Although cultivated rice originated from the tropical region, a long process of domestication and human selection has enabled cultivated rice to grow in a wide range of geographical regions. Diversification of photoperiodic flowering provides a foundation for this diverse adaptation. Intensive studies have focused on elucidating how japonica varieties lost photoperiodic sensitivity (PS) to expand their cultivation areas to high latitudes, where rice is grown in a short season when day-length is long (Fujino et al, 2013; Naranjo et al, 2014; Gomez-Ariza et al, 2015; Li et al, 2015, 2018; Goretti et al, 2017; Ye et al, 2018). By contrast, limited attention has been paid to the genetic architecture of heading date (HD) variation among varieties in middle and low latitudes. The southern China rice region, located in middle and low latitudes, occupies the most important rice cultivation region in China. This region is predominantly planted with indica varieties, which presents a rich diversity of regional and seasonal adaptations. In the present study, improved varieties and landraces used in this region were analyzed for allelic variations of 12 cloned QTLs controlling HD, as well as for the genotypic effects of these genes on HD and PS. Our objective was to clarify predominant genetic factors influencing eco- geographical adaption of rice varieties in southern China by comparing improved varieties with landraces.
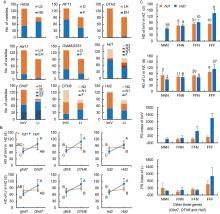
A total of 224 indica varieties were tested, including 132 improved varieties and 92 landraces (Tables S1 and S2). They were grown under natural short-day (NSD) conditions in Lingshui (LS), Hainan, and under natural long-day (NLD) conditions in Hangzhou (HZ), Zhejiang, China. The rice varieties were genotyped using gene-specific markers of 12 flowering genes (Table S3). Nine flowering genes showed allelic variations (Fig. 1-A). Five of them, Hd3a, RFT1, DTH2, Hd17 and OsMADS51, had two allelic types. The other four, Hd1, Ghd7, DTH8 and Hd2, had multiple allelic types. A single genotype was detected for the remaining three flowering genes, including Ehd1, Hd6 and Hd18.
The nine genes having different allelic types were analyzed for their effects on HD in HZ, HD in LS, and PS between HZ and LS. Among the five genes having two allelic types, significant genotypic effects were detected at Hd3a, Hd17 and OsMADS51, but not at RFT1 and DTH2 by using one-way analysis of variance (ANOVA) (Table S4). For HD in HZ that was the primary target trait of this study, Hd3a and OsMADS51 showed differences between the two collections (landrace and improved variety). At Hd3a, the effect was only significant in landrace, explaining 11.0% of phenotypic variance. At OsMADS51, the effect was only significant in improved variety, explaining 11.1% of phenotypic variance.
| Table S4. Phenotypic differences between two genotypic groups of indica rice at nine gene loci. |
For the four genes having multiple allelic types, Hd1, Ghd7, DTH8 and Hd2, the Duncan’ s multiple test was firstly performed, and the results were applied to re-classify the varieties into two groups. At Hd1, different non-functional types of the same collection showed no significant difference on all the three traits, but they differed significantly from the functional counterpart in LS (Table S5). Thus, different non-functional types were combined as a single non-functional group (Group 2) and the functional type remained as Group 1. Like Hd1, results of Duncan’ s multiple tests for Ghd7 and DTH8 matched the classification of non-functional and functional types according to previous reports (Table S5). Therefore, the two genes were also classified into functional group (Group 1) and non-functional group (Group 2). For Hd2, results of Duncan’ s multiple tests did not match the original classification of functional and non-functional types. In both collections, phenotypic differences were more distinguishable between the two non-functional types than between non- functional-2 and the functional type (Fig. S1). Thus, the functional type and non-functional-2 were combined as the functional group (Group 1), and non-functional-1 remained as Group 2.
