Drought stress is a serious limiting factor to rice production, which results in huge economic losses. It is becoming a more serious issue with respect to the global climate change. Keeping in view of the current and forecasted global food demand, it has become essential to enhance the crop productivity on the drought-prone rainfed lands with priority. In order to achieve the production target from rainfed areas, there is a requirement of rice varieties with drought tolerance, and genetic improvement for drought tolerant should be a high priority theme of research in the future. Breeding for drought tolerant rice varieties is a thought-provoking task because of the complex nature and multigenic control of drought tolerant traits would be a major bottleneck for the current research. A great progress has been made during last two decades in our understanding of the mechanisms involved in adaptation and tolerance to drought stress in rice. In this review, we highlighted the recent progresses in physiological, biochemical and molecular adaptation of rice to drought tolerance. A brief discussion on the molecular genetics and breeding approaches for drought tolerance in rice will be focused for the future crop improvement program for development of drought tolerant rice varieties.
Rice (Oryza sativaL.) is the most widely consumed staple food for a large part of the world’ s human population, especially in Asia (Samal et al, 2018). Asia is on the top in terms of production and consumption of rice. According to FAO report (2016-2017), average production of rice is estimated as 5.0 × 108 t, and due to rise in population, the requirement is expected to increase up to 2.0 × 109 t by the year 2030. The present and foreseen worldwide sustenance requests a momentous enhancement in crop productivity on the less favourable rainfed lands. Climate change, influencing the regularity and level of hydrological fluctuations, is a major threat to agriculture particularly in developing nations, and causes various abiotic stresses for plants (Turral et al, 2011). Amongst the abiotic factors that have created plant evolution, drought is the most imperative and major limitation for rice production in rainfed ecosystems (Nelson et al, 2014; Pandey and Shukla, 2015). From the agricultural aspect, drought is a time span with low average precipitation/poor rain or higher evaporation rates causing a downfall in crop growth and yield (Rollins et al, 2013). The intensity/ severity of drought is very complex and is dependent on different reasons like frequency of rainfall, evaporation and soil moisture (Hao et al, 2018; Oladosu et al, 2019). More than one third of the world’ s total cultivated area is affected by drought stress. Within that area, 33% (9.9 × 107 hm2) belongs to developing countries, 25% (6.0 × 107 hm2) belongs to developed nations and 42% (12.6 × 107 hm2) belongs to under developed countries (Rijsberman, 2006). In Asia alone, about 3.4 × 107 hm2 of rainfed lowland and 8.0 × 106 hm2 of upland rice exposed to drought stress (Singh et al, 2016). Breeding rice varieties with tolerance to drought stress offers an economically viable and sustainable option to improve rice productivity (Pandey and Shukla, 2015). Breeding for rice plants with drought tolerance has previously been attempted by a number of researchers, but progress is slow because of lack of suitable donors with a high level of drought tolerance. Screening of thousands of germplasm has been conducted earlier for drought resistance in various corners of the world, however, only a few drought-tolerant varieties are yet recognized (Singh et al, 2016). The main reasons for the minimal success are non-availability of truly drought-tolerant genotypes and lack of suitable screening methods (Pandey and Shukla, 2015). During the last two decades, scientists from the International Rice Research Institute (IRRI), the Philippines, screened nearly 1000 Genebank accessions originated from 47 countries for drought tolerance (Bin Rahman and Zhang, 2016). They have identified 65 more drought-tolerant accessions, which are either ausor indica(Torres et al, 2013). The highest number of drought-tolerant ausaccessions are originated from Bangladesh (19), followed by India (7), whereas the highest number of drought-tolerant indicaaccessions are originated from India (16), followed by Bangladesh (3) and Sri Lanka (3). Molecular genetics and characterization for drought tolerance is a vital requirement for using these rice accessions in the future crop improvement programs. The responses of rice plants to drought are believed to be complex that involves various physiological, biochemical and molecular changes (Upadhyaya and Panda, 2019; Gupta et al, 2020; Melandri et al, 2020). Therefore, the present review described the effects of drought stress on rice plants and highlighted the recent progresses in physiological, biochemical and molecular adaptation of rice to drought tolerance.
