Md. Anamul HOQUE (anamul71@yahoo.com)
In order to elucidate the role of antioxidant responses in salinity tolerance in rice genotypes under salt stress, experiments were conducted using four rice varieties, including salt-sensitive BRRI dhan 28 and three salt-tolerant varieties BRRI dhan 47, BINA dhan 8 and BINA dhan 10. Thirty-day-old rice seedlings were transplanted into pots. At the active tillering stage (35 d after transplanting), plants were exposed to different salinity levels (0, 20, 40 and 60 mmol/L NaCl). Salt stress caused a significant reduction in growth for all the rice genotypes. Growth reduction was higher in the salt-sensitive genotype than in the salt-tolerant ones, and BINA dhan 10 showed higher salt tolerance in all measured physiological parameters. The reduction in shoot and root biomass was found to be minimal in BINA dhan 10. Chlorophyll content significantly decreased under salt stress except for BINA dhan 10. Proline content significantly increased in salt-tolerant rice genotypes with increased salt concentration, and the highest proline content was obtained from BINA dhan 10 under salt stress. Catalase and ascorbate peroxidase activities significantly decreased in salt-sensitive genotype whereas significantly increased in salt-tolerant ones with increasing salt concentration. However, salt stress significantly decreased guaiacol peroxidase activity in all the rice genotypes irrespective of salt tolerance. K+/Na+ ratio also significantly decreased in shoots and roots of all the rice genotypes. The salt-tolerant genotype BINA dhan 10 maintained higher levels of chlorophyll and proline contents as well as catalase and ascorbate peroxidase activities under salt stress, thus, this might be the underlying mechanism for salt tolerance.
Climate change is a serious environmental threat that causes sea level rise and thereby affects coastal areas of Bangladesh. Salinity is one of the major abiotic factors, limiting crop production worldwide. Approximately 7% of the world’ s total land area, 20% of the world’ s cultivated land area and nearly 50% of the world’ s irrigated land area are affected by salinity (Zhu, 2001). In Bangladesh, about 1.06 million hectares of arable lands are affected by soil salinity (SRDI, 2010). Salinity is a serious threat to rice production in the southern part of Bangladesh. Salinity imposes both ionic toxicity and osmotic stress to plants (Hasegawa et al, 2000; Zhu, 2003). Salt stress disturbs cytoplasmic K+/Na+ homeostasis, causing a decrease in K+/Na+ ratio in the cytosol (Zhu, 2003). Accumulation of excess Na+ and Cl- causes ionic imbalance that may impair the selectivity of root membranes and induce K+ deficiency in plants.
Rice is the most important cereal crop in the world, yielding one-third of the total carbohydrate sources. The total rice growing area in Bangladesh is about 10.83 million hectares, leading to the production of 33.54 million metric tons of rice. On the contrary, rice yield is very low in the salt soil. To increase rice yield per acre, it is imperative to know about physiological and biochemical changes in plants under salt stress.
Plants have evolved a variety of adaptive mechanisms to respond to salt stress. One of the main adaptive mechanisms is the accumulation of compatible solutes (Sharma and Dietz, 2006; Ashraf and Foolad, 2007), and proline is the most common one. Increased levels of endogenous proline accumulation in plants is correlated with enhanced salt tolerance (Hasegawa et al, 2000; Ashraf and Foolad, 2007; Boscaiu et al, 2012; Sripinyowanich et al, 2013), and induce oxidative stress tolerance by modulating the activities of antioxidant enzymes (Saeedipour, 2013). Proline accumulation also plays a regulatory role during plant growth under salt stress (Mattioli et al, 2008).
Salinity induces the production of reactive oxygen species (ROS) in plant cells (Hasegawa et al, 2000; Banu et al, 2009, 2010). ROS can act as signaling molecules, mediating many key physiological processes. Excess production of ROS is toxic to plants and causes oxidative damage to cellular constituents, leading to cell death (Noctor and Foyer, 1998; Banu et al, 2009, 2010). Plants possess enzymatic and non-enzymatic antioxidant defense systems to protect cells against the damaging effects of ROS. The major ROS-scavenging antioxidant enzymes are catalase (CAT), guaiacol peroxidase (POX) and ascorbate peroxidase (APX). Salt stress shows different effects on the components of antioxidant defense systems in plants (Hasegawa et al, 2000; Mittova et al, 2003; Demiral and Turkan, 2005; El-Shabrawi et al, 2010; Hasanuzzaman et al, 2014).