| Table S5. Heading date (Hd) and photoperiodic sensitivity (PS) index of multiple allelic types of indica rice at DTH8, Ghd7andHd1. |
Based on the two-group’ s classification, effects of Hd1, Ghd7, DTH8 and Hd2 were determined with one-way ANOVA. All the four genes showed important effects, among which differences between the two collections were observed (Table S4). At Hd1, the early-heading group of improved variety was the functional type in both locations, but that of landrace changed from the non-functional type in HZ to the functional type in LS. Phenotypic variances explained (R2) in improved variety and landrace were 8.2% and 13.3% in HZ and 69.5% and 27.9% in LS, respectively. At Ghd7, major effects on HD were detected in improved variety with R2 of 42.3% and 55.6% in HZ and LS, respectively. However, the effects were non-significant in landrace, which may be partly caused by the unbalanced distribution of 85 functional and 7 non-functional cultivars. At DTH8 and Hd2, flowering in HZ was significantly promoted in the functional group compared with the non-functional group in each collection, with R2 ranging from 23.4% to 46.7%. In LS, the effects of DTH8 and Hd2 were only significant in landrace and improved variety, respectively.
It has been known that Ghd7, DTH8 and Hd2 are three main regulators of Hd1 (Nemoto et al, 2016; Du et al, 2017; Zhang et al, 2019). Following the two-group classification of functional and non-functional genotypes at the four loci, each rice collection used in the present study was classified into four genotypic groups for gene pairs Hd1-Ghd7, Hd1-DTH8 and Hd1-Hd2, respectively. Phenotypic differences on HD in HZ among the four genotypic groups of the same series were analyzed using the Duncan’ s multiple test. The differences revealed a consistent pattern of digenic interaction across all the three gene pairs in the two collections (Fig. 1-B). The functional Hd1 delayed flowering with functional Ghd7, DTH8 and Hd2, but promoted flowering with non-functional ghd7, dth8 and hd2. It would be also important to determine the joint effects of the three digenic interactions on the function of Hd1.
Rice cultivars in each collection were further classified into different groups according to the functional type of Hd1 and the total number of genes showing functional genotypes at the Ghd7, DTH8 and Hd2 loci (Fig. 1-C). In improved variety, only seven groups were available because no cultivar had non- functional genotypes at all the four loci. In landrace, all the eight groups were available. In each collection, the effects of Hd1 on HD in HZ and PS index were analyzed with changes of the total number of functional Ghd7, DTH8 and Hd2. In improved variety, functional Hd1 delayed flowering in HZ by 53 d when Ghd7, DTH8 and Hd2 all had functional genotypes, but this effect almost completely disappeared when only one or two of the three background genes had functional genotypes. Consistent results were observed on PS index despite of the presence of a residual effect when two background genes had functional genotypes. In landrace, similar but less obvious tendency was detected for the two traits.
Ecotypes of the improved varieties and landraces were defined by the primary cropping region and season, followed by classification of functional groups according to the functional type of Hd1 and the total number of functional Ghd7, DTH8 and Hd2 (Table 1). In improved variety, the seven functional groups showed a clear differentiation on growing seasons. In the group having functional Hd1 and non-functional ghd7, dth8 and hd2, all the 55 varieties were early-season varieties. In the groups having functional Hd1 with two or three other functional genes, all the 15 varieties were late-season varieties. In the three other groups having one or two functional genes, the 45 varieties were all early- or middle-season varieties, except one late-season variety used in central China. The last group, which had non-functional hd1 and functional Ghd7, DTH8 and Hd2, contained five, three and two early-, middle- and late-season varieties, respectively. In landrace variety, differentiation of the functional groups on growing seasons was also observed but less distinguishable than in improved variety. Landraces having functional genotypic at all the Ghd7, DTH8 and Hd2 loci were found in 29 of 31 late-season cultivars, but only in 4 of 50 early/middle cultivars.