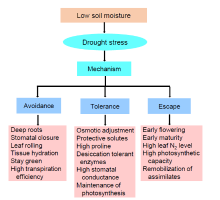
Drought resistance is the capability of a plant to produce its maximum economic yield under water limited conditions (Rollins et al, 2013). It is a complex trait depends on the action and interaction of different morphological, biochemical and physiological responses (Kumar et al, 2016). Drought escape is defined as ‘ the ability of a plant to complete its life cycle before the development of serious soil water deficits’ . Drought avoidance is defined by Kumar et al (2016) as ‘ the ability of plants to maintain relatively high tissue water potential despite a shortage of soil moisture’ . Drought tolerance is defined as ‘ the ability of plants to survive under low tissue water content’ (Kumar et al, 2016). Drought has detrimental effects on crop growth parameters and ultimately declines yield. Such damage is dependent on the scale, interlude of the stress and the growth stage of the plant. The detrimental results are reflected for changes in morphological, physiological, biochemical and molecular processes of the plant and their responses under drought stress are shown in Fig. 1.
Alterations in early morphology of rice are seen when rice are exposed to water stress. Founding of a timely and optimal crop stand is vital for normal productivity. The principal impact of drought stress is blighted germination and reduced growth (Farooq et al, 2012; Kadam et al, 2017; Mishra and Panda, 2017). Severe reduction in germination and seedling growth is observed under drought stress due to the scarcity of water (Vibhuti et al, 2015). Unlike some other crops, rice is extremely sensitive to drought conditions during the germination and early seedling growth stage. Seed germination needs appropriate temperature and soil humidity. Drought negatively affects germination process through inhibition of water uptake and reduces the strength of seedling (Vibhuti et al, 2015). Drought stress causes trouble of water balance, and damages metabolic process at cell level impairment of membrane transport, and decreases ATP production and respiration, leading to poor seed germination (Kadam et al, 2017). There are several reports indicated that water stress causes decreases in plant height, leaf area and biomass (Sarvestani et al, 2008; Mishra and Panda, 2017; Hussain et al, 2018).
Leaf growth is reduced due to limited water potential under drought stress (Zhu et al, 2020). Disrupted flow of water towards another cell from xylem, including lower turgor pressure due to water deficiency, responds in form of poor cell development and diminished leaf area in crops (Hussain et al, 2018). The anatomy of leaf and its ultra-structure are changed in drought stressed conditions (Upadhyaya and Panda, 2019). These changes are shrinkage of leaf size, reduction in number of stomata, bulky cell wall, cutinisation on leaf surface, and poor development of conducting system (Rollins et al, 2013). Rolling of leaf and initiation of early senescence are other important characteristics seen under drought stress (Anjum et al, 2011). Several leaf traits have been used for the screening of drought tolerant variety, i.e. higher flag leaf area, leaf area index, leaf relative water content and leaf pigment content (Farooq et al, 2009; Mishra and Panda, 2017; Hussain et al, 2018).
Root characteristics of the plants are the vital attributes for enhancing production under drought stress. Crop function under water stress is determined by the constitution and formation of rice root system. Rice production under water stress can be forecasted by taking root mass (dry) and length into account (Comas et al, 2013). Diversified and varied responses are observed on root growth characteristics under water stress. Manivannan et al (2007) observed the increase in the length of rice root under drought stress because of rise in abscisic acid concentration in the roots. Generally, rice varieties with profound and prolific root system show better adaptability in drought (Mishra et al, 2019; Kim et al, 2020). In case of rice, the genotypes having profound root system, coarse roots, capacity of producing many branches and high root and shoot ratio are important for drought tolerance (Kim et al, 2020). The morpho-physiological characteristics of rice roots play a major role in determining shoot growth and overall grain yield under drought stress (Kim et al, 2020).
During drought stress, different physiological processes are negatively impacted, and plants respond to drought in order to acclimatize in adverse states. It is essential to optimize the physiological parameters and processes prior to breeding program for enhanced yield under drought conditions (Dash et al, 2018; Barik et al, 2019; Gupta et al, 2020). Scarcity of water negatively affects the physiological characteristics of rice in innumerable ways, such as decreases in net photosynthetic rate, transpiration rate, stomatal conductance, water use efficiency, internal CO2 concentration, photosystem II (PSII) activity, relative water content and membrane stability index (Farooq et al, 2009; Dash et al, 2018; Mishra et al, 2018; Zhu et al, 2020).
