Grain shape is one of the important agronomic traits, which is closely related to the yield of rice. A new rice mutant, named small and round grain( srg1), was isolated from an indica cultivar Zhenong 34 by ethyl methane sulfonate (EMS) mutagenesis. The microscopic analysis showed that the cell length of spikelet in srg1 was decreased compared with that in wild type (WT), which caused the grain length short. Meanwhile, the grains of srg1 were wider than those of WT because of the increased cell layers in spikelet in the lateral direction. Therefore, the inhibition of cell expansion and increased cell proliferation collectively led to the small and round grain. By map-based cloning, the gene SRG1 was located on the short arm of chromosome 9, which encodes a kinesin-4 protein, represented by the gene LOC_Os09g02650. A single nucleotide polymorphism, occurred in the 16th exon of SRG1, led to premature translation stop in mutant. The cell cycle-related genes were up-regulated in srg1, which conferred that SRG1 controlled grain width through the cell proliferation. Since the role of SRG1 in regulating grain shape was not clarified well before, it is valuable to explore the mechanism of grain development. This study could, hence, provide a morphogenesis and molecular basis for elucidating the function of SRG1, as well as a new germplasm resource for the further study of grain development.
Rice is one of the main food crops in the world, which feeds more than half of world population. Food security is becoming more urgent and important due to the increasing global population, declining arable land and dramatic climate change. In rice breeding, the yield of rice was obviously improved by dwarfing breeding and heterosis utilization. But now, the rice production improvement meets a plateau, and more excellent genes are required to realize the value in pyramiding breeding. The rice yield was determined by three main components including 1000-grain weight, grain number per panicle and panicle number per plant (Xing and Zhang, 2010). Grain weight associates with grain shape that depends on the length, width and thickness of grain. In-depth exploration of the genes controlling grain shape is helpful for the further improvement of rice yield.
Previous studies have successfully isolated and characterized some genes for determining grain shape in rice, including GS3 (Fan et al, 2006), GW2 (Song et al, 2007), qSW5 (Shomura et al, 2008), SRS3 (Kitagawa et al, 2010), qGW8 (Wang et al, 2012), SMG1 (Duan et al, 2014), GS2(Hu et al, 2015) andWTG1 (Huang et al, 2017), which are explored by quantitative trait locus analysis or map-based cloning. GW2, encoding a cyclic E3 ubiquitin ligase protein, negatively regulates cell division. The defect of GW2 could promote the division of the glumes, thereby increasing the width of the glumes (Song et al, 2007; Wang et al, 2012). The mutants related to grain shape are useful materials to study the morphogenesis and development of rice organs (Hong et al, 2005; Duan et al, 2014). The wtg1-1 mutant shows wide, thick, short and heavy grains together with an increased grain number per panicle (Huang et al, 2017). Using map-based cloning and whole-genome sequencing, the gene WTG1, LOC_Os08g42540, is finally located on chromosome 8. It encodes an otubain-like protease and determines grain shape mainly by influencing cell expansion.
Recently, some genes related to the grain shape were reported as their involvements in the pathways of plant hormone. The genesXIAO, SG1, SMG1, GS2 and some DWARF genes play important roles in regulating the grain shape and are related to brassinosteroid (BR) and other hormones (Tanabe et al, 2005; Jiang et al, 2012; Nakagawa et al, 2012; Duan et al, 2014; Hu et al, 2015). Therefore, the mutants of these kind genes generally show the pleiotropic phenotype with decreased plant height or small organs. SMG1 encodes a mitogen activated protein kinase OsMKK4, and it might be involved in the response of BR (Duan et al, 2014). Furthermore, the expression levels of cell cycle genes CYCA2; 1, CYCD4; 1 and CYCD7; 1are reduced in mutant than these in wild type (WT), which indicates SMG1 controlling grain size by cell proliferation. The D1 gene encodes the α subunit of GTP binding protein, and its mutant is insensitive to exogenous gibberellin and BR (Ueguchi-Tanaka et al, 2000; Wang et al, 2006). BRD2, D11 and D61 are characterized to be involved in the synthesis and signal transduction pathways of BR (Yamamuro et al, 2000; Hong et al, 2005; Tanabe et al, 2005). Except of the small grain shape, the mutants defective in these genes generally display dwarfism, erect leaves and other abnormities. However, some genes mainly control grain shape and have no effect on the plant architecture, such as GW2, qSW5, TGW6, GW7and GL7 (Song et al, 2007; Shomura et al, 2008; Ishimaru et al, 2013; Wang S K et al, 2015; Wang Y X et al, 2015).
