
Rice Science ›› 2025, Vol. 32 ›› Issue (6): 831-844.DOI: 10.1016/j.rsci.2025.08.004
• Research Papers • Previous Articles Next Articles
Pattanapong Jaisue1,2, Chalongrat Daengngam3,4, Panuwat Pengphorm3,4, Surapa Nutthapornnitchakul5, Sompop Pinit6, Lompong Klinnawee1,2( )
)
Received:2025-04-10
Accepted:2025-08-01
Online:2025-11-28
Published:2025-12-04
Contact:
Lompong Klinnawee (Pattanapong Jaisue, Chalongrat Daengngam, Panuwat Pengphorm, Surapa Nutthapornnitchakul, Sompop Pinit, Lompong Klinnawee. Enhanced Chlorophyll Accumulation is Early Response of Rice to Phosphorus Deficiency[J]. Rice Science, 2025, 32(6): 831-844.
Add to citation manager EndNote|Ris|BibTeX

Fig. 1. Effect of environmental inorganic phosphate (Pi) concentrations on photosynthetic pigment content, photosynthesis, and oxidative stress indicators. A-I, Contents of Pi (A), photosynthetic pigments [chlorophyll a (B), chlorophyll b (C), and carotenoids (D)], photosynthetic parameters [the quantum yield of photosystem II (Phi2, E) and non-photochemical quenching (PhiNPQ, F)], and oxidative stress indicators [H2O2 (G), malondialdehyde (MDA, H), and phenolic (I) contents] were measured in the first (L1) and second (L2) fully expanded leaves in rice seedlings grown hydroponically for 2 weeks with Pi concentrations varying from 0 to 500 µmol/L. Data are mean ± SD (n = 3). Different lowercase letters above bars indicate significant differences among the treatments in each leaf age by one-way analysis of variance, with the Least Significant Difference test.
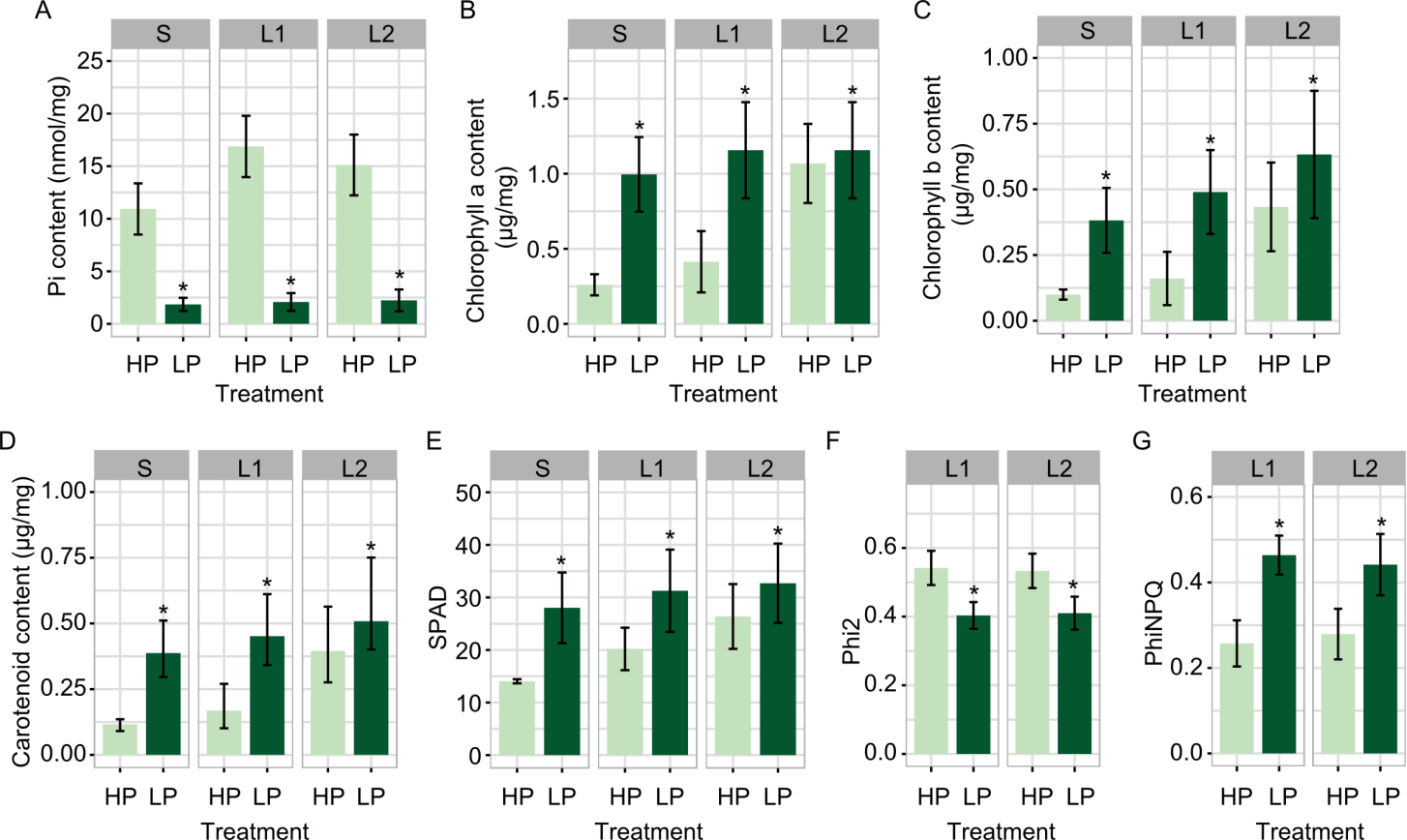
Fig. 2. Effect of phosphorus (P) deficiency on photosynthetic pigment content and photosynthesis of KDML 105 rice at the tillering stage. A-G, Contents of inorganic phosphate (Pi, A), photosynthetic pigments [chlorophyll a (B), chlorophyll b (C), and carotenoids (D)], and SPAD (Soil plant analysis development) value (E) were assessed in shoot (S), and the first (L1) and second (L2) fully expanded leaves, and photosynthetic parameters [the quantum yield of photosystem II (Phi2, F) and non-photochemical quenching (PhiNPQ, G)] were determined in L1 and L2 of 6-week-old KDML105 rice seedlings grown in pots under high (HP) and low P (LP) conditions. Data are mean ± SD (n = 3). * represents significant differences at the 0.05 level between the HP and LP treatments using the Student’s t-test.