Photosynthesis is one of the prime metabolic processes that determine the crop growth and production, and it is affected by water deficit/drought stress. Water stress changes the standard pace of photosynthesis as well as the gas exchange characteristics in plant (Zhu et al, 2020). Stomata are closed in environmental conditions of limited water, reducing carbon dioxide influx to leaves and driving extra electrons for formation of reactive oxygen species (Farooq et al, 2009; Mishra et al, 2018). Several factors are involved in the declining of photosynthesis, such as stomatal closure, decline of turgor pressure, reduction in leaf gas exchange and decrease in CO2 assimilation, ultimately damaging photosynthetic apparatus (Farooq et al, 2009; Gupta et al, 2020; Zhu et al, 2020). Photosynthetic capacities of leaves and water availability to the root zones are very important factors that reduce yield in susceptible rice genotypes under drought stress condition in rice (Zhu et al, 2020). During drought stress, inequity is observed between capture and use of light, reduction and impairment in Rubisco activity, pigments and photosynthetic machinery (Farooq et al, 2009), which are the reasons for photosynthesis diminutions. Water stress damages the normal functions of PSI and PSII (Zlatev, 2009; Mishra and Panda, 2017). PSII function is very important in reduction reaction and generation of ATP. Several studies have been carried out in vivo and observed that drought causes significant detrimental decrease in centers for oxygen evolution along with photosystem, leading to inhibition of electron transport chain and subsequent inactivation of PSII (Mishra and Panda, 2017; Mishra et al, 2018). Plant pigments, namely chlorophyll, are the vital predecessors of photosynthesis, mostly for obtaining light and generation of reducing powers (Mishra et al, 2019). Water deficiency causes reduction in the potential of mesophyll cell to utilize the carbon dioxide present in it. As a consequence, the amount of lively chlorophyll declines (Sarwar et al, 2013). Fall in chlorophyll and utmost quantum generations of PSII (Fv/Fm) are described in water stressed rice plants (Mishra and Panda, 2017; Mishra et al, 2018; Zhu et al, 2020). Carotenoids are the essential components for photoprotection and act as a precursor in directing signals for the growth of plants under stress conditions. Therefore, currently special attention is being taken by plant biologists to improve the carotenoid contents in plants either by breeding or genetic manipulation (Ashraf and Harris, 2013).
Plant and water relationship can be depicted through different representations such as water potential of the leaf and relative water content (RWC) (Farooq et al, 2009). Water use efficiency is considered to be an important component to determine yield potential of plants under water stress conditions. It can be considered as an approach for improving crop performance in drought (Mishra et al, 2019). RWC is an important attribute of water relations in the plants and is considered as the best integrated measurement of plant water status, which represents the variations in water potential and turgor potential of the plants (Gupta et al, 2020). Water stress is one amongst the several factors that negatively affects the RWC, turgor pressure and transpiration in many crops (Rao and Chaitanya, 2016). Choudhary et al (2009) screened four-week-old rice seedlings against drought and all the tested rice cultivars demonstrated a uniform rise in RWC around 48-72 h due to osmotic adjustment as result of increased proline content. Maintenance of stability in membrane index of the plant under water scarcity is a well-accepted tolerance phenomenon (Pandey and Shukla, 2015). Under water stressed conditions, exclusion of water from the membrane causes damage and dislocation of the usual lipid structure, and as a result, the membrane becomes exceptionally permeable during desiccation. Traits of cell membrane stability/membrane stability index have been used to know its correlation with yield of rice under drought stress (Upadhyaya and Panda, 2019).
Under drought stress, the plants try to maintain the cell turgor by accumulation of organic and inorganic solutes that lower the osmotic potential. Accumulation of osmoprotectants, such as proline, glycinebetaine and soluble sugar, provides osmotic adjustments for the plants (Kumar et al, 2016; Upadhyaya and Panda, 2019). Protein content and profiling along with increase in the antioxidant activity for scavenging reactive oxygen species improve drought tolerance (Pandey and Shukla, 2015). Tissue- and time-specific expression of drought-response traits, such as abscisic acid, brassinosteroids and ethylene phytohormone pathways, improves drought response without depressing yield (Gupta et al, 2020).
Osmoregulation is the major process in plants, and the decrease in turgor gives rise to accumulation of osmoprotectants. Accumulation of various osmolytes, such as proline, soluble sugar, phenolic and total free amino acids, increases under water deficit and it has important function for drought tolerance in plants (Anjum et al, 2017). Proline is a proteinogenic five- carbon α -amino acid as osmolyte in plants (Hayat et al, 2012). The free proline was first reported in rye grasses under water deficit stress by Kemble and Macpherson (1954). The proline accumulation increases under drought conditions as judged against well-watered condition in all the rice varieties (Mishra et al, 2018). Higher accumulation of proline is usually associated with drought tolerance and it helps for maintenance of leaf turgor and progress in stomatal conductance (Kumar et al, 2016). Thus, proline content can act as a biochemical marker under drought screening of plants (Pandey and Shukla, 2015; Mishra et al, 2019).