There is increasing evidence that salt stress has a significant effect on physiological and biochemical attributes of plants. Better understanding of physiological and biochemical characteristics of plants is vital for improving salt tolerance under salinity. To clarify the physiological and biochemical responses of salt-sensitive and salt-tolerant rice genotypes, we investigated the plant growth, chlorophyll content, proline content and activity of antioxidant enzymes in four contrasting rice genotypes exposed to salt stress, which can be used as index for in vitrosalt tolerance in rice.
Four rice genotypes, including one salt-sensitive variety (BRRI dhan 28) and three salt-tolerant varieties (BRRI dhan 47, BINA dhan 8 and BINA dhan 10), were exposed to different NaCl levels (0, 20, 40 and 60 mmol/L). The experiment was laid out in a randomized complete block design with four replications. Considerable spacing was maintained among the pots for the ease of management practices.
Pot experiments were carried out at a net-house (average temperature 24 º C and relative humidity 60%) of the Department of Soil Science, Bangladesh Agricultural University, Bangladesh to investigate the effects of salt stress on the growth as well as the physiological and biochemical characteristics of salt-sensitive and salt-tolerant rice.
Soils were collected from the Soil Science Field Laboratory, Bangladesh Agricultural University. The initial soil sample was silt loam having exchangeable Na 0.383 meq/100 g and exchangeable K 0.082 meq/100 g. Each 15 L plastic pot was filled with 8 kg soil and 5 L water, ensuring enough space to maintain flooded conditions. Thirty-day-old seedlings were transplanted into pots. No NaCl was added to soils for control treatment. For 20, 40 and 60 mmol/L NaCl treatments, 15.24, 30.47 and 45.71 g pure NaCl were dissolved in 1 000 mL water and then added to the pots, respectively, at the active tillering stage (five weeks after transplanting). The soil in the pot was flooded with salt solution and the EC values of soil for control, 20, 40 and 60 mmol/L NaCl treatments were 1.00, 2.25, 5.60 and 6.58 dS/m, respectively. The crops were harvested at two weeks after salt treatment. Whole plants (with root attached) were carefully uprooted from the soil so that the root system of the plants remained unaffected.
Plant height, root length, shoot fresh weight, shoot dry weight, root fresh weight, root dry weight and number of tillers per hill were recorded.
Assay of chlorophyll content
Chlorophyll content was measured according to Porra et al (1989). An aliquot of fresh leaf (0.5 g) was suspended in 10 mL of 80% acetone, mixed well and kept at room temperature in the dark for 7 d. The supernatant was collected after centrifugation at 5 000 r/min for 15 min. The sample absorbance was recorded at 645 and 663 nm using a spectrophotometer (Model 336001, Spectronic Instruments, USA).
Assay of proline content
Proline content was measured according to Bates et al (1973). An aliquot of fresh leaf (0.5 g) was homogenized in 10 mL of 3% sulfosalicylic acid, and the homogenate was centrifuged at 5 000 r/min for 15 min. A total of 2 mL supernatant was incubated with 2 mL acid ninhydrin (1.25 g ninhydrin dissolved in 30 mL glacial acetic acid and 20 mL of 6 mol/L phosphoric acid) and 2 mL glacial acetic acid for 1 h at 100 º C, and then quickly cooled in an ice bath. The colored reaction mixture was extracted with 4 mL toluene, and the absorbance was recorded at 520 nm.
Preparation of enzyme extract and assay of antioxidant enzymes
An aliquot fresh leaf (0.5 g) was homogenized with 5 mL of 50 mmol/L Tris-HCl buffer (pH 8.0) for CAT and 50 mmol/L KH2PO4 buffer (pH 7.0) for POX and APX. The homogenate was centrifuged at 5 000 r/min for 20 min, and the supernatant was then used for enzyme activity assays. CAT (EC: 1.11.1.6) activity was assayed as described by Islam et al (2009). POX (EC: 1.11.1.7) and APX (EC: 1.11.1.11) activities were assayed as described by Hoque et al (2007a, b).