In addition, several mutants showing small and round grain have been identified, such as srs1, srs3, srs5 and also the BR-deficient mutant d11/cpb1 (Abe et al, 2010; Kitagawa et al, 2010; Segami et al, 2012; Wu et al, 2016). The small and round shape of mutant srs1-1is caused by the decrease in both cell length and cell number in the longitudinal direction, and the cell elongation of lemma in the lateral direction (Abe et al, 2010).
Even some genes related to grain shape have been identified, the molecular regulating mechanisms of grain shape are still not enough. Therefore, more genes need to be identified to further explore the mechanism of grain shape formation and it is also valuable to provide more beneficial genetic resources for rice molecular breeding. In present study, a new mutant named small and round grain(srg1) was used to identify the srg1 locus by map-based cloning method and analyze the mechanism in regulating the grain shape. The genetic characteristic of mutant, SRG1 gene expression pattern and the expression levels of related genes were also determined. The findings could provide some valuable information for the role of SRG1 in regulating grain shape, as well as the material basis for the rice breeding.
A rice (Oryza sativa L.) mutant, small and round grain(srg1), was found from an indicacultivar Zhenong 34 by ethyl methane sulfonate (EMS) mutagenesis. After successive generations of planting, the mutant traits could be inherited stably. The F2segregating populations for genetic analysis and gene mapping were made from the crossings of srg1 with Zhenong 34 and Zhenongda 104 (japonicacultivar), respectively. All the plants of F2 were cultivated in the rice paddy field of Zhejiang University in Hangzhou, China, during the usual rice growing seasons from March to October in 2016 and 2017, respectively.
At the maturity stage, randomly 10 plants from the WT and mutant srg1 were respectively selected for analyzing the agronomic traits, including plant height (cm), panicle length (cm), seed-setting rate (%) and grain number per panicle. The dried grains were selected for measuring the grain traits including length (mm), width (mm) and 1000-grain weight (g). Randomly 10 grains were measured for the grain length and width with a vernier caliper. The 1000-grain weight was calculated based on the weight of 200 grains with three replications. After grain scanned, the roundness values were obtained from the analysis of rice appearance quality inspection system (SC-E).
For paraffin section, the fresh samples were fixed in FAA (3.7% formaldehyde, 50% ethanol and 5.0% glacial acetic acid) overnight at 4 º C. The fixed spikelet samples were dehydrated using a graded ethanol series (50%, 70%, 85% and 95% for 90 min each and 100% for 60 min) and finally embedded in paraffin. The embedded tissue sections (10 μ m) were de-paraffined with xylene, and then stained with safranine and fast green. Finally, digital images were collected by light microscopy (LEICA DMI4000, Germany).
For scanning electron microscopy (SEM) and transverse electric and magnetic (TEM), sample segments were fixed with 2.5% glutaraldehyde overnight at 4 º C. In the SEM assay, the spikelet samples were washed with a sodium phosphate buffer (0.1 mol/L, pH 7.2) for three times. The samples were fixed in 1% osmic acid for 1.5 h and then dehydrated through an ethanol series. After incubation in the ethanol-isoamyl acetate (1:1) and isoamyl acetate, in turn, the samples were dried, mounted and coated with gold powder. Finally, it was observed and photographed by a scanning electronic microscope (HITACHI TM-1000, Japan). The TEM assay was performed as the following method. The fixed leaf samples were washed three times (15 min for each) with phosphate buffer (0.1 mol/L, pH 7.0), fixed them in 1% osmic acid for 2 h and rinsed three times with phosphate buffer (0.1 mol/L, pH 7.0), dehydrated in increasing grades of ethanol (30%, 50%, 70%, 80%, 90%, 95% and 100%) for 15 min at each step, then transferred to the ethanol-isoamyl acetate mixture (1:1) for 30 min and isoamyl acetate at least 1 h, at last, dried at critical point and coated with gold powder for observing the ultra-structure of cell wall under the TEM (HITACHI, H-7650, Japan).