The structural unit, which provides energy to hold-up plant biomass, is named as carbohydrates/ soluble sugar. Under abiotic stress, basically three types of water-soluble carbohydrates, viz. disaccharides, oligosaccharides and fructans, play a vital role for stress tolerance (Keunen et al, 2013). Soluble sugars are highly important for balancing many physiological processes like photosynthesis and mitochondrial respiration (Gill and Tuteja, 2010a). Sugars have various roles in plants as they use numerous sugar- based strategies to acclimatize with environmental stress (Krasensky and Jonak, 2012). The abundance of mannitol, sorbitol and trehalose plays important roles in proper growth and metabolic function of the plant. Drought induces accumulation of soluble sugars that even acts as osmoprotectants under unfavorable conditions and protects the plants to a certain extent (Kumar et al, 2016; Upadhyaya and Panda, 2019).
During the aerobic metabolism, reactive oxygen species (ROS) is a natural byproduct. However, various biotic and abiotic stresses often stimulate extreme production of ROS that damages cells and causes death of the plants (Gill and Tuteja, 2010b). Photosynthesis and respiration produce ROS in various segments of the cytosol. Under unfavorable conditions, the over- reduction of electron transport chain of mitochondria and chloroplasts leads to the over-production of ROS (Melandri et al, 2020). Drought can also cause imbalance between ROS production and it’ s quenching in rice reacting with proteins, lipids and deoxyribonucleic acid causing oxidative damage and adversely affecting the plant life cycle (Gill and Tuteja, 2010b). Photosynthesis is adversely affected with electron leakage to 1O2 generating ROS by Mehler reaction. Excess amounts of superoxide radical (O2· -), hydrogen peroxide (H2O2) and hydroxyl radical (OH· ) are produced due to adverse effects of photo-respiratory pathway during drought (Gill and Tuteja, 2010a). These are highly toxic radicals that damage various cell components in drought stress like lipid peroxidation, protein and membrane damage ultimately leading to cellular death (Gill and Tuteja, 2010a). Therefore, the efficient way to enhance drought tolerance in rice is to reduce ROS over-production or enhance antioxidant activity in rice organs. The mode of ROS production, hazardous consequences of oxidative stress, cell damage that leads to plant death and different antioxidative systems scavenging ROS are presented in Fig. 2.
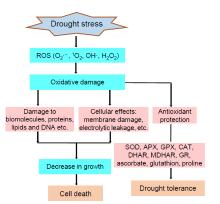 | Fig. 2. Schematic representation of reactive oxygen species (ROS) damage and antioxidant protection of rice plants under drought stress (Gill and Tuteja, 2010b). APX, Ascorbate peroxidase; CAT, Catalase; DHAR, Dehydroascorbate reductase; GR, Glutathione reductase; GPX, Guaiacol peroxidase; MDHAR, Monodehydroascorbate reductase; SOD, Superoxide dismutase. |
Plants possess antioxidant defense system as a protection against oxidative damage (Gill and Tuteja, 2010b). It comprises enzymatic and non-enzymatic antioxidants. The enzymatic antioxidants are superoxide dismutase (SOD), catalase (CAT), guaiacol peroxidase (GPX), ascorbate peroxidase (APX), monodehy- droascorbate reductase (MDHAR), dehydroascorbate reductase (DHAR) and glutathione reductase (GR), etc. The non-enzymatic antioxidants include ascorbate (AsA) and glutathione (GSH). The enhancement in the expression of antioxidative system can advance tolerance against drought stress and it can be a strategy against oxidative stress and increase drought tolerance in rice (Mishra and Panda, 2017). SOD acts as the primary defense line for oxidative stress, found in almost all cellular components of all organisms (Gill and Tuteja, 2010b). Increase of SOD level under drought stress is observed in various plants viz. rice, pea, wheat, sunflower, bean and sweet potato (Csiszá r et al, 2005; Zlatev et al, 2006; Lu et al, 2010; Mishra and Panda, 2017; Melandri et al, 2020). CAT is a heme containing enzyme and directly catalyses the dismutation of H2O2 into H2O and O2 (Gill and Tuteja, 2010b). It is located in the peroxisomes, mitochondria and other cellular components. CAT activity can increase or decrease during stress conditions as per the severity of the stress (Gill and Tuteja, 2010b). However, the decline in the CAT activity is the common response to many stresses (Qureshi et al, 2018). This decrease in CAT is presumed to occur as a result of suppression of the enzyme active sites under stress conditions. However, under drought stress, CAT activity is heterogeneous, and can increase, decrease or remain unchanged (Mishra and Panda, 2017). GPX is an enzyme which is composed of 40-50 kDa monomers. These monomers use hydrogen peroxide for oxidizing different substrates (Gill and Tuteja, 2010b). GPX is a great scavenger of ROS and produces related compounds like lignin, guaiacol and payragallol that help as electron donor for scavenging hydrogen peroxide both intra- and extra-cellular. Many studies reveal that GPX level rises under drought conditions in various plants such as wheat (Csiszá r et al, 2005) and rice (Mishra and Panda, 2017). The increase in GPX activity in rice under drought stress is universally studied and established as a good screening tool for tolerance characters (Bhattacharje and Dey, 2018; Qureshi et al, 2018).