Determination of K+ and Na+
Grain and straw samples were dried in an oven at about 65 º C for 48 h, and then samples were ground in a grinding machine to pass through a 20-mesh sieve. Ground sample (0.5 g) was added to 10 mL di-acid mixture (HNO3:HClO4= 2:1) in a 100 mL digestion vessel. After a brief incubation at room temperature, the vessels were heated slowly to 200 º C. Heating was stopped when a dense white fume of HClO4 was observed. After cooling, the digested tissue was transferred into a 50 mL volumetric flask and brought to a final volume of 50 mL with distilled water. K+ and Na+ contents were determined using a flame photometer (Jencon PFP 7, JENCONS-PLS, UK) following to Brown and Lilleland (1946).
Data were analyzed using the statistical package MStatC. The mean differences were adjudged by Duncan’ s multiple range test (Gomez and Gomez, 1984).
Salt stress caused significant reductions in shoot and root growth of all the rice genotypes (Table 1). Except salt-sensitive genotype, all salt-tolerant rice genotypes are survived at the highest NaCl level (60 mmol/L). BINA dhan 10 showed 100% survival and growth at 60 mmol/L NaCl stress whereas the other tolerant genotypes BRRI dhan 47 and BINA dhan 8 displayed tolerance up to 40 mmol/L NaCl. The sensitive genotype (BRRI dhan 28) survived only at low salinity (20 mmol/L NaCl). At 60 mmol/L NaCl treatment, the highest fresh and dry weights of root and shoot were obtained in BINA dhan 10, followed by BRRI dhan 47 and BINA dhan 8. BINA dhan 10 showed reduction in shoot fresh weight by 12%, 19%, 46% and shoot dry weight by 2%, 26% and 48% at 20, 40 and 60 mmol/L NaCl, respectively. In the salt-sensitive genotype BRRI dhan 28, shoot fresh weight was reduced by 61% and shoot dry weight was reduced by 60% at 40 mmol/L NaCl. The reduction in root fresh weight was the highest in BRRI dhan 28 (77%) and the lowest in BINA dhan 10 (57%) at 60 mmol/L NaCl treatment.
| Table 1 Effects of salinity on fresh weight and dry weight in salt-sensitive and salt-tolerant rice genotypes. g |
Soil salinity also caused significant reductions in plant height, root length and number of tillers per hill in all the rice varieties. Plant height significantly decreased in all the varieties with increasing salinity levels. At the highest salt stress (60 mmol/L NaCl), the tallest plants (58.5 cm) were obtained in BRRI dhan 47 and the shortest plants (45.0 cm) in BRRI dhan 28 (Table 2). At 20 mmol/L NaCl, root length was significantly decreased in all the rice genotypes compared to the control, but no significant differences were observed between 40 and 60 mmol/L NaCl treatments in BINA dhan 10 and BRRI dhan 47. The salt-sensitive genotype showed a significant decrease in number of tillers per hill at 20 mmol/L NaCl treatment. The highest number of tillers per hill was obtained in BINA dhan 10 among the four rice genotypes irrespective of salinity level.
| Table 2 Effects of salinity on plant traits in different rice genotypes. |
Chlorophyll a content in rice showed different responses to salinity stress. The salt-sensitive rice genotype BRRI dhan 28 showed a decrease in chlorophyll a content at 40 and 60 mmol/L NaCl. In salt-tolerant rice genotypes, chlorophyll a content increased with salinity level up to 40 mmol/L NaCl, but decreased at 60 mmol/L NaCl (Table 3). Chlorophyll b content decreased in all the rice genotypes with increasing salt concentrations. A reduction in total chlorophyll content was observed with increasing salt concentration in all the genotypes but this reduction was more pronounced in the salt-sensitive genotype than the tolerant ones. Compared to the control, BRRI dhan 28, BRRI dhan 47 and BINA dhan 8 showed reduction of total chlorophyll content by 38%, 32% and 42% at 60 mmol/L NaCl, respectively. It is important to note that only 2% reduction in total chlorophyll content was recorded in BINA dhan 10 at 60 mmol/L NaCl treatment.
| Table 3 Effects of salinity on chlorophyll content in different rice genotypes. mg/g |
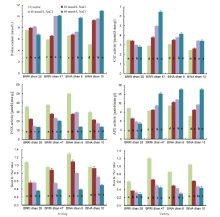
The proline content in all the salt-tolerant genotypes increased with increasing salinity levels (Fig. 1). However, proline content in the salt-sensitive genotype decreased significantly at 60 mmol/L NaCl. At 60 mmol/L NaCl, the highest proline content was obtained in BINA dhan 10 whereas the lowest content was found in salt-sensitive rice (BRRI dhan 28). BINA dhan 10 accumulated approximately 2.2-fold higher proline while BRRI dhan 47 and BINA dhan 8 accumulated 1.7- and 1.4-fold higher proline as compared to the control, respectively.
To investigate whether soil salinity influenced the antioxidant defense system in rice, the activities of major ROS-scavenging antioxidant enzymes, including CAT, POX and APX, were measured. Activities of antioxidant enzymes were significantly affected in response to salinity stress. CAT and APX activities in salt-tolerant genotypes increased with increasing salinity levels (Fig. 1). POX activities in all the rice genotypes showed a decreasing trend with increasing salt concentrations irrespective of salt tolerance levels (Fig. 1). CAT and APX activities in salt-sensitive rice decreased with increased salinity. At any given concentration of NaCl, the decrease in enzyme activities was more pronounced in the salt-sensitive rice genotype than the salt-tolerant ones.
K+/Na+ ratio in the shoots and roots of all the rice genotypes significantly decreased due to salt stress (Fig. 1). BINA dhan 10 showed a higher K+/Na+ ratio compared to the other salt-tolerant genotypes. In plants treated with 60 mmol/L NaCl, K+/Na+ ratio was approximately one-third (shoot) and half (root) of that in control plants in the salt-sensitive genotype.
Reduced growth is a common phenomenon when plants are grown in saline conditions. Inhibition of growth due to salt stress has been observed even in tolerant plant species (Mittler et al, 2001). Rice is considered as a salt-sensitive crop (Shereen et al, 2005; Darwish et al, 2009). In this study, significantly higher growth was observed in salt-tolerant genotype BINA dhan 10. Inhibitions of shoot and root growth are the most important agricultural indices of salt tolerance (Tuna et al, 2008). The present study demonstrated that shoot and root growth significantly decreased due to increased level of salinity (Table 1). Shannon and Grieve (1998) also reported decreased shoot and root biomass of rice under increased salt stress. The results from this study also indicated significant declines in plant height, root length and number of tillers per hill as concentration of NaCl increased (Table 2). Similar results in rice as well as in other crops have been reported (Dadkhah et al, 2001; Momayezi et al, 2010).
Chlorophyll is one of the most important plant pigments, supporting photosynthetic ability. Chlorophyll content can vary due to salinity level, eventually affecting plant growth and development. A reduction in plant chlorophyll content was observed with increasing salt concentration in all the genotypes, however, the reduction was more pronounced in the salt-sensitive genotype than in the salt-tolerant ones (Table 3). The concentration of the pigment fractions (chlorophyll a and chlorophyll b) in the leaves of all the rice varieties clearly demonstrated that the biosynthesis of photosynthetic pigments was affected by NaCl stress (Table 3). These results are in agreement with those of Islam et al (2009) and Abeer et al (2013) in rice. The decrease in chlorophyll content is an indicative response across different plants subjected to salinity stress (Roy and Basu, 2008). Parida and Das (2005) suggested that the decrease in chlorophyll content in response to salt stress is a widespread phenomenon. Chen and Yu (2007) also observed a significant decrease in chlorophyll content at high NaCl level.
Increased accumulation of proline in plants is correlated with improved salinity tolerance (Ashraf and Foolad, 2007; Hasanuzzaman et al, 2014). Proline content significantly increased in all the genotypes with increasing salt concentration (Fig. 1). Among the salt-tolerant rice genotypes, BINA dhan 10 accumulated 2.2-fold higher proline while the other genotypes BINA dhan 8 and BRRI dhan 47 accumulated 1.5- and 1.7-fold higher proline at 60 mmol/L NaCl compared to the control, respectively. The proline accumulation was also higher at 20 and 40 mmol/L NaCl in BRRI dhan 28, however, the accumulation decreased at a higher level of salinity (60 mmol/L NaCl) (Fig. 1). The decrease in proline accumulation in the salt-sensitive rice genotype was observed probably due to low synthesis of proline or higher degradation of proline under high salinity stress. In many studies, a positive correlation between the accumulation of proline and stress tolerance in plants has been found (Lutts et al, 1996; Kumar et al, 2003). Higher proline content in BINA dhan 10 might be one reason for the observed higher salt tolerance when compared to the other genotypes. Demiral and Turkan (2005) also reported that enhanced salt tolerance of rice is correlated with increased capacity of antioxidant system. Proline has been suggested to function as an antioxidant in protecting cells against various abiotic stress, since proline scavenges free radicals and suppresses ROS accumulation.
Increased plant antioxidant response has been shown to be positively associated with decreased oxidative damage and improved salinity tolerance (Hasegawa et al, 2000; Mittova et al, 2003; Demiral and Turkan, 2005; Hoque et al, 2007a, b, 2008; Banu et al, 2009; El-Shabrawi et al, 2010). In order to evaluate the relationship between salinity tolerance and antioxidant response, the activity of ROS-scavenging antioxidant enzymes was measured. The activity of antioxidant enzymes differed significantly in response to salinity stress. In this study, CAT and APX activities increased with increased salt concentrations in salt-tolerant rice genotypes but the activities in the salt-sensitive genotype (BRRI dhan 28) decreased with increased salinity, as compared to the control (Fig. 1). Previous reports support that increased salinity level decreases the activity of antioxidant enzymes including CAT in salt-sensitive rice, but increases CAT activity in salt-tolerant rice (Demiral and Turkan, 2005; El-Shabrawi et al, 2010; Hasanuzzaman et al, 2014). POX activity was considerably decreased in response to increased salinity in all the genotypes irrespective of their tolerance levels (Fig. 1). Dogan et al (2011) also showed that high salinity significantly decreases APX activity in salt-sensitive soybean. Our results are consistent with the observations of many researchers who reported that APX activity coordinated with CAT and POX activities confers salt tolerance in rice as well as in cotton (Meloni et al, 2003; Vaidyanathan et al, 2003; Demirel and Turkan, 2005).
A high cytosolic K+/Na+ ratio is essential for reducing salinity-induced oxidative damage. Enhanced influx of Na+ and inhibition of K+ uptake by plants under salt stress caused disturbances in K+/Na+ homeostasis, leading to toxic effects on plants (Zhu, 2003). The K+/Na+ ratio in both shoots and roots was significantly higher in the salt-tolerant genotypes than in the salt-sensitive one (Fig. 1). In the present study, differences in K+/Na+ ratio between rice genotypes suggest that Na+ exclusion from leaf tissues appears to play an important role in the salt tolerance of rice by maintaining the optimal K+/Na+ ratio. High salt (Na+) uptake competes with the uptake of other nutrient ions, especially K+, leading to K+ deficiency. It is often found that many glycophytes exhibiting enhanced tolerance to salinity stress have higher ability for sodium exclusion, maintaining high levels of K+/Na+ ratio (Zhu, 2001).
It is clear that increased antioxidant enzyme (CAT and APX) activity, chlorophyll content, proline content and K+/Na+ ratio in rice could be some of the physiological and biochemical mechanisms underlying salinity tolerance in plants. It is evident that no single parameter could be responsible for salinity tolerance in any of the studied rice genotypes whereas a combination of different characteristics contributes to the salinity tolerance. These findings may serve as in vitroselection criteria for salt tolerance in rice. On the basis of growth performance and biochemical assays, BINA dhan 10 showed a higher tolerance to salinity stress than the other genotypes. Therefore, further research should be focused on bio-molecular mechanisms involved in salinity stress tolerance for the determination of key pathways controlling salinity tolerance in plants.
This study was supported by a grant from the Ministry of Education, Government of Bangladesh. The authors are grateful to Gretchen Elizabeth Dykes, PhD, Department of Plant and Soil Sciences, University of Delaware, USA, for English editing of this article.
The authors have declared that no competing interests exist.
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
|
| 6 |
|
| 7 |
|
| 8 |
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
|
| 20 |
|
| 21 |
|
| 22 |
|
| 23 |
|
| 24 |
|
| 25 |
|
| 26 |
|
| 27 |
|
| 28 |
|
| 29 |
|
| 30 |
|
| 31 |
|
| 32 |
|
| 33 |
|
| 34 |
|
| 35 |
|
| 36 |
|
| 37 |
|
| 38 |
|
| 39 |
|
| 40 |
|
| 41 |
|
| 42 |
|