The culm at the heading stage was harvested to measure the cellulose, hemicelluloses and lignin contents. After dried and grind into powder, the cell wall components of samples were measured by crude fiber tester (CXC-06, China) and Fiber Analyzer (VELP ScientificaTM FIWE6, Italy), which was performed as previously described by van Soest et al (1991).
For genetic analysis, the phenotypes of mutant and WT from the F2 population were counted at 25 d after heading, respectively and tested by Chi square test for their segregation pattern. Gene mapping was carried out using the bulked segregate analysis (BSA) method to select linked markers and the candidate gene (Zhang et al, 1994). The DNASTAR and Primer 5 softwares were used to design new polymorphic primers (Supplemental Table 1). The Database of the Rice Genome Annotation Project (http://rice.plantbiology. msu.edu/cgi-bin/gbrowse/rice/) was used to obtain the functional annotation of each candidate gene in the target region. Sequencing for the candidate genes was performed between mutant and WT, and the mutation site was confirmed using sequence alignment website (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
| Table 1 Agronomic traits of wild type and srg1 mutant. |
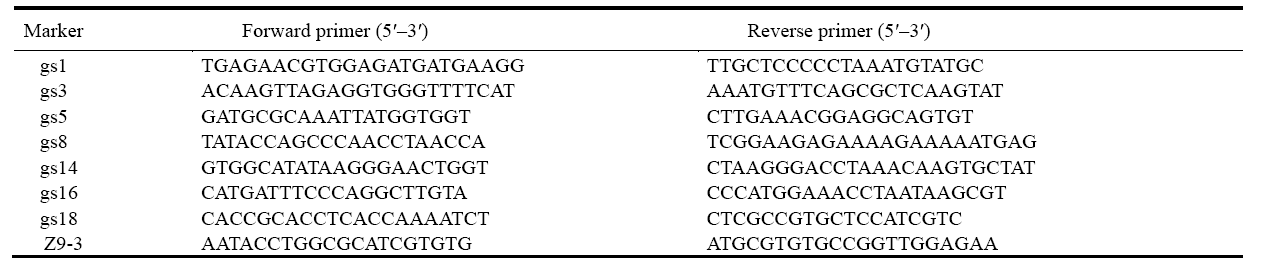
| Supplemental Table 1 Primer list for gene mapping. |
The fresh samples for qRT-PCR analysis were kept in -80 º C. Total RNAs were extracted from the tested samples using Trizol reagent (Invitrogen, USA) as prescribed by the manufacturer. For qRT-PCR, PrimeScript RT Reagent Kit with gDNA Eraser and SYBR Premix Ex Taq II were used as described by instruction (Takara, Japan). The qRT-PCR program was run on the LightCycler 96 system (Roche, Switzerland). The procedure was the three-step protocol: activation at 95 º C for 30 s, 40 cycles of denaturation at 95 º C for 5 s, annealing at 55 º C for 20 s and extension at 72 º C for 10 s. All the primers used for qRT-PCR were listed in Supplemental Table 2. The 2-ρ ρ CT method suggested by Schmittgen and Livak (2008) was used for calculating the relative expression levels.
| Table 2 Functional predications of candidate genes of srg1. |
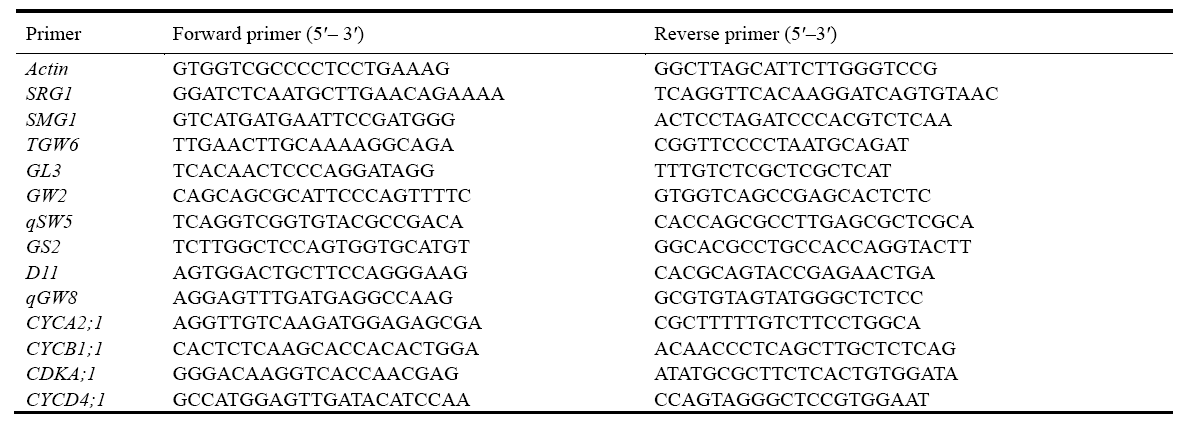
| Supplemental Table 2 Primer list for quantitative real-time PCR. |
The phylogenetic tree analysis was done for presenting the relatedness and evolutionary relationship among the SRG1 homologs. The 32 homologs included 4 from Oryza and others from a wide range of different plant species. The sequences of these homologous proteins were obtained by a BLASTP in NCBI (https://blast.ncbi.nlm.nih.gov/ Blast.cgi?PROGRAM=blastp& PAGE_TYPE=BlastSearch& LINK_LOC=blasthome) against the amino acid sequence of SRG1. Then, an alignment was performed with software MEGA6.0. And the phylogenetic tree was constructed by the Neighbor-Joining (NJ) algorithm with the parameter of 1000 replications for bootstrap values.
The srg1mutant showed a significantly different grain shape compared with WT. The grains of srg1were much shorter and wider than those of WT, and the rate of length/width of the mutant grain was smaller than that of WT correspondingly, which led to a round-like grain shape in the mutant srg1 (Fig. 1-A, -B and Table 1). The grain shape was scanned to analyze the roundness and the image was shown in Supplemental Fig. 1. The roundness value of mutant obtained from the scanner system was 0.61, which was significantly higher than that of WT (0.46) (Table 1). In addition, srg1 had pleiotropic phenotypes such as dwarfism and brittle culm (Fig. 1-C to -E). All the internodes of srg1were shorter than those of WT (Fig. 1-D). The culm of srg1 can be easily broken apart through bending by hand, while those of WT were steady under such mechanical stress for the entity of sturdy fibers (Fig. l-E). Meanwhile, the other yield-related traits altered dramatically in srg1. Herein, the panicle length, grain number per panicle, seed-setting rate and 1000-grain weight were reduced significantly in srg1(Table 1).
For understanding the role of SRG1 on grain shape, assays of histologic sectioning and SEM were performed. The SEM observation showed that the cell lengths of both outer and inner surfaces in the srg1 lemma were much decreased than those in WT, which might be the causal effect of shorter grain shape in srg1 (Fig. 2-A to -D). The cross section of srg1lemma revealed that the outer cell layers were more than those of WT, which might cause the lemma expanding in a lateral level (Fig. 2-E and -F). Analogously, the cell widths of both surfaces of srg1 lemma were also shortened. In view of the grain’ s fatter shape, the wider grain size of srg1 might be due to the increase in cell number. In that case, we checked the expression levels of several cell cycle-related genes. The expression levels ofCYCA2; 1, CYCB1; 1 and CDKA; 1 were significantly increased in srg1 compared to those in WT, while CYCD4; 1 showed litter difference (Fig. 2-G). This indicated that the mutation of SRG1 affected the expressing of cell cycle-related genes.