The highly important function for the antioxidant association till date is the AsA-GSH (Halliwell-Asada) pathway. It takes place in the chloroplast where it provides photo-protection by scavenging hydrogen peroxide (Noctor and Foyer, 1998). The series of steps in the AsA-GSH cycle are: H2O2 is reduced by APX where AsA acts as electron donor. Later, the oxidized AsA is reduced by the help of reduced GSH. GR plays a vital role in producing this reduced GSH from oxidized glutathione (GSSG) by utilizing NADPH. These enzymes play vital roles in stress tolerance of plants. For rice plants, the enhanced activities of these enzymes are considered as one of the highly important protective mechanisms for oxidative stress during drought conditions (Gill and Tuteja, 2010b). In addition, enhancement of the activity of AsA-GSH cycle is directly correlated to the increase in the levels of ascorbic acid and GSH in rice plants under drought stress (Sharma and Dubey, 2005; Bhattacharje and Dey, 2018). There are many studied that have established importance of this pathway to battle water deficit in rice (Bhattacharje and Dey, 2018; Qureshi et al, 2018). Melandri et al (2020) studied the biomarker for yield loss in rice under drought stress and suggested that integrated network of antioxidant enzymes particularly the AsA-GSH cycle shows tolerance that can minimize grain yield losses under drought stress.
AsA holds an important stand for scavenging H2O2 via AsA-GSH cycle (Gill and Tuteja, 2010b). Basically, two steps are involved for the oxidation of AsA, viz. production of monodehydroascorbate (MDHA) and then the dehydroascorbate (DHA). The increase in ascorbic acid content is highly related to oxidative tolerance in rice (Bhattacharje and Dey, 2018) and hence broadly used as an important screening parameter for drought tolerance in rice plants (Upadhyaya and Panda, 2019). GSH is highly important against drought stress and over-production of ROS (Bhattacharje and Dey, 2018). GSH unswervingly functions as a free radical scavenger and can defend proteins, lipids and DNA. Under drought stress, reduced GSH is extensively studied in rice crop, and remarkable increase was observed as a response (Farooq et al, 2009; Gill and Tuteja, 2010b). Many researchers have suggested for reduction of GSH content for determining or screening plants for drought stress tolerance (Pandey and Shukla, 2015; Bhattacharje and Dey, 2018).
The environmental drought stimuli are caught by sensors on the membranes which are still to be very much portrayed, and after that the signals are transmitted down through various signal transduction pathways, bringing about the outflow of drought responsive qualities with appropriate gene functions and tolerance towards the drought (Dash et al, 2018; Oladosu et al, 2019). Drought is a multifaceted phenomenon (Zargar et al, 2011; Kumar et al, 2016). Hence, hybridization and selection strategies can not give precise results with respect to drought tolerance. Nevertheless, utilization for DNA markers in molecular studies can affix the procedure providing exact outcomes. Also, these molecular markers can be a boon for the screening of drought tolerant germplasms from a mass and using for further crop improvement purposes. Many works have been dedicated to establish some qualitative trait loci (QTLs) related to various traits (Kumar et al, 2016; Barik et al, 2019; Upadhyaya and Panda, 2019). The primary methodologies used for distinguishing genes engaged with drought resilience in rice are DNA studies based on marker-based phenotyping. Despite the progress, only a few traits practically have been approved for having drought resistance capacity (Zargar et al, 2011; Prakash et al, 2019). In this way, molecular breeding can lead in improvement of crop varieties and enhancement of yield assortments, and create productive harvests that are safe and have high agronomic legitimacy.
There are certain genes available in the plant genome that have very precise quantitative traits and these genes are known as QTLs. Different QTLs linked to different agronomic traits under drought are shown in Table 1. Earlier molecular genetic studies identified numerous QTLs linked to different physiological and biochemical traits (Alexandrov et al, 2014; Todaka et al, 2015; Dixit et al, 2017; Barik et al, 2019), but failed to identify genes that regulate these traits, because of low mapping resolution and weak phenotypic effect (Bin Rahman and Zhang, 2016; Singh et al, 2016). Identifying these QTLs related to selective traits helps in stress screening programs for plants (Vikram et al, 2011). Many QTLs linked to different physiological and growth traits under drought have been identified and used extensively for selection of tolerant rice genotypes (Vikram et al, 2011, 2016; Venuprasad et al, 2012; Dixit et al, 2017; Vinod et al, 2019). The classification of QTLs at various growth stages of rice is also studied (Dixit et al, 2012; Venuprasad et al, 2012; Ramchander et al, 2016). Considering yield to be a definitive point, ongoing investigation centers around the world concentrate basically in mapping QTLs for grain yield of rice under drought stress (Pandey and Shukla, 2015). Hence, unique QTLs could be identified for drought tolerance and could be used for breeding drought-tolerant rice varieties. Till date, the QTLs identified in rice for drought tolerance are mostly from non-elite genotypes. The QTL qDTY1.1 is extensively used as a yield trait under drought stress of rice plants (Vikram et al, 2011). Other important QTLs identified in different rice lines are qDTY2.1 (Dixit et al, 2012), qDTY2.2 (Dixit et al, 2014), qDTHI2.3(Lin et al, 2007), qDTY3.1 and qDTY6.1(Venuprasad et al, 2012), qDTR8 (Ramchander et al, 2016), qDLR8.1(Qu et al, 2008), qDTY9.1A (Dixit et al, 2012) and qDTY12.1 (Mishra et al, 2013). Various SSR markers linked to these QTLs are also reported (Vikram et al, 2011). Hence, using these markers for molecular screening of new rice genotypes for drought tolerance would be helpful for quick and precise profiling of the rice lines. Barik et al (2019) studied the genetic mapping of morpho-physiological traits related with drought tolerance during reproductive stage in rice, and reported five QTLs such as qLR9.1, qLD9.1, qHI9.1, qSF9.1 and qRWC9.1 in rice that controlling leaf rolling, leaf drying, harvest index, spikelet fertility and relative water content, respectively, under drought stress at reproductive stage.
| Table 1. QTLs associated with yield and its component traits in rice with respect to drought tolerance. |
After exposure to drought stress in rice, many different types of genes are differentially expressed with about 5000 genes upregulated and 6000 downregulated (Bin Rahman and Zhang, 2016; Joshi et al, 2016). Some of the genes and their functions associated with drought tolerance in rice are presented in Table 2. These genes are categorised into three major categories such as genes related with membrane transport, genes related to signalling and genes related with transcriptional control (Upadhyaya and Panda, 2019; Kim et al, 2020). Their expression controls most of the biochemical, physiological and molecular mechanisms under drought stress in rice (Dash et al, 2018; Gupta et al, 2020). Many different genes/ transcription factors reported by Kumar et al (2016) and Upadhyaya and Panda (2019) show differential expression in rice and are used for transgenic plants in relation to drought stress. Most of the genes regulated under drought are ABA-independent as well as ABA independent regulatory systems (Du et al, 2018; Gupta et al, 2020). OsJAZ1 attenuates drought tolerance by ABA signalling in rice that orchestrates plant responses to growth and development under drought stress (Fu et al, 2017). Several genes are also associated with osmoregulation and late embryogenesis abundant (LEA) proteins that impart tolerance to water deficit in rice (Dash et al, 2018; Upadhyaya and Panda, 2019). The geneDRO1induces root elongation and deeper rooting in transgenic rice (Uga et al, 2013), Other genes such as OsPYL/RCAR5 and EcNAC67 delay leaf rolling, and induce higher root and shoot mass in rice under water deficit conditions (Kