Seed germination is associated with grain yield and quality in crop production. Gibberellic acid (GA) serves as a major phytohormone in the promotion of seed germination. It is synthesized in the embryos and transmitted to the aleurone layers, where GA triggers the synthesis and secretion of a set of hydrolases, especially α -amylase. Subsequently, the storage nutrients such as starch in the endosperm are digested by these hydrolases and absorbed by the embryo to sustain seed germination and early seedling establishment (Kaneko et al, 2002). The detailed GA biosynthesis process has been well studied and thoroughly reviewed in several literatures (Sakamoto et al, 2004; Reinecke et al, 2013). Briefly, geranylgeranyl diphosphate (GGDP) is turned into ent-kaurene by two terpene synthases, ent-copalyl diphosphate synthase (CPS) and ent-kaurene synthase (KS). Subsequently, the conversion of GA precursor ent-kaurene to ent-kaurenoic acid is catalyzed by ent-kaurene oxidase (KO), and that from ent-kaurenoic acid to GA12 is catalyzed by ent-kaurenoic acid oxidase (KAO). Ultimately, GA12 is converted to various GA intermediates and bioactive GAs by GA20-oxidase (GA20ox) and GA3-oxidase (GA3ox), respectively.
WRKY transcription factors (TFs), one of the largest TF families in higher plants, usually bind to the W-box motif (T)(T)TGAC(C/T) in the promoter of the downstream target genes (Eulgem et al, 2000; Ulker and Somssich, 2004). Several WRKY family members have been reported to participate in GA-mediated seed germination in the past decade. In Arabidopsis, AtWRKY27, which is directly regulated by GA signaling component RGA, is involved in GA-mediated seed germination (Zentella et al, 2007). Rushton et al (1995) reported that AfWRKY1 and AfWRKY2 inhibit the expression of α -amylase, therefore delay seed germination in Avena fatua. OsWRKY51 and OsWRKY71, which are homologous of AfWRKY1 and AfWRKY2, function as heterologous dimers and interact with GA signal positive regulator GAMYB to inhibit the expression of α -amylase in rice (Zhang et al, 2004; Xie et al, 2006). Here we report that WRKY72 acts as a negative regulator in rice seed germination by restricting GA accumulation through modulating ‘ LRK1-OsKO2’ pathway, which would provide novel insights into the finely regulated mechanism of WRKY72-mediated seed germination in rice.
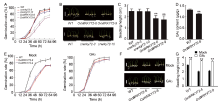
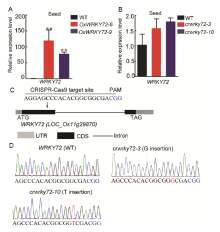
Previous studies have shown that WRKY72 is predominantly expressed in rice developing seeds, especially in aleurone layers, indicating it can participate in the regulation of seed maturation or germination (Xie et al, 2005; Hou et al, 2019). In this study, we mainly focused on the role of WRKY72 in rice seed germination process. Firstly, WRKY72 over-expression lines (OxWRKY72) and wrky72 mutants (crwrky72) were generated. Two independent WRKY72over-expression lines (OxWRKY72-6 and -9) showed about 90-fold higher transcript level compared with the wild type (WT) (Fig. S1-A). Two crwrky72 mutants (crwrky72-3 and -10) harbored a G insertion and a T insertion in the 1st exon of WRKY72respectively, which shifted the open reading frame, though the transcript level of WRKY72 remained unchanged (Fig. S1-B to -D). Seeds of T2 generation from both OxWRKY72 andcrwrky72 lines were subjected to seed germination assay. The germination rates of OxWRKY72 lines were significantly lower than that of the WT (Fig. 1-A). In consistent with the retarded seed germination, the seedling heights of OxWRKY72 lines were also lower than that of the WT (Fig. 1-B and -C). However, the germination rates and seedling growths of crwrky72 lines were similar to the WT, possibly due to its functional redundancy with other WRKY family members (Fig. 1-A to -C). As GA is a major activating phytohormone in seed germination, the retarded seed germination of OxWRKY72 seeds intrigued us to measure the endogenous GA level in OxWRKY72 as well as the WT. The results showed that the GA3 content was significantly reduced in the OxWRKY72-6 germinating embryos, indicating that OxWRKY72 defected in GA accumulation, rather than GA signaling (Fig. 1-D). As expected, the retarded germination rates and seedling growths of OxWRKY72 lines were restored to the same level as the WT when 1.5 µ mol/L exogenous GA3 was applied (Fig. 1-E to -G). Hence, the suggestion is that WRKY72 inhibits seed germination at least partly by blocking GA accumulation.
