Interactions between plants and soil microorganisms can influence the other interactions in which plants participate, including interactions with herbivores. Many fungi, including arbuscular mycorrhizal fungi (AMF), form symbiotic relationships with the roots they inhabit, and potentially alter defense against pests. The objective of this study was to document the extent of root colonization by AMF on non-flooded rice plants grown under conditions typical of commercial fields. We hypothesized that AMF naturally colonized rice plants in different rice producing field locations. Rice plant samples were collected from areas across the southern United States, including Texas, Mississippi, Arkansas and two research stations in Louisiana. We quantified the amount of AMF colonization in insecticide-free rice plants over three consecutive years (2014-2016). The results revealed natural colonization of AMF in all rice producing areas. In all the three years of survey, rice-AMF associations were the greatest in Arkansas followed by Mississippi and Texas. This research will help draw attention to natural colonization of AMF in rice producing areas that can impact future rice research and production by facilitating agricultural exploitation of the symbiosis.
Arbuscular mycorrhizal fungi (AMF, Phylum Glomeromycota) are important components of soil microbial communities. AMF form mutualistic associations with roots of most terrestrial plants, including many agricultural crops. In many agricultural plants, these mutualistic associations have shown the potential to increase crop productivity, thereby playing a key role in the functioning and sustainability of agroecosystems (Gianinazzi et al, 2010). The most important function of these symbiotic associations involves the transfer of nutrients such as organic carbon (C), in the form of sugars and lipids (Jiang et al, 2017; Luginbuehl et al, 2017), to the fungi by the plants, and the transfer of phosphorus (P) and nitrogen (N) to the plants by the fungi (Smith and Read, 2008). AMF-mediated improvement in mineral uptake may lead to increased growth and development of plants, and may confer resistance to abiotic and biotic stress (Liu et al, 2007; Smith and Read, 2008; Gianinazzi et al, 2010). In addition to these benefits to plants, AMF may improve soil structure, ameliorate drought and salinity stress, and affect the diversity of plant communities (van der Heijden et al, 1998, 2010; Rillig and Mummey, 2006; Smith et al, 2010). The benefits of AMF may be critical to increasing agricultural yields and productivity in a low-input manner.
AMF share a long history of coevolution with plants in various ecosystems, resulting in their adaptation to specific areas (Gosling et al, 2006). The majority of research on AMF associations involves laboratory or greenhouse experiments, in which plants are cultivated in sterilized soil, with particular AMF species. They ignore indigenous AMF species that could alter plant responses or compete with the AMF inoculant (Munkvold et al, 2004). In addition, these studies ignore the complexity of soil biological communities that could influence the establishment of the AMF symbiosis and its impact on plant fitness (Lekberg and Koide, 2005).
During the last two decades, different aspects of the association of crop plants with AMF have been studied extensively in different geographical regions and under different agricultural conditions (Srivastava et al, 1996; Gianinazzi et al, 2010). Those studies have shown variable effects of AMF on crop plants, ranging from mutualistic to parasitic. The effects of AMF can depend on soil moisture, the inorganic nutrient available in the soil, pH, species of AMF and the host plant species. Along with these factors, a number of agricultural management practices affect the soil environment, and therefore, mycorrhizal abundance and activity.
Rice (Oryza sativa L.) is one of the world’ s most important cereal crops. In the United States of America, it is cultivated in two distinct regions, California and several southern states, including Arkansas, Louisiana, Mississippi, Missouri and Texas. In the southern region, the majority of rice acreage is grown under a delayed-flood cultural system in which rice is drill-seeded and surface-irrigated as necessary to establish a stand (Hamm et al, 2010). Timing of the permanent flood in this system varies, but flooding is generally delayed until rice begins to tiller, four to five weeks after planting. The period from seeding to flooding favors the colonization of root systems by AMF (Dhillion, 1992; Secilia and Bagyaraj, 1994).
Colonization by indigenous or native AMF species in cereal crops in general and rice in particular has been reported earlier (Maiti et al, 1995; Sawers et al, 2008; Campos-Soriano et al, 2010; Cosme et al, 2011). Despite this, in USA, almost no attention has been paid to AMF associations in rice. In another study, we showed that the performance of insects and a pathogen on rice is enhanced when plants are colonized by AMF, and AMF colonization can be manipulated by inoculating plots with a commercial AMF product (unpublished data). It will be necessary to evaluate the natural association of AMF with rice plants, particularly in regions where rice is produced, to facilitate agricultural exploitation of the symbiosis.
Given the paucity of information on the natural association of AMF with rice in different production areas of the United States, our goal was to survey rice fields from several locations in the southern United States to determine the extent of AMF colonization associated with commercial varieties before flooding. We tested the hypotheses that AMF establish natural association with rice roots, and that the AMF colonization would differ among locations. Unlike previous studies of natural AMF colonization in rice (Dhillion, 1992; Dhillion and Ampornpan, 1992), this study was carried out in most of the rice-producing areas of the southern United States and demonstrated natural colonization of AMF in rice fields, which may have practical implications for increasing rice production and sustainability.
Sampling to determine the extent of natural AMF colonization was conducted over three production seasons from 2014 to 2016. Four (2014 and 2015) or five (2016) collection sites were included in each year to represent a range of production environments in the southern United States (Table 1). The climate in the rice-producing regions of the southern United States belongs to the humid subtropical type, with average annual rainfall of 1 000 to 1 600 mm. In these areas, the summers are warm and humid, and the daily maximum temperatures usually range from 32 º C to 37 º C during the growing season. Average temperatures in late spring are about 23 º C, while 28 º C in summer and about 25 º C in early fall (US Climate Data, 2018).
Environmental conditions and cultural practices varied from year to year and site to site, but in all cases were typical of rice fields in the southern USA. In all these environments, rice was grown as a single crop per year, drill-seeded and irrigated. Plot sizes at all the sites were at least 1.5 m × 6.0 m. At the Winnsboro (WB) site, rice was grown in experimental plots in fields that had been under a continuous rice cultivation system for several years; for the Crowley (CR) and Beaumont (BM) sites, rice was grown in fields that had been in a rice-fallow rotation for the past 50 years; for the Stuttgart (ST) and Stoneville (SV) sites, rice was grown in rotation with soybeans. Planting dates were within the range of normal planting dates for each site, ranging from March or April at CR and BM sites to May at WB, ST and SV sites. Fertilization practices at each site were performed based on soil test results (Blanche et al, 2009). Only in CR and WB sites, all nitrogen was applied pre-flood, whereas split applications were employed at the other sites. As is typical for a survey spanning a large region, each site has a different soil type (Table 1).
| Table 1 Arbuscular mycorrhizal fungi (AMF) colonization percentage (presence of hyphae, arbuscular and vesicles) in fields during 2014-2016. |
Rice samples, consisting of leaves, roots, and soil, were collected from each site four to five weeks after seeding but before application of permanent flood. Seven to ten samples of rice plants at the early tillering stage were collected by pulling out plants from soil by hand or using a metal core sampler measuring 9.2 cm (diameter) × 7.6 cm (depth) and attached to a metal handle. Each sample contained three to four whole plants. The roots of plants were washed under pressure over a sieve to remove soil. The roots of each sample were immediately wrapped in moist paper towels, and the entire plant was loosely wrapped in newspaper for shipping. Each sample was placed in plastic bags and shipped overnight to Louisiana State University, Baton Rouge, USA. Samples were processed in the lab as described below.
The percentages of roots exhibiting signs of AMF colonization at each site were determined using the trypan blue staining method of Koske and Gemma (1989) with minor modifications. Color and texture were used to distinguish live roots from dead roots (dead roots were darker than live ones, and the great majority of roots survived the sampling and shipping process with little damage). Roots from each collected sample were cut into 2-cm-long segments and placed in tissue processing cassettes (Ted Pella, Redding, CA). Approximately 200 of these small root pieces per sample were cleared in 10% KOH at 90 º C for 30 min in a water bath. Cleared pieces of roots were rinsed five times with tap water to remove KOH, and roots were immersed in 2% HCl at room temperature for 15-20 min to ensure the roots were adequately acidified for staining. Cassettes containing roots were immediately stained with 0.05% trypan blue (Sigma-Aldrich, St. Louis, USA) by incubation overnight and then transferred to vials containing lactoglycerol at 4 º C to allow excess stain to leach out of the roots. Stained root samples were stored in lactoglycerol solution for 48 h before being mounted in the same solution on a microscopic slide.
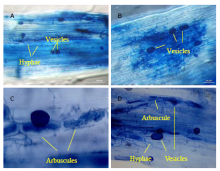
Mycorrhizal colonization by AMF structures was determined by assessing five slides with ten segments per slide from each sample and scoring the amount of colonization using the magnified intersections method of McGonigle et al (1990) with minor modifications. A total of 50 stained root segments per sample were examined with a compound microscope (Olympus CH2, Tokyo, Japan) at 40× to 100× magnification for confirmation of mycorrhizal colonization of rice plants. Root pieces showing presence of blue-stained mycorrhizal structures including arbuscules, hyphae or vesicles were scored as positive for AMF. All microscopic examinations were carried out by the same individual. Photos of mycorrhizal structures on colonized roots were taken using a microscope-mounted 5.0 megapixel digital camera (Leica DFC480, Cambridge, UK). Root colonization percentage was averaged for the seven to ten samples at each site and calculated by the following formula:
Root colonization percentage (%) = No. of segments colonized with AMF / No. of segments observed × 100
All the rice samples collected from multiple locations over multiple years were colonized by AMF, with root colonization percentage ranging from 1.8% to 61.4% (Table 1). AMF structures typical of plant-AMF symbioses such as hyphae, vesicles and arbuscules were present in all screened rice roots at each location (Fig. 1). The most common structures were hyphae, which appeared in all samples. Few arbuscules were observed in our survey because these structures tended to degrade quickly (Parniske, 2008); vesicles were found in greater number.
SV and ST sites had the highest colonization percentage in all years (Table 1). AMF colonization in Cocodrie increased substantially in CR from 2014 to 2016. Texas, Mississippi and Arkansas samples showed consistent AMF colonization, with slight fluctuations between years. In 2014, the highest mycorrhizal colonization was recorded in SV (16.7%) followed by ST (11.8%), and the lowest colonization was found in CR in Lemont (1.8%) (Table 1). In 2015, the highest colonization percentage was found in ST (61.4%) and the lowest colonization was found again in CR (19.0%). In 2016, CR and ST had the highest colonization percentages (59.3% and 61.2%, respectively), followed by Cheniere (58.0%) in CR and the lowest colonization was in WB (16.0%) (Table 1).
The presence and importance of AMF in rice production systems have received some recent attention (Vallino et al, 2014; Wang et al, 2015; Zhang et al, 2015), but no study has addressed the questions of whether AMF naturally colonize rice in commercial farming systems and to what extent natural colonization by AMF differs among conventional rice farming regions in the southern United States. The present survey was carried out over three years at four or five representative sites of rice production areas in the southern United States. Natural AMF colonization was found in all the sampling sites, confirming our hypothesis that natural AMF colonization is widespread in unflooded rice across the southern USA.