| Table 1. Ecotypic distribution of different combinations of Hd1, Ghd7, DTH8 and Hd2 functional types. |
Double cropping of early- and late-season rice varieties in the southern China rice region plays a critical role in ensuring the food security in China. It has been recognized that early-season varieties used in a large scale of the commercial rice production do not show sensibility to photoperiod or only have weak sensitivity, although they generally carry functional Hd1 having a strong sensitivity to photoperiod (Lin et al, 2000; Xu et al, 2010). Of the 86 early-season improved varieties for which genotypes of Hd1, Ghd7, DTH8and Hd2 were all clear, 62 carried functional Hd1 (Table 1). However, all the 62 varieties were not included either in the only group showing strong PS in the two collections, Hd1+FFF (functional Hd1 with functional genotypic at all the Ghd7, DTH8 and Hd2 loci), or in the group having residual PS in improved variety, Hd1+FFN (functional Hd1 with two functional genotypes at the Ghd7, DTH8 and Hd2 loci). Obviously, loss of the strong photoperiodic sensitivity of functional Hd1 in early-season rice varieties can be ascribed to the absence of sufficient complementation from Ghd7, DTH8 and Hd2. In the meantime, presence of the functional Hd1 in most early-season rice varieties was beneficial due to its favorable association with early heading in the presence of non-functional ghd7, dth8 and hd2. It was also found 55 of the 62 early-season improved varieties were included in the Hd1+NNN group (functional Hd1 with non-functional genotype at the Ghd7, DTH8 and Hd2 loci) which showed no PS, whereas only two landraces belonged to this group (Table 1). These results suggest that the combination of functional Hd1 with non-functional ghd7, dth8 and hd2has undergone intensive artificial selection in modern rice breeding.
Taking advantage of the climate conditions in winter and spring in Hainan Province of China, shuttle breeding from other regions of China is implemented in Hainan during the cold season (Zhou et al, 2014). The rice materials are grown and tested in Hainan under NSD conditions, but most of the varieties developed are to be used under NLD conditions. Such a difference may lead to inappropriate selection on the agronomical traits especially flowering time (Wang et al, 2001; Qin et al, 2016; Zhao et al, 2017). Based on the comparison between HD of the 132 improved varieties in HZ and LS, it appears that day-length difference between the two regions may be no longer a great problem. As shown in Fig. S2-A, discrepancy on HD between the two regions was only observed in five varieties in the Hd1+FFF group and several varieties in the Hd1+FFN group, and all of them are late-season varieties grown under NLD conditions in their original cultivating areas. On the other hand, discrepancy was observed for many more landraces (Fig. S2-B). These results indicated that intensive artificial selection in modern rice breeding not only shaped rice varieties to adapt to the eco-geographical conditions in southern China, but also brought about the adaptation to shuttle breeding between Hainan and other regions of China.
In conclusion, the effects of Hd1 conditioned by its interactions with Ghd7, DTH8 and Hd2 largely determined the eco-geographical adaptation of rice varieties in southern China. Intensive artificial selection in modern rice breeding has resulted in the accumulation of the genotypic combination of functional Hd1 with non-functional ghd7, dth8 and hd2 in early-season varieties in southern China.
This study was supported by the National Natural Science Foundation of China (Grant No. 31571637) and the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Science (CAAS-ASTIP-2013-CNRRI). We thank the National Medium Rice Genebank at the China National Rice Research Institute for providing rice varieties.
The following materials are available in the online version of this article at http://www.sciencedirect.com/science/journal/ rice-science; http://www.ricescience.org.
File S1. Methods.
Fig. S1. Heading date and photoperiodic sensitivity index of three allelic types of Hd2 in rice.
Fig. S2. Comparison of heading date between Hangzhou and Lingshui.
Table S1. Improved varieties of indica rice used in this study.
Table S2. Landraces of indica rice used in this study.
Table S3. DNA markers used in this study.
Table S4. Heading date and photoperiodic sensitivity of multiple allelic types of indica rice at Hd1, Ghd7 and DTH8.
Rice materials
A total of 224 indica varieties used in southern China were tested, including 132 improved varieties and 92 landraces. The improved varieties selected from varieties that were planted in large areas in southern China, key backbone parents of inbred varieties, or main parents of hybrid rice (Table S1). The landraces were selected from previous study, representing the major rice-growing regions of southern China (Table S2) (Lu et al, 2015).