Whether SRG1was related to the regulatory pathway of grain development or not, the expression levels of related genes were examined. The results showed that the expression levels of grain shape-related genes GW2, qSW5, GS2, D11 and qGW8were significantly increased in srg1 compared to those in WT. The TGW6gene expression showed no significant difference between WT and the mutant. However, the SMG1 expression level was decreased (Fig. 2-H). This result might suggest that SRG1could control the grain shape relying on the regulation pathway of the related genes.
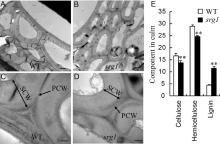
As presented in Fig. 1, srg1 showed pleiotropic effects on plant height and culm strength. Mechanical strength was determined mainly by the properties of the secondary cell wall and reduced cellulose content. The TEM result showed that the cell walls of sclerenchyma were thinner in srg1 than those in WT, which was mostly contributed by the decreased thickness of secondary cell wall (Fig. 3-A to -D). Meanwhile, the stratification between the primary cell wall and secondary cell wall was not clear in WT, while it was visible in srg1. This might also affect the mechanical strength of culm. In addition, the contents of cellulose, hemicellulose and lignin, which are the main components of cell wall, also revealed obvious alterations in the srg1. Both of the cellulose and hemicelluloses contents were significantly reduced in the srg1 culm compared to those in WT. However, the lignin content was higher in the srg1 culm than that in WT (Fig. 3-E).
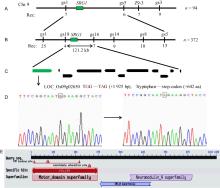
Totally 185 plants with normal grain shape and 71 plants with small and round grain shape were obtained from the F2 population from the crossing of srg1 and its wild type (Zhenong 34), which accorded with the segregation ratio of 3:1 (χ 2 = 1.10 < χ 0.052 = 3.84). This result confirmed that the small and round grain shape was controlled by a single recessive gene. An F2 population from a cross between srg1and Zhenongda 104 was used for the gene mapping of SRG1. Firstly, one marker Z9-3 on the short arm of chromosome 9 was found to be linked to the target gene SRG1using the BSA method. In reference with the genome sequences of the indica and japonica, we designed another three InDel markers gs1, gs3 and gs5 with long distance from Z9-3 to determine the region of target gene using 94 individuals which exhibited the phenotype of small and round grain. Then, the gene was primarily located between markers gs1 and gs5 (Fig. 4-A). In order to fine map the gene SRG1, additional 372 mutant individuals were detected by polymorphic markers such as markers gs8, gs14, gs16 and gs18. With the expanded population and increased molecular markers, the gene was finally fine plotted to an interval of 121.2 kb flanked by markers gs18 and gs16 (Fig. 4-B). Refer to the Rice Genome Annotation Project (RGAP), there were 11 open reading frames on this region (Fig. 4-C) and their annotation information was provided in Table 2. No difference was found in those gene sequences between srg1 and WT except the locus LOC_Os09g02650. Therefore, LOC_Os09g02650 was identified as the putative candidate gene. The sequencing result showed that one bp substitution (G to A) occurred in the 16th exon (+1 925 bp) of srg1 (Fig. 4-D). This mutation led to the amino acid tryptophan (+642 aa, TGG) change to a stop codon (TGA), and the protein translation met a premature stop. The full lengths of genome DNA and cDNA of SRG1are 9 792 bp and 3 108 bp in span, respectively, and the gDNA is composed of 25 exons and 24 introns. SRG1 encoded a kinesin-4 protein with 1 035 amino acid residues, which contained three functional domains the kinesin motor domain, the neuromodulin N-terminal region and SMC (structural maintenance of chromosomes) N-terminal domain (Fig. 4-E). The amino acid mutation that led to a premature stopped translation was located on both of neuromodulin protein N-terminal region and SMC N-terminal domain.
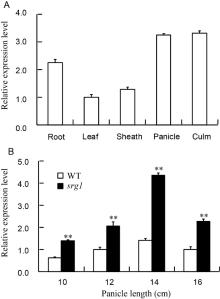
The differential expression levels of SRG1 were found among all the tested organs including leaf, culm, sheath panicle and root, which indicated that SRG1 was constitutively expressed in different tissues of rice (Fig. 5-A). Moreover, it was highly expressed in culm, panicle and root. In addition, the expression levels of SRG1 in spikelets at different stage were also observed. Along with the development of spikelet, the expression levels were increased, while it declined after the panicle length of 14 cm (Fig. 5-B). Compared to WT, the significant higher expression in spikelets from different panicle lengths of srg1 was detected, which might be the reason of the small and round grain of mutant on mRNA level.
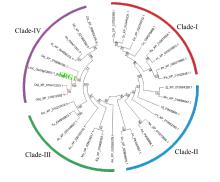
The phylogenetic analysis presented the evolutionary relationship among the SRG1 homologs (Fig. 6). The 32 homologs included 4 from Oryza and others from a wide range of different plant species. The homologs were divided into four clades (I-IV) with high bootstrap support. The clade IV contained homologs from Panicum hallii, Zea mays, Sorghum bicolor, Setaria italica, Oryza brachyantha and Oryza sativa. Among them, the SRG1 protein from indica shared high similarity with the homolog from japonica.
Grain shape in rice is one of the vital agronomic traits. Although many genes influencing grain shape have been identified, the molecular mechanisms of grain shape are still little known in rice. In this study, we reported the gene SRG1as an important factor involved in grain shape formation, the locus of which was identified by map-based cloning. The sequencing data and functional annotation in RGAP revealed that SRG1 is the mutated gene, which encodes a kinesin-4 protein with three functional domains. SRG1is found to be allelic to the gene BC12/GDD1/MTB1, which is involved in controlling plant height, tillering and cell wall properties in rice (Zhang et al, 2010; Li et al, 2011; Yu et al, 2016). The single base mutation from G to A in srg1 caused the alteration of genetic code TGG to TAG (a stop codon), which led to a premature stop of protein translation. This amino acid mutation occurred in both of neuromodulin protein N-terminal region and SMC N-terminal domain. The proteins, belonging to SMC superfamily, have ATP-binding domains at the N- and C-termini, which are involved in chromatin and DNA dynamics. Generally, SMC family also includes the RecF and RecN proteins that are involved in DNA metabolism and recombination (Cobbe et al, 2004).
The kinesin motor domain is another domain of SRG1, which plays important roles in the protein functions. Plant kinesins are vital apparatus of mitotic mechanism. The common utility of kinesins is concerned in microtubule association negotiation in mitotic process (Preuss et al, 2004; Persson et al, 2007; Li et al, 2012). The perturbation of kinesins might result in damaged morphogenesis and/or an irregular cellulose deposition pattern (Reddy and Reddy, 2002; Zhang et al, 2010). Kitagawa et al (2010) reported that the defect of kinesin-13, a member of kinesin subfamily, also brought about the small and round grain in srs3 due to the reduced cell length in the longitudinal direction. Here, the mutant showed the similar grain shape like srs3, which was caused by the inhibition of cell expansion in the longitudinal direction of lemma and the increased cell proliferation in lateral direction. Correspondingly, CYCA2; 1, CYCB1; 1 and CDKA; 1, involved in cell proliferation, showed higher expression levels in spikelets of the mutant than those of WT. These results indicated that SRG1 controlled the grain shape though the regulations of cell elongation and cell proliferation.