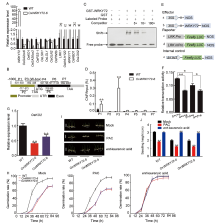
Germinating embryos of OxWRKY72 and the WT grown on half-strength Murashige and Skoog (MS) medium for 2 d were collected for RNA-sequencing (RNA-seq) assay to clarify the regulatory mechanism underlying the WRKY72-governed seed germination. As a result, we totally identified 2 457 differentially expressed genes (DEGs), including 727 down-regulated and 1 730 up-regulated genes in OxWRKY72 (|log2 ratio| ≥ 1; False discovery rate < 0.01) (Table S1). To validate the transcriptome analysis, 13 DEGs, which are functionally relevant to GA biosynthesis or seed germination, were selected for gene transcript abundance verification (Table S2). As shown in Fig. 2-A, the transcript levels of most of the selected genes were consistent with the RNA-seq results, suggesting the high-reliability of the RNA-seq data. Interestingly, among these detected DEGs, several have been reported to be functionally involved in GA biosynthesis or metabolism. For example, OsGA20ox2 (gibberellin 20 oxidase 2, a major GA biosynthesis enzyme) (LOC_Os01g66100) was down-regulated in OxWRKY72, and mutation of OsGA20ox2 reduces GA biosynthesis and thereby delays seed germination (Ye et al, 2015). OsLOL1 (a C2C2-type zinc finger protein) (LOC_Os08g06280) was also reduced in OxWRKY72, and it can interact with OsbZIP58 to promote seed germination through activating the gibberellin biosynthesis gene OsKO2 (Wu et al, 2014). LRK1 (a leucine- rich repeat receptor-like kinase, LRR-RLKs) (LOC_Os02g05980) was significantly elevated in OxWRKY72, and it represses GA biosynthesis through inhibiting the activity of the GA biosynthesis enzyme OsKO2 (Itoh et al, 2004; Yang et al, 2013). We further analyzed the cis-element distribution in the promoter region of these selected DEGs using the online tool PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/), and found that only OsGA2ox6 and LRK1 contain W-box (TTGAC[C/T]) or W-box like (TGAC[C/T])cis-elements (Fig. S2). The increased transcript level of LRK1 in OxWRKY72 indicated that LRK1 might be involved in WRKY72-mediated GA biosynthesis repression (Fig. 2-A). Therefore, we mainly focused on whether LRK1 can be the direct target of WRKY72. To test this hypothesis, the EMSA (electrophoresis mobility shift assay) was firstly performed to detect the DNA binding ability of WRKY72 with LRK1 in vitro. As shown in Fig. 2-B and -C, GST-WRKY72 protein can bind to the probe 3 (P3), which contains a conserved W-box motif close to the transcription starting site, and the shift band signal was gradually weakened by the addition of unlabeled, competitive P3 probe in a dosage-dependent manner, suggesting that this binding is highly specific (Fig. 2-B and -C). Subsequently, ChIP-qPCR (chromatin immunoprecipitation-quantitative PCR) assay was performed to validate the binding pattern of WRKY72 on LRK1promoter in vivo. In consistent with the results of EMSA, WRKY72 was significantly enriched in the P3 region of LRK1promoter, while there was no significant enrichment in the other fragments, except that P1 region located in LRK1 promoter exhibited slightly WRKY72 enrichment, strongly suggesting that the W-box in the P3 region acts as a core binding site for WRKY72 (Fig. 2-B and -D). Finally, a dual-luciferase (LUC) transient transcriptional activity assay was performed to determine the regulatory effect of WRKY72 on LRK1transcription (Fig. 2-E and -F). In comparison with the empty effector, pro35S: WRKY72:tNOS drastically elevated the transcript level of proLRK1:firefly LUC reporter, but such induction was significantly reduced when the W-box in the P3 promoter region of LRK1 was mutated, which was in accordance with the transcription pattern of LRK1 in OxWRKY72 transgenic lines (Fig. 2-A, -E and -F). Taken together, these experiments clearly demonstrated that WRKY72 specifically binds to the LRK1 promoter containing a W-box cis-element and induces the latter’ s transcription.
It is reported that LRK1 restricts rice internode elongation through suppressing OsKO2and thereby results in reduced endogenous GA level (Yang et al, 2013). OsKO2, a key ent-kaurene oxidase, promotes GA biosynthesis by catalyzing GA precursor ent-kaurene into ent-kaurenoic acid, and the mutation of OsKO2 causes severe GA deficiency and dwarf phenotype (Itoh et al, 2004). These evidences intrigued us to speculate that the function of OsKO2 could be interrupted by WRKY72. In consistent with the up-regulation of LRK1, the OsKO2 transcription was significantly reduced in OxWRKY72 (Fig. 2-G). Moreover, the effects of ent-kaurenoic acid and paclobutrazol (PAC, a KO inhibitor) (Swain et al, 2005) were further determined on the seed germination of OxWRKY72. Interestingly, ent-kaurenoic acid, the product of OsKO2 catalyzed reaction, fully restored the delayed seed germination and seedling growth of OxWRKY72 (Fig. 2-H to -J). On the contrary, PAC significantly inhibited the seed germination and seedling growth of all the tested seeds (Fig. 2-H to -J). These results strongly suggested that WRKY72 negatively regulates seed germination and GA accumulation via the ‘ WRKY72- LRK1-OsKO2’ pathway.
Up to date, over 100 WRKY gene family members have been identified in rice (Ramamoorthy et al, 2008). Rice WRKY proteins have been shown to regulate the cross-talk between multiple hormone-mediated signaling pathways in various biological processes, but most notably in biotic stress responses (Qiu et al, 2007; Peng et al, 2012; Wang et al, 2015). Previous studies have shown that WRKY72 is induced by polyethylene glycol, NaCl, naphthalene acetic acid, abscisic acid (ABA) and heat stress in rice, indicating the versatile roles of WRKY72 in multiple physiological processes (Song et al, 2010). Very recently, our group revealed that WRKY72 acts negatively in rice resistance to bacterial blast disease through repressing jasmonic acid (JA) accumulation (Hou et al, 2019). WRKY72 can directly bind to the promoter of a key JA biosynthesis gene AOS1, and repress the AOS1 transcription possibly via a RNA-directed DNA methylation mechanism. Meanwhile, the WRKY72 transrepression activity depends on its phosphory- lation status mediated by SAPK10, which is a core component in ABA signaling (Hou et al, 2019). Hence, WRKY72 likely serves as an important node in the ABA-JA interaction. Due to its predominant expression pattern in rice developing seeds, especially in aleurone layers, WRKY72 might also participate in the regulation of seed maturation or germination (Xie et al, 2005; Hou et al, 2019). Indeed, when WRKY72 is ectopically expressed in Arabidopsis, seed germination of the transgenic lines is drastically retarded (Song et al, 2010). Nevertheless, how WRKY72 functions in rice remains unclear. In this study, we revealed that over-expression of WRKY72inhibited seed germination and seedling growth (Fig. 1-A to -C). Several cases have demonstrated that WRKYs involve in seed germination by interfering GA biosynthesis or signaling. For example, heterologous dimmers of OsWRKY51 and OsWRKY71 are found to negatively regulate GA signaling through direct interacting with GAMYB, a GA signal positive regulator, and ultimately inhibit the expression of α -amylase (Zhang et al, 2004; Xie et al, 2006). In our case, it is clear that GA-deficiency resulted in the retarded germination and seedling growth of OxWRKY72, because OxWRKY72 exhibited reduced endogenous GA level, and the addition of GA completely restored the phenotype (Fig. 1-D to -G). Therefore, WRKY72 can be a key player in the interaction of phythormones including ABA, JA and GA.
Since WRKY72 is annotated as a transcription factor, identifying its direct target gene is crucial to clarify the regulatory mechanism underlying the WRKY72-governed seed germination. Our RNA-seq and qRT-PCR analyses identified a long list of DEGs which are functionally related to GA biosynthesis and metabolism. Among the DEGs, a leucine-rich repeat receptor-like kinase (LRR-RLKs)LRK1, which is up-regulated in OxWRKY72, is of particular interest (Fig. 2-A). EMSA experiment in vitro, ChIP-qPCR and rice protoplasts transient transcriptional activity assay in vivo demonstrated that WRKY72 can specifically bind to the W-box cis-element of LRK1 promoter and activate its transcription, suggesting that LRK1is a direct target of WRKY72 (Fig. 2-B to -D). It is reported that LRK1 restricts rice internode elongation through suppressing the ent-kaurene oxidase OsKO2 and thereby results in reduced endogenous GA level (Yang et al, 2013). In agreement with the up-regulation of LRK1, OsKO2 was significantly reduced in OxWRKY72 (Fig. 2-G). OsKO2 has been known as a key enzyme catalyzing the conversion of ent-kaurene to ent-kaurenoic acid, and mutation of OsKO2 causes severe GA deficiency and dwarf phenotype (Itoh et al, 2004). This hypothesis is further supported by the fact that addition of ent-kaurenoic acid, the product of OsKO2 catalyzed reaction, fully rescued the retarded germination of OxWRKY72 (Fig. 2-H to -J). Thus, WRKY72 inhibits seed germination and GA accumulation via the ‘ WRKY72-LRK1-OsKO2’ pathway.
This study was supported by the National Natural Science Foundation of China (Grant No. 31701395), the special research funds for the Central Public Research Institute of the China National Rice Research Institute (Grant No. 2017RG002-5) and the special research funds of State Key Laboratory of Rice Biology (Grant No. 2017ZZKT10105).
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/journal/16726308; http://www.ricescience.org.
Fig. S1. Molecular characterization of OxWRKY72 and crwrky72 mutants.
Fig. S2. Occurrence of cis-regulatory elements in promoters ofOsGA2ox6 and LRK1.
Table S1. Differentially expressed genes between wild type and OxWRKY72-6.
Table S2. Selected differentially expressed genes used for RNA-seq verification.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|