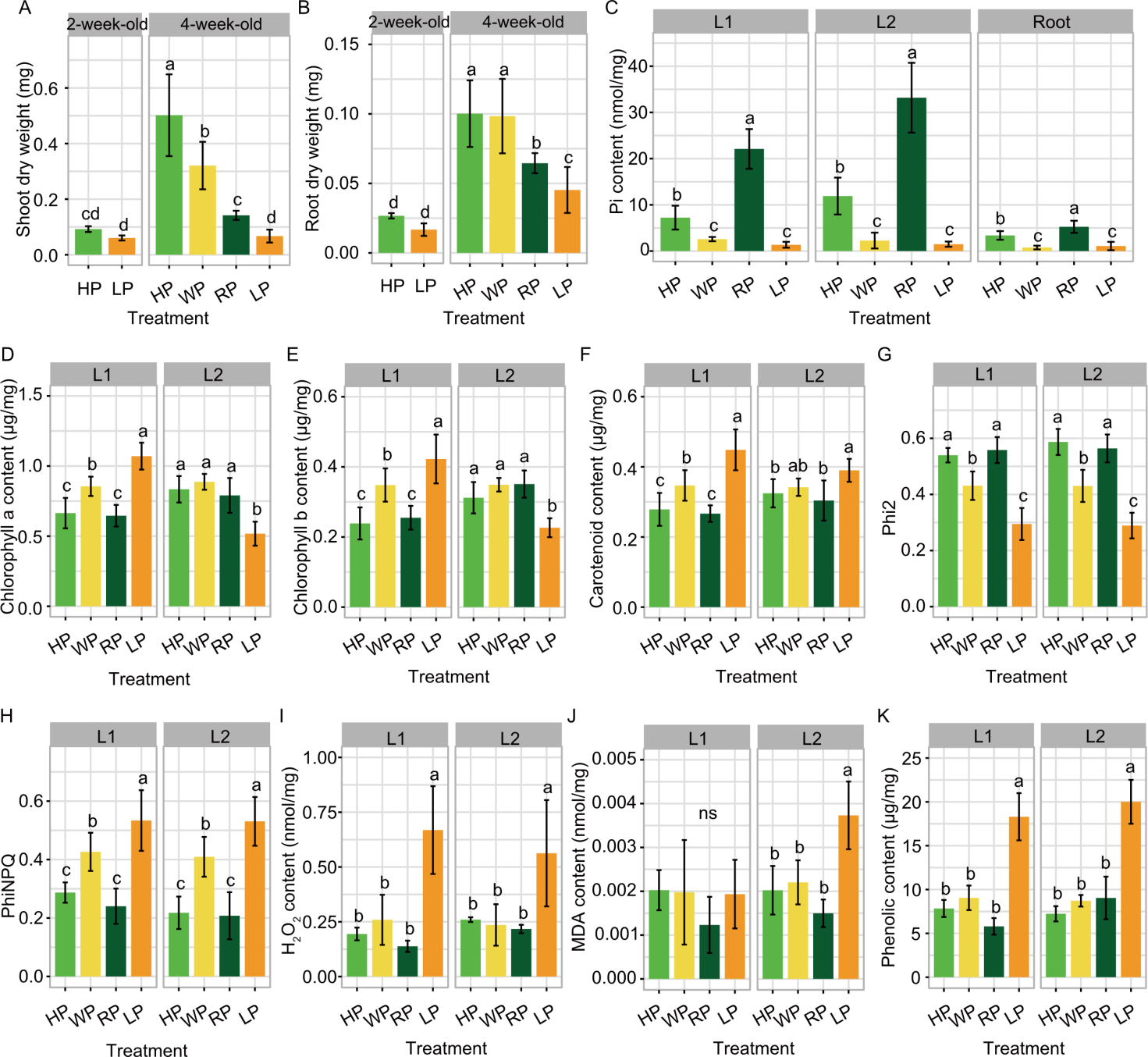
Fig. 3. Effects of various phosphorus (P) conditions on photosynthetic pigment content, photosynthetic parameters, and oxidative stresses in KDML 105 rice seedlings. A and B, Shoot dry weights (A) and root dry weights (B) were recorded in 2-week-old and 4-week-old seedlings. C, Inorganic phosphate (Pi) contents in the first (L1) and second (L2) fully expanded leaves and roots of rice seedlings. D-K, Photosynthetic pigments [chlorophyll a (D), chlorophyll b (E), and carotenoids (F)], photosynthetic parameters [the quantum yield of photosystem II (Phi2, G) and non-photochemical quenching (PhiNPQ, H)], and oxidative stresses [H2O2 (I), malondialdehyde (MDA, J), and phenolic (K) contents] were measured in L1 and L2 leaves of rice seedlings grown under hydroponic conditions for 4 weeks. Rice seedlings were subjected to the high P (HP), withdrawn P (WP), resupplied P (RP), and low P (LP) conditions. Data are mean ± SD (n = 3). Different lowercase letters above bars indicate significant differences among the treatments in each leaf age or root by one-way analysis of variance, with the Least Significant Difference test.