In our study, the mutant srg1 was a new allele, which was derived from the indica rice variety Zhenong 34 and had different phenotype, mutation way and genetic background from its alleles bc12-1(C418, japonica), bc12-2 (Nipponbare, japonica), gdd1(Zhonghua 10, japonica) and mtd1(W7, japonica) (Zhang et al, 2010; Li et al, 2011; Yu et al, 2016). DNA sequencing results confirmed that the gene in mutant srg1 has one bp substitution (G to A) in the 16th exon of LOC_Os09g02650, which results in a premature stop of protein translation, and the mutation way is found in mtd1 (Yu et al, 2016). The bc12-1 and bc12-2 have a 26-bp deletion in the 4th exon and Tos17 insertion in the 23th exon, respectively (Zhang et al, 2010), which belong to the kinesin motor domain and the neuromodulin protein N-terminal region. A deletion of 27-bp is observed in gdd1 with covering the intersection of the 19th intron and 20th exon of LOC_Os09g02650, leading a premature stop of protein translation during the neuromodulin protein N-terminal region (Li et al, 2011). All of them show the similar phenotypes of dwarfism and brittle culm. Except of these two traits, the mutant mtd1 also shows more tillers, which is not observed on other alleles, even the mutant srg1 here shared the similar mutation to mtd1. Moreover, srg1exhibits small and round grain with decreased grain length and increased grain width. Mutant gdd1 also displays the short grain size, but the grain width does not change much, and there is no further research to explore how GDD1/BC12 regulates the grain size (Yu et al, 2016). In addition, it is divergent about the role of BC12 in controlling the plant height among two kinds of mutants bc12 and gdd1 (Zhang et al, 2010; Li et al, 2011). The bc12mutant displays dwarfism resulting from a significant reduction in cell number, while the dwarfism ofgdd1is due to the cell elongation. Here, the short grain of srg1 was resulted from the inhibition of cell elongation, and the wider grain was due to the increased cell proliferation. It indicated that the gene could work on both cell elongation and proliferation and by that produced different effects on grain size. Therefore, the different mutation sites and genetic background might lead to morphological and cytological phenotypes with different mechanisms.
As the cell wall is the skeleton of plant, cellulose, hemicellulose and lignin are three major components of cell wall. The contents of these components and cell wall structure could affect the plant mechanical strength. In general, rice brittle culm mutants usually accompany with the decreases in both cellulose and hemicellulose contents, increase in lignin content, as well as a decrease in the thickness of secondary cell wall of sclerenchyma (Xu et al, 2008). But for some brittle mutants likebc1andbc6, the cellulose contents are reduced much, while hemicellulose contents are significantly increased or without changes (Li et al, 2003; Kotake et al, 2011). This might be explained by the existing compensation regulation mechanism, in which the content of hemicellulose is increased when the plant faces the decrease of cellulose content. Furthermore, some mutants such as bc1 and bc5 have higher lignin levels but with less cellulose contents (Li et al, 2003; Aohara et al, 2009). Like in the common brittle mutants bc1, bc3, bc6 and bc14, the cellulose and hemicellulose contents are much decreased in srg1 than those in WT, and the lignin content is significantly higher in srg1(Li et al, 2003; Hirano et al, 2010; Kotake et al, 2011; Zhang et al, 2011).The cell wall of sclerenchyma was thinner in srg1 than that in WT, which was mostly contributed to the decreased thickness of secondary cell wall of srg1. Consistent with the other brittle mutants bc1 and bc5, the lignin content of bc12, as well as srg1, was much higher than that of WT. But the cellulose content and the structure of cell wall in bc12 showed no obvious differences compared with those in WT. As a result, there may be some differences about the mechanism of cell wall formation between srg1 and bc12, it might be attributed to the genetic background and different mutated way as well as other reasons. In conclusion, srg1 was a new allele of BC12 gene, which could control the mechanical strength in a different way. The expression pattern of SRG1 displayed different expression levels during the panicle and grain development, implying that the expression of SRG1 was spatiotemporal. It is known that the grain development was initiated from the spikelet meristem, and the bloom period of cell elongation and proliferation was before rice flowering. The expression pattern of SRG1during spikelet development was expressed higher with the increasing panicle length and declined when the rice flowering.
In conclusion, the factor of grain shape, SRG1, is responsible for the small and round grain shape of mutant. As this gene has not been reported for regulating grain size before, this study thus might provide a morphogenesis and molecular basis for elucidating the role of SRG1 in controlling grain shape.
This work was supported by the Science and Technology Office of Zhejiang Province (Grant Nos. 2012C12901-2, 2016C32085 and 2016C02050-6).
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/ journal/16726308; http://www.ricescience.org.
Supplemental Table 1. Primers list for gene mapping.
Supplemental Table 2. Primers list for quantitative real-time PCR.
Supplemental Fig. 1. Scanned images of the grain shape of wild type (A) and srg1 mutant (B).
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|