All the varieties were planted in Lingshui (LS), Hainan of China from December in 2017 to May in 2018 and Hangzhou (HZ), Zhejiang Province, China from May to October in 2018. During the period of floral transition of the rice materials, day-length in LS and HZ corresponds to natural short-day (NSD) and natural long-day (NLD) conditions, respectively (Zhang et al, 2012).
The varieties were planted using spacing of 16.7 cm between plants and 26.7 cm between rows. Field management followed the normal agricultural practice. For each variety, eight plants were planted in one row, and the middle six plants were scored. Heading date (HD) was recorded when a panicle emerged, and the mean value over six plants was used. Difference of effective accumulated temperature (AT) between HZ and LS was adopted as the photoperiodic sensitivity (PS) index.
Genotyping
The rice varieties were genotyped using gene-specific markers of 12 cloned QTLs for HD, including Hd3a, RFT1, Ehd1, Hd1, Ghd7, DTH8, Hd2, Hd6, Hd17, Hd18, OsMADS51and DTH2. Total DNA was extracted following the method of Zheng et al (1995). PCR amplification was performed according to Chen et al (1997). The products were separated on non-denaturing polyacrylamide gels and visualized using silver staining or separated on agarose gels and visualized using GelRed staining. The primers were selected from previous studies or designed using Oligo Primer Analysis Software Version 7.0 (Molecular Biology Insights, Inc.) based on functional nucleotide polymorphisms reported previously. As shown in Table S3 and described below, the markers differentiated the rice varieties into two allelic types for eight genes (Hd3a, RFT1, Ehd1, Hd6, Hd17, Hd18, OsMADS51, and DTH2), and into multiple types for four genes (Hd1, Ghd7, DTH8, and Hd2)..
For DTH2, a CAPS marker was developed according to single nucleotide polymorphism (SNP) G25A (Wu et al, 2013) and named as Tw30095. When the PCR products were subjected to Pst I digestion, allele A2 (early heading) can be cut into two fragments, whereas allele A1 (late heading) remains intact. For Hd6, a CAPS marker (Hd6) reported by Ebana et al (2011) was used. When the PCR products were subjected to Hind III digestion, the functional allele can be cut into two fragments, whereas the non-functional allele remains intact. For Hd17, a CAPS marker was developed according to an SNP T1673C (Matsubara et al, 2012) and named as Si2235. When the PCR products were subjected to Hpy188 I digestion, alleleOsELF3(S) (late heading) can be cut into two fragments, whereas allele OsELF3(L) (early heading) remains intact. For Hd18, a derived cleaved amplified polymorphic sequences (dCAPS) marker was developed according to an SNP A673G (Shibaya et al, 2016) and named as Ei2388. When the PCR products were subjected to Fau I digestion, the G673-type (late heading) can be cut into two fragments, whereas the A673-type (early heading) remains intact. For OsMADS51, an allele-specific marker (Wn44142) reported by Chen et al (2018) was used. A 992-bp fragment can be amplified from the MY46-type (functional) whereas no product can be amplified from the ZS97-type (defective).
For Hd1, 10 functional and 9 non-functional types were found in indica rice (Zhang et al, 2015). Three types of nucleotide change, a 1-bp deletion at position 321, a 2-bp deletion at positions 871 and 872, and a 4-bp deletion at positions 1089-1092, were the major non-functional types. These three types jointly occupy 96.4% of indica varieties with non-functional hd1. InDel markers Si9336, Si9337-1 and Si9337-2 were developed according to the 1-bp, 2-bp and 4-bp deletions, which can amplify 90-bp, 72-bp and 145-bp fragments for the three major non-functional haplotypes, respectively. The other haplotype has 91-bp, 74-bp and 149-bp fragments at Si9336, Si9337-1 and Si9337-2, respectively, which was regarded as the functional type in the present study.
For Ghd7, four allelic types in indica were reported by Xue et al (2008). Among them, Ghd7-0 was non-functional, Ghd7-1and Ghd7-3 were strongly functional, and Ghd7-2 was weakly functional. The gene was fully deleted in Ghd7-0, while Ghd7-2 and Ghd7-3 carried substitutions at positions 365 and 697 compared with Ghd7-1, respectively. An allele-specific marker (Se9153) was developed according to the SNP at position 365, and a CAPS marker (Se9151) was developed according to the SNP at position 697. By combining genotypic information produced with the two markers, all the four allelic types can be distinguished.
For DTH8, five functional and six non-functional types were found in cultivated rice (Zhang et al, 2015). Two types of nucleotide change, a 105-bp deletion and a 1-bp deletion, are the major non-functional types, jointly occupying 95.8% of rice varieties with non-functional dth8. InDel markers Ei4332 and Ei4334 were developed according to the 105-bp and 1-bp deletions, which can amplify a 423-bp and a 108-bp fragments for the two major non-functional haplotypes, respectively. The other haplotype has 1539-bp and 109-bp fragments at Ei4332 and Ei4334, respectively, which was regarded as the functional type in the present study.
For Hd2, four allelic types were reported by Koo et al (2013), including a functional allele PRR37-1 and nonfunctional alleles PRR37-1a, PRR37-1b and PRR37-1c. Compared with PRR37-1, PRR37-1a carries an 8-bp deletion, and PRR37-1b and PRR37-1c have substitutions T2010C and C2113T, respectively. Two markers were used. One was InDel marker Se29626 developed previously (Zhang et al, 2016), which can amplify a 224-bp fragment for PRR37-1aand a 232-bp fragment for the other three alleles. The other one was CAPS marker Se29628 developed according to the two SNPs. When the PCR products were subjected to Kpn I digestion, the functional PRR37-1 can be cut into two fragments, whereas PRR37-1b and PRR37-1c remain intact. By combining genotypic information produced with the two markers, three allelic types can be distinguished, including PRR37-1, PRR37-1a and PRR37-1b/1c.
Data analysis
At each locus, genotypes of the 224 rice varieties were firstly recoded based on each marker and then classified into allelic types. Ambiguous results due to unclear products were treated as missing data, which included one improved variety at Hd3a, one landrace at Hd1, one improved variety and four landraces at DTH8, and seven improved varieties and six landraces at Hd2.
The two rice collections, improved variety and landrace, were separately computed for allelic frequencies of the 12 genes and tested for genotypic effects of 9 polymorphic genes. One-way analysis of variance (ANOVA) and Duncan’ s multiple range test were used to determine the genotypic effects. For the five genes having two allelic types, Hd3a, RFT1, DTH2, Hd17 and OsMADS51, phenotypic differences between two genotypic groups were straightforwardly tested with one-way ANOVA. For the four genes having multiple allelic types, Hd1, Ghd7, DTH8 and Hd2, the Duncan’ s multiple test was firstly performed, and the results were applied to re-classify the varieties into two groups for one-way ANOVA. In addition, two-way ANOVA was used to determine genetic interactions of Hd1 with three other genes, Ghd7, DTH8 and Hd2. These analyses were performed using the SAS procedure GLM (SAS Institute, 1999). The threshold used for claiming a significant difference was P < 0.01.
REFERENCES
Chen J Y, Zhang H W, Zhang H L, Ying J Z, Ma L Y, Zhuang J Y. 2018. Natural variation at qHd1 affects heading date acceleration at high temperatures with pleiotropism for yield traits in rice. BMC Plant Biol, 18(1): 112.
Chen X, Temnykh S, Xu Y, Cho Y G, McCouch S R. 1997. Development of a microsatellite framework map providing genome-wide coverage in rice (Oryza sativa L.). Theor Appl Genet, 95(4): 553-567.
Ebana K, Shibaya T, Wu J, Matsubara K, Kanamori H, Yamane H, Yamanouchi U, Mizubayashi T, Kono I, Shomura A, Ito S, Ando T, Hori K, Matsumoto T, Yano M. 2011. Uncovering of major genetic factors generating naturally occurring variation in heading date among Asian rice cultivars. Theor Appl Genet, 122(6): 1199-1210.
Koo B H, Yoo S C, Park J W, Kwon C T, Lee B D, An G, Zhang Z, Li, J, Li Z, Paek N C. 2013. Natural variation in OsPRR37 regulates heading date and contributes to rice cultivation at a wide range of latitudes. Mol Plant, 6(6): 1877-1888.
Lu Q, Zhang M C, Niu X J, Wang S, Xu Q, Feng Y, Wang C H, Deng H Z, Yuan X P, Yu H Y, Wang Y P, Wei X H. 2015. Genetic variation and association mapping for 12 agronomic traits inindica rice. BMC Genom, 16: 1067.
Matsubara K, Ogiso-Tanaka E, Hori K, Ebana K, Ando T, Yano M. 2012. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol, 53(4): 709-716.
SAS Institute Inc. 1999. SAS/STAT User’ s Guide. Cary: SAS Institute.
Shibaya T, Hori K, Ogiso-Tanaka E, Yamanouchi U, Shu K, Kitazawa N, Shomura A, Ando T, Ebana K, Wu J, Yamazaki T, Yano M. 2016. Hd18, encoding histone acetylase related to Arabidopsis FLOWERING LOCUS D, is involved in the control of flowering time in rice.Plant Cell Physiol, 57(9): 1828-1838.
Takahashi Y, Teshima K M, Yokoi S, Innan H, Shimamoto K. 2009. Variations in Hd1 proteins, Hd3apromoters, and Ehd1expression levels contribute to diversity of flowering time in cultivated rice. Proc Natl Acad Sci USA, 106(11): 4555-4560.
Wu W X, Zheng X M, Lu G W, Zhong Z Z, Gao H, Chen L P, Wu C Y, Wang H J, Wang Q, Zhou K N, Wang J L, Wu F Q, Zhang X, Guo X P, Cheng Z J, Lei C L, Lin Q B, Jiang L, Wang H Y, Ge S, Wan J M. 2013. Association of functional nucleotide polymorphisms at DTH2 with the northward expansion of rice cultivation in Asia. Proc Natl Acad Sci USA, 110(8): 2775-2780.
Xue W Y, Xing Y Z, Weng X Y, Zhao Y, Tang W J, Wang L, Zhou H J, Yu S B, Xu C G, Li X H, Zhang Q F. 2008. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet, 40(6): 761-767.
Zhang J, Zhou X C, Yan W H, Zhang Z Y, Lu L, Han Z M, Zhao H, Liu H Y, Song P, Hu Y, Shen G J, He Q, Guo S B, Gao G Q, Wang G W, Xing Y Z. 2015. Combinations of the Ghd7, Ghd8 and Hd1 genes largely define the ecogeographical adaptation and yield potential of cultivated rice. New Phytol, 208(4): 1056-1066.
Zhang Z H, Wang K, Guo L, Zhu Y J, Fan Y Y, Cheng S H, Zhuang J Y. 2012. Pleiotropism of the photoperiod-insensitive allele of Hd1 on heading date, plant height and yield traits in rice. PloS One, 7(12): e52538.
Zhang Z H, Cao L Y, Chen J Y, Zhang Y X, Zhuang J Y, Cheng S H. 2016. Effects of Hd2in the presence of the photoperiod-insensitive functional allele of Hd1 in rice. Biol Open, 5(11): 1719-1726.
Zheng K L, Huang N, Bennett J, Khush G S. 1995. PCR-Based Marker-Assisted Selection in Rice Breeding: IRRI Discussion Paper Series No. 12. Los Banos: International Rice Research Institute.
Zhu Y J, Fan Y Y, Wang K, Huang D R, Liu W Z, Ying J Z, Zhuang J Y. 2017.Rice Flowering Locus T 1 plays an important role in heading date influencing yield traits in rice.Sci Rep, 7(1): 4918.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|