Our results are in agreement with Watanarojanaporn et al (2013) and Wang et al (2015), who showed that AMF are commonly present in rice roots from conventional rice fields (paddy wetlands) in Thailand and China at early growth stages and before flooding. However, our results differ from those of Vallino et al (2009) and Lumini et al (2011), who showed that AMF colonization was lower or absent, respectively, in rice roots grown under a conventional cultivation system in Italy. Rice roots in agricultural fields exhibit higher levels of colonization by native AMF than other crops in a different environment such as winter wheat in southern Switzerland (Mä der et al, 2000). Because environmental conditions and cultural practices were not manipulated over years and at the different locations in our survey, the factors responsible for variation in levels of AMF colonization in our study cannot be unequivocally determined. However, likely contributors to this variation include rice variety, AMF species and soil fertility.
In this study, the extent of AMF colonization was relatively stable over the three years at each location. Some sites showed consistently higher AMF colonization than the others. For example, colonization at the ST site was consistently higher than those at the others. The exception to this seemed to be the CR site, where colonization increased over the years. Differences in rice varieties could be among the factors that contributed to the differences among sites. Alternatively, differences among sites may have been due to differences in abundance and geographic distribution of AMF species, which in turn may have resulted from differences among sites in soil fertility, soil type, environmental conditions such as temperature and precipitation (soil moisture), past use of pesticides (fungicides) and crop rotational history. Environmental factors and agricultural management practices in rice fields such as fertilizer input and water management have been shown to influence both symbiosis and diversity of AMF communities (Gosling et al, 2006; Lumini et al, 2011; Barber et al, 2013).
One environmental factor in particular that is known to have a negative impact on AMF symbiosis is P availability. At lower soil P concentrations, when plants are P-limited, they tend to allocate a higher fraction of available carbon to AMF, thus stimulating AMF colonization (Johnson, 2010). At higher soil P concentrations, less carbon is allocated to AMF from the host plant and AMF can become carbon-limited. As a result, low colonization is expected at high P concentrations (Treseder and Allen, 2002). We hypothesize that the high rates of AMF colonization at the AR and CR field sites were due to the low-medium levels of P in the soil. In contrast, low rates of root colonization in some rice fields of our study may be due to higher levels of P in the soil due to addition of P fertilizer. However, we did not have soil nutrition analyses from the different field sites. Therefore, we recommend more studies to develop a better understanding of the relationship between soil fertility and AMF colonization.
At present, there are only few studies that provide information of the effects of AMF colonization on rice growth and physiology, and there is still no clear picture of the potential direct benefit of this association on crop yield in rice. van der Heijden et al (2006) showed that growth responses of plants to different species of AMF are temporally variable and plant-species dependent, where Lotus and Trifolium perform better with one AMF species in the first growing season, but grow best with a mixture of several AMF in the second season. Future work will be needed to understand the composition of the microbial communities on rice roots by identifying colonizing AMF species present in rice fields of the southern United States. Currently, we are assessing the benefits of AMF colonization on rice growth and yields in a field trial. Preliminary data reveal that colonization by AMF influences plant performance by increasing shoot biomass. Yields of field grown rice were up to 13% higher in rice plants inoculated with AMF than non-inoculated plants (unpublished data). This information will give a better idea of the beneficial effects of AMF in rice-producing areas.
This study demonstrated for the first time the natural association of AMF in rice in commercial fields in the southern U.S. Fungi living in intimate relationship with rice plants may have effects on their host ranging from beneficial to detrimental, depending on the partners involved and other biotic and abiotic factors in a highly context-dependent manner. This work built on an earlier study, in which we showed that inoculation of rice plots with a commercial AMF inoculum influences plant- herbivore and plant-pathogen interactions as well as rice growth (unpublished data). The information gathered from this study can be used to further investigate the impact of the symbiotic relationship between AMF and rice, which is becoming increasingly relevant for sustainable agriculture, where soil organisms may be useful for plant production (Berruti et al, 2016).
We are grateful to the Louisiana Rice Research Board for funding this work under the Entomology Program. The authors thank the support from Dr. Sebe Brown for collecting and sending rice samples in 2016.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|