Fig. 4. Hyperspectral analyses of rice seedling leaves treated with different phosphorus (P) regimes. A and B, Reflectance spectra of the first (L1, A) and second (L2, B) fully explanded leaves across different treatments. C and D, Principal component (PC) analysis score plots for the first three components are shown for L1 (C) and L2 (D) samples, where color labels represent the different treatments. E and F, Percentage-based confusion matrices represent classification accuracy across P treatments for L1 (E) and L2 (F) samples. Data are from three biological replicates. Rice seedlings were hydroponically grown for 4 weeks in four different P treatments: high P (HP), P withdrawal (WP), P resupply (RP), and low P (LP).
| [1] | Abbas M, Shah J A, Irfan M, et al. 2018. Remobilization and utilization of phosphorus in wheat cultivars under induced phosphorus deficiency. J Plant Nutr, 41(12): 1522-1533. |
| [2] | Adem G D, Ueda Y, Hayes P E, et al. 2020. Genetic and physiological traits for internal phosphorus utilization efficiency in rice. PLoS One, 15(11): e0241842. |
| [3] | Agathokleous E, Feng Z Z, Peñuelas J. 2020. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants. Sci Total Environ, 726: 138637. |
| [4] | Calatayud A, Barreno E. 2004. Response to ozone in two lettuce varieties on chlorophyll a fluorescence, photosynthetic pigments and lipid peroxidation. Plant Physiol Biochem, 42(6): 549-555. |
| [5] | Carstensen A, Herdean A, Schmidt S B, et al. 2018. The impacts of phosphorus deficiency on the photosynthetic electron transport chain. Plant Physiol, 177(1): 271-284. |
| [6] | Chiera J, Thomas J, Rufty T. 2002. Leaf initiation and development in soybean under phosphorus stress. J Exp Bot, 53: 473-481. |
| [7] | Fu Y Q, Yang X J, Shen H. 2014. The physiological mechanism of enhanced oxidizing capacity of rice (Oryza sativa L.) roots induced by phosphorus deficiency. Acta Physiol Plant, 36(1): 179-190. |
| [8] | Goufo P, Trindade H. 2014. Rice antioxidants: Phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, γ-oryzanol, and phytic acid. Food Sci Nutr, 2(2): 75-104. |
| [9] | Hou W F, Tränkner M, Lu J W, et al. 2020. Diagnosis of nitrogen nutrition in rice leaves influenced by potassium levels. Front Plant Sci, 11: 165. |
| [10] | Huang J, Wang H M, Dai Q, et al. 2014. Analysis of NDVI data for crop identification and yield estimation. IEEE J Sel Top Appl Earth Observ Remote Sens, 7(11): 4374-4384. |
| [11] | Irfan M, Aziz T, Maqsood M A, et al. 2019. Differential performance of lowland rice cultivars for phosphorus uptake and utilization efficiency under hydroponic and soil conditions. Int J Agric Biol, 21(3): 703-710. |
| [12] | Irfan M, Aziz T, Maqsood M A, et al. 2020. Phosphorus (P) use efficiency in rice is linked to tissue-specific biomass and P allocation patterns. Sci Rep, 10(1): 4278. |
| [13] | Jamaludin R, Mat N, Mohd K S, et al. 2020. Influence of exogenous hydrogen peroxide on plant physiology, leaf anatomy and Rubisco gene expression of the Ficus deltoidea Jack var. deltoidea. Agronomy, 10(4): 497. |
| [14] | Jiang R, Sanchez-Azofeifa A, Laakso K, et al. 2021. UAV-based partially sampling system for rapid NDVI mapping in the evaluation of rice nitrogen use efficiency. J Clean Prod, 289: 125705. |
| [15] | Kaur A, Zhawar V, Dhillon B. 2023. Phosphorus uptake relates to vegetative growth, grain yield and grain quality in phosphorus deprived rice genotypes. Iran J Plant Physiol, 5(4): 4689. |
