μmol/L Cd (H), Ni (J), Se6+ and Ni (L), Se6+, 5 μmol/L Cd, and Ni (N), as well as Se6+, 20 μmol/L Cd, and Ni (P). The concentrations used for Se6+ and Ni are 5 and 20 μmol/L, respectively. Scale bars, 5 mm.

Fig. 7. Distribution of selenium (Se) and nickel (Ni) in leaves and roots of rice plants treated with 5 μmol/L Se4+ and different concentrations of cadmium (Cd) and Ni. A, C, E, G, I, K, M, and O, Distribution of Se in leaves and roots of rice plants treated with control (A), Se4+ (C), Se4+ and 5 μmol/L Cd (E), Se4+ and 20 μmol/L Cd (G), Ni (I), Se4+ and Ni (K), Se4+, 5 μmol/L Cd, and Ni (M), as well as Se4+, 20 μmol/L Cd, and Ni (O). B, D, F, H, J, L, N, and P, Distribution of Ni in leaves and roots of rice plants treated with control (B), Se4+ (D), Se4+ and 5 μmol/L Cd (F), Se4+ and 20 μmol/L Cd (H), Ni (J), Se4+ and Ni (L), Se4+, 5 μmol/L Cd, and Ni (N), as well as Se4+, 20 μmol/L Cd, and Ni (P). The concentrations used for Se4+ and Ni are 5 and 20 μmol/L, respectively. Scale bars, 5 mm.
| [1] | Ali W, Mao K, Zhang H, et al. 2020. Comprehensive review of the basic chemical behaviours, sources, processes, and endpoints of trace element contamination in paddy soil-rice systems in rice-growing countries. J Hazard Mater, 397: 122720. |
| [2] | Boesenberg U, Ryan C G, Kirkham R, et al. 2016. Fast X-ray microfluorescence imaging with submicrometer-resolution integrating a Maia detector at beamline P06 at PETRA III. J Synchrot Radiat, 23(6): 1550-1560. |
| [3] | Cai Y X, Zhang S H, Cai K Z, et al. 2020. Cd accumulation, biomass and yield of rice are varied with silicon application at different growth phases under high concentration cadmium-contaminated soil. Chemosphere, 242: 125128. |
| [4] | DalCorso G, Farinati S, Maistri S, et al. 2008. How plants cope with cadmium: Staking all on metabolism and gene expression. J Integr Plant Biol, 50(10): 1268-1280. |
| [5] | El Mehdawi A F, Jiang Y, Guignardi Z S, et al. 2018. Influence of sulfate supply on selenium uptake dynamics and expression of sulfate/selenate transporters in selenium hyperaccumulator and nonhyperaccumulator Brassicaceae. New Phytol, 217(1): 194-205. |
| [6] | Ellis D R, Salt D E. 2003. Plants, selenium and human health. Curr Opin Plant Biol, 6(3): 273-279. |
| [7] | European Union.. 2024 Commission Regulation (EU) 2024. 2024/1987 of 30 July 2024 amending Regulation (EU) 2023/915 as regards maximum levels of nickel in certain foodstuffs. [2024-10-22]. https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=OJ:L_202401987. |
| [8] | Farooq M U, Tang Z C, Zheng T D, et al. 2019. Cross-talk between cadmium and selenium at elevated cadmium stress determines the fate of selenium uptake in rice. Biomolecules, 9(6): 247. |
| [9] | Feng R W, Wei C Y, Tu S X, et al. 2013. A dual role of Se on Cd toxicity: Evidences from the uptake of Cd and some essential elements and the growth responses in paddy rice. Biol Trace Elem Res, 151(1): 113-121. |
| [10] | Genchi G, Carocci A, Lauria G, et al. 2020. Nickel: Human health and environmental toxicology. Int J Environ Res Public Health, 17(3): 679. |
| [11] | Guo Y K, Mao K, Cao H R, et al. 2021. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ Pollut, 268 (Pt A): 115829. |
| [12] | Gupta M, Gupta S. 2017. An overview of selenium uptake, metabolism, and toxicity in plants. Front Plant Sci, 7: 02074. |
| [13] | Hasanuzzaman M, Borhannuddin Bhuyan M H M, Raza A, et al. 2020. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants, 9(12): 1711. |
| [14] | Hawrylak-Nowak B, Matraszek-Gawron R. 2020. Difference between selenite and selenate in the regulation of growth and physiological parameters of nickel-exposed lettuce. Biology, 9(12): 465. |
| [15] | Hodges D M, DeLong J M, Forney C F, et al. 1999. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta, 207(4): 604-611. |
| [16] | Huang F Y, Chen L, Zhou Y, et al. 2024. Exogenous selenium promotes cadmium reduction and selenium enrichment in rice: Evidence, mechanisms, and perspectives. J Hazard Mater, 476: 135043. |
| [17] | Huang Q Q, Liu Y Y, Qin X, et al. 2019. Selenite mitigates cadmium-induced oxidative stress and affects Cd uptake in rice seedlings under different water management systems. Ecotoxicol Environ Saf, 168: 486-494. |
| [18] | Jing H N, Yang W T, Chen Y L, et al. 2023. Exploring the mechanism of Cd uptake and translocation in rice: Future perspectives of rice safety. Sci Total Environ, 897: 165369. |
| [19] | Khan M S, Soyk A, Wolf I, et al. 2022. Discriminative long-distance transport of selenate and selenite triggers glutathione oxidation in specific subcellular compartments of root and shoot cells in Arabidopsis. Front Plant Sci, 13: 894479. |
| [20] | Kolbert Z, Molnár Á, Feigl G, et al. 2019. Plant selenium toxicity: Proteome in the crosshairs. J Plant Physiol, 232: 291-300. |
| [21] | Kumarathilaka P, Seneweera S, Meharg A, et al. 2018. Arsenic speciation dynamics in paddy rice soil-water environment: Sources, physico-chemical, and biological factors: A review. Water Res, 140: 403-414. |
| [22] | Li Y X, Zhu N L, Liang X J, et al. 2020. A comparative study on the accumulation, translocation and transformation of selenite, selenate, and SeNPs in a hydroponic-plant system. Ecotoxicol Environ Saf, 189: 109955. |
| [23] | Li Z M, Liang Y, Hu H W, et al. 2021. Speciation, transportation, and pathways of cadmium in soil-rice systems: A review on the environmental implications and remediation approaches for food safety. Environ Int, 156: 106749. |
| [24] | Liang T S, Ding H, Wang G D, et al. 2016. Sulfur decreases cadmium translocation and enhances cadmium tolerance by promoting sulfur assimilation and glutathione metabolism in Brassica chinensis L. Ecotoxicol Environ Saf, 124: 129-137. |
| [25] | Liu J L, Feng X Y, Qiu G Y, et al. 2023. Inhibition roles of calcium in cadmium uptake and translocation in rice: A review. Int J Mol Sci, 24(14): 11587. |
| [26] | Liu R, Deng Y, Zheng M L, et al. 2022. Nano selenium repairs the fruit growth and flavor quality of tomato under the stress of penthiopyrad. Plant Physiol Biochem, 184: 126-136. |
| [27] | Liu R, Zhou W J, Yu D, et al. 2024. Spatial distribution and enrichment characteristics of selenium in paddy soil and rice around the Dongting Lake. Environ Pollut, 359: 124552. |
| [28] | Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant, 15(3): 473-497. |
| [29] | Nakanishi H, Ogawa I, Ishimaru Y, et al. 2006. Iron deficiency enhances cadmium uptake and translocation mediated by the Fe2+ transporters OsIRT1 and OsIRT2 in rice. Soil Sci Plant Nutr, 52(4): 464-469. |
| [30] | Nishida S, Tsuzuki C, Kato A, et al. 2011. AtIRT1, the primary iron uptake transporter in the root, mediates excess nickel accumulation in Arabidopsis thaliana. Plant Cell Physiol, 52(8): 1433-1442. |
| [31] | Nishida S, Kato A, Tsuzuki C, et al. 2015. Induction of nickel accumulation in response to zinc deficiency in Arabidopsis thaliana. Int J Mol Sci, 16(5): 9420-9430. |
| [32] | Padoan E, Passarella I, Prati M, et al. 2020. The suitability of short rotation coppice crops for phytoremediation of urban soils. Appl Sci, 10(1): 307. |
| [33] | Pilon-Smits E A, Quinn C F, Tapken W, et al. 2009. Physiological functions of beneficial elements. Curr Opin Plant Biol, 12(3): 267-274. |
| [34] | Purwadi I, Gei V, Erskine P D, et al. 2020. Tools for the discovery of hyperaccumulator plant species in the field and in the herbarium. In: van der Ent A, Baker A J M, Echevarria G, et al. Agromining: Farming for Metals: Mineral Resource Reviews series. 2nd edition. Springer International Publishing: 183-195. |
| [35] | Qu L L, Xu J Y, Dai Z H, et al. 2023. Selenium in soil-plant system: Transport, detoxification and bioremediation. J Hazard Mater, 452: 131272. |
| [36] | Rayman M P. 2020. Selenium intake, status, and health: A complex relationship. Hormones, 19(1): 9-14. |
| [37] | Rinklebe J, Shaheen S M. 2017. Redox chemistry of nickel in soils and sediments: A review. Chemosphere, 179: 265-278. |
| [38] | Riyazuddin R, Nisha N, Ejaz B, et al. 2022. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules, 12(1): 43. |
| [39] | Sarwar N, Saifullah, Malhi S S, et al. 2010. Role of mineral nutrition in minimizing cadmium accumulation by plants. J Sci Food Agric, 90(6): 925-937. |
| [40] | Sasaki A, Yamaji N, Yokosho K, et al. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell, 24(5): 2155-2167. |
| [41] | Schaaf G, Honsbein A, Meda A R, et al. 2006. AtIREG2 encodes a tonoplast transport protein involved in iron-dependent nickel detoxification in Arabidopsis thaliana roots. J Biol Chem, 281: 25532-25540. |
| [42] | Schiavon M, Berto C, Malagoli M, et al. 2016. Selenium biofortification in radish enhances nutritional quality via accumulation of methyl-selenocysteine and promotion of transcripts and metabolites related to glucosinolates, phenolics, and amino acids. Front Plant Sci, 7: 1371. |
| [43] | Schiavon M, Pilon-Smits E A H. 2017. The fascinating facets of plant selenium accumulation: Biochemistry, physiology, evolution and ecology. New Phytol, 213(4): 1582-1596. |
| [44] | Schiavon M, Nardi S, Dalla Vecchia F, et al. 2020. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil, 453(1/2): 245-270. |
| [45] | Schneider C A, Rasband W S, Eliceiri K W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9(7): 671-675. |
| [46] | Schoonjans T, Brunetti A, Golosio B, et al. 2011. The xraylib library for x-ray-matter interactions: New developments and applications. In: DelRio M S, Chubar O. Advances in Computational Methods for X-Ray Optics II. Bellingham, WA, USA: SPIE-Int Soc Optical Engineering: 776-784. |
| [47] | Sharma S, Kaur I, Nagpal A K. 2021. Contamination of rice crop with potentially toxic elements and associated human health risks: A review. Environ Sci Pollut Res Int, 28(10): 12282-12299. |
| [48] | Siemianowski O, Barabasz A, Kendziorek M, et al. 2014. HMA4 expression in tobacco reduces Cd accumulation due to the induction of the apoplastic barrier. J Exp Bot, 65(4): 1125-1139. |
| [49] | Solé V A, Papillon E, Cotte M, et al. 2007. A multiplatform code for the analysis of energy-dispersive X-ray fluorescence spectra. Spectroc Acta Pt B: Atom Spectr, 62(1): 63-68. |
| [50] | Somagattu P, Chinnannan K, Yammanuru H, et al. 2024. Selenium dynamics in plants: Uptake, transport, toxicity, and sustainable management strategies. Sci Total Environ, 949: 175033. |
| [51] | Takahashi R, Ishimaru Y, Senoura T, et al. 2011. The OsNRAMP1 iron transporter is involved in Cd accumulation in rice. J Exp Bot, 62(14): 4843-4850. |
| [52] | Tang H, Li T X, Yu H Y, et al. 2016. Cadmium accumulation characteristics and removal potentials of high cadmium accumulating rice line grown in cadmium-contaminated soils. Environ Sci Pollut Res Int, 23(15): 15351-15357. |
| [53] | Tolu J, Bouchet S, Helfenstein J, et al. 2022. Understanding soil selenium accumulation and bioavailability through size resolved and elemental characterization of soil extracts. Nat Commun, 13(1): 6974. |
| [54] | Trippe 3rd R C, Pilon-Smits E A H. 2021. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J Hazard Mater, 404(Pt B): 124178. |
| [55] | van der Ent A, Przybyłowicz W J, de Jonge M D, et al. 2018. X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phytol, 218(2): 432-452. |
| [56] | van der Ent A, Salinitro M, Brueckner D, et al. 2023. Differences and similarities in selenium biopathways in Astragalus, Neptunia (Fabaceae) and Stanleya (Brassicaceae) hyperaccumulators. Ann Bot, 132(2): 349-361. |
| [57] | van der Pas L, Ingle R A. 2019. Towards an understanding of the molecular basis of nickel hyperaccumulation in plants. Plants, 8(1): 11. |
| [58] | Wan N, Xu Z, Chi Q R, et al. 2019. microRNA-33-3p involved in selenium deficiency-induced apoptosis via targeting ADAM10 in the chicken kidney. J Cell Physiol, 234(8): 13693-13704. |
| [59] | Wan Y N, Yu Y, Wang Q, et al. 2016. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol Environ Saf, 133: 127-134. |
| [60] | White P J. 2018. Selenium metabolism in plants. Biochim Biophys Acta: Gen Subj, 1862(11): 2333-2342. |
| [61] | Wu J W, Li R J, Lu Y, et al. 2021. Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review. J Environ Manage, 295: 113081. |
| [62] | Wu W J, Qi D Q, Chen Y L, et al. 2024. Exogenous selenium mitigates cadmium uptake and accumulation in two rice (Oryza sativa L.) varieties in cadmium-contaminated soil. Sci Rep, 14(1): 21248. |
| [63] | Yang H, Yang X F, Ning Z P, et al. 2022. The beneficial and hazardous effects of selenium on the health of the soil-plant-human system: An overview. J Hazard Mater, 422: 126876. |
| [64] | Zhang L H, Chu C C. 2022. Selenium uptake, transport, metabolism, reutilization, and biofortification in rice. Rice, 15(1): 30. |
| [65] | Zhang M, Tang S H, Huang X, et al. 2014. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ Exp Bot, 107: 39-45. |
| [66] | Zhang Z X, Lu Y, Li H P, et al. 2023. The role of nickel in cadmium accumulation in rice. Sci Total Environ, 859(Pt 2): 160421. |
| [67] | Zhao Y L, Zhang C B, Wang C R, et al. 2020. Increasing phosphate inhibits cadmium uptake in plants and promotes synthesis of amino acids in grains of rice. Environ Pollut, 257: 113496. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||