| [16] | Kaur S, Kumari A, Sharma N, et al. 2022. Physiological and molecular response of colored wheat seedlings against phosphate deficiency is linked to accumulation of distinct anthocyanins. Plant Physiol Biochem, 170: 338-349. |
| [17] | Kavanová M, Lattanzi F A, Grimoldi A A, et al. 2006. Phosphorus deficiency decreases cell division and elongation in grass leaves. Plant Physiol, 141(2): 766-775. |
| [18] | Klinnawee L, Kaewchumnong K, Nualtem K. 2023. Effect of phosphorus deficiency on allelopathic activity of lowland indica rice. Scienceasia, 49(2): 184-191. |
| [19] | Kuhlgert S, Austic G, Zegarac R, et al. 2016. MultispeQ Beta: A tool for large-scale plant phenotyping connected to the open PhotosynQ network. R Soc Open Sci, 3(10): 160592. |
| [20] | Lanfer-Marquez U M, Barros R M C, Sinnecker P. 2005. Antioxidant activity of chlorophylls and their derivatives. Food Res Int, 38(8/9): 885-891. |
| [21] | Li X, Yang R, Li L, et al. 2023. Physiological and molecular responses of wheat to low light intensity. Agronomy, 13(1): 272. |
| [22] | Lichtenthaler H K. 1987. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Meth Enzymol, 148: 350-382. |
| [23] | Ma J Y, Chen T T, Lin J, et al. 2022. Functions of nitrogen, phosphorus and potassium in energy status and their influences on rice growth and development. Rice Sci, 29(2): 166-178. |
| [24] | Ma P, Zhou L, Liao X H, et al. 2023. Effects of low light after heading on the yield of direct seeding rice and its physiological response mechanism. Plants, 12(24): 4077. |
| [25] | Mahlein A K, Steiner U, Hillnhütter C, et al. 2012. Hyperspectral imaging for small-scale analysis of symptoms caused by different sugar beet diseases. Plant Methods, 8(1): 1-13. |
| [26] | Meng X, Chen W W, Wang Y Y, et al. 2021. Effects of phosphorus deficiency on the absorption of mineral nutrients, photosynthetic system performance and antioxidant metabolism in Citrus grandis. PLoS One, 16(2): e0246944. |
| [27] | Mishra V, Srivastava G, Prasad S M. 2009. Antioxidant response of bitter gourd (Momordica charantia L.) seedlings to interactive effect of dimethoate and UV-B irradiation. Sci Hortic, 120(3): 373-378. |
| [28] | Mostofa M G, Hossain M A, Fujita M, et al. 2015. Physiological and biochemical mechanisms associated with trehalose-induced copper-stress tolerance in rice. Sci Rep, 5: 11433. |
| [29] | Pamuta D, Siangliw M, Sanitchon J, et al. 2022. Physio-biochemical traits in improved ‘KDML105’ jasmine rice lines containing drought and salt tolerance gene under drought and salt stress. Chil J Agric Res, 82(1): 97-110. |
| [30] | Pengphorm P, Thongrom S, Daengngam C, et al. 2024. Optimal-band analysis for chlorophyll quantification in rice leaves using a custom hyperspectral imaging system. Plants, 13(2): 259. |
| [31] | Pérez-Gálvez A, Viera I, Roca M. 2020. Carotenoids and chlorophylls as antioxidants. Antioxidants, 9(6): 505. |
| [32] | Pinit S, Chadchawan S, Chaiwanon J. 2020. A simple high-throughput protocol for the extraction and quantification of inorganic phosphate in rice leaves. Appl Plant Sci, 8(10): e11395. |
| [33] | Pinit S, Ruengchaijatuporn N, Sriswasdi S, et al. 2022. Hyperspectral and genome-wide association analyses of leaf phosphorus status in local Thai indica rice. PLoS One, 17(4): e0267304. |
| [34] | Prathap V, Kumar S, Meena N L, et al. 2023. Phosphorus starvation tolerance in rice through combined physiological, biochemical, and proteome analyses. Rice Sci, 30(6): 613-631. |
| [35] | R Core Team. 2024. R: A language and environment for statistical computing (Version 5.12.10). [2025-03-05]. https://www.r-project.org/.. |
| [36] | Rao I M, Terry N. 1989. Leaf phosphate status, photosynthesis, and carbon partitioning in sugar beet: I. Changes in growth, gas exchange, and calvin cycle enzymes. Plant Physiol, 90(3): 814-819. |
| [37] | Saengwilai P J, Bootti P, Klinnawee L. 2023. Responses of rubber tree seedlings (Hevea brasiliensis) to phosphorus deficient soils. Soil Sci Plant Nutr, 69(2): 78-87. |
| [38] | Sara L, Saeheng S, Puttarak P, et al. 2024. Changes in metabolites and allelopathic effects of non-pigmented and black-pigmented lowland indica rice varieties in phosphorus deficiency. Rice Sci, 31(4): 434-448. |
| [39] | Sergiev I, Todorova D, Shopova E, et al. 2018. Effects of auxin analogues and heat stress on garden pea. Zemdirbyste, 105(3): 243-248. |
| [40] | Sonobe R, Sano T, Horie H. 2018. Using spectral reflectance to estimate leaf chlorophyll content of tea with shading treatments. Biosyst Eng, 175: 168-182. |
| [41] | Soree T, Sharma P B, Kaonongbua W, et al. 2024. The negative mycorrhizal growth response of host plants to Acaulospora cf. morrowiae irrespective of soil P availability. Rhizosphere, 30: 100909. |
| [42] | Thomas S, Kuska M T, Bohnenkamp D, et al. 2018. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J Plant Dis Prot, 125(1): 5-20. |
| [43] | Tougaard S L, Szameitat A, Møs P, et al. 2023. Leaf age and light stress affect the ability to diagnose P status in field grown potatoes. Front Plant Sci, 14: 1100318. |
| [44] | Trejo-Téllez L I, Estrada-Ortiz E, Gómez-Merino F C, et al. 2019. Flavonoid, nitrate and glucosinolate concentrations in Brassica species are differentially affected by photosynthetically active radiation, phosphate and phosphite. Front Plant Sci, 10: 371. |
| [45] | Valentine A J, Osborne B A, Mitchell D T. 2001. Interactions between phosphorus supply and total nutrient availability on mycorrhizal colonization, growth and photosynthesis of cucumber. Sci Hortic, 88(3): 177-189. |
| [46] | Vejchasarn P, Lynch J P, Brown K M. 2016. Genetic variability in phosphorus responses of rice root phenotypes. Rice, 9(1): 29. |
| [47] | Veronica N, Subrahmanyam D, Vishnu Kiran T, et al. 2017. Influence of low phosphorus concentration on leaf photosynthetic characteristics and antioxidant response of rice genotypes. Photosynthetica, 55(2): 285-293. |
| [48] | Wickham H. 2016. ggplot2: Elegant Graphics for Data Analysis (Version 3.5.1). New York, USA: Springer-Verlag. |
| [49] | Xu H X, Weng X Y, Yang Y. 2007. Effect of phosphorus deficiency on the photosynthetic characteristics of rice plants. Russ J Plant Physiol, 54(6): 741-748. |
| [50] | Zhang Q, Xing D K, Wu Y Y, et al. 2024. Effects of low-phosphorus stress on use of leaf intracellular water and nutrients, photosynthesis, and growth of Brassica napus L. Horticulturae, 10(8): 821. |
| [51] | Zhu D, Luo F, Zou R, et al. 2021. Integrated physiological and chloroplast proteome analysis of wheat seedling leaves under salt and osmotic stresses. J Proteomics, 234: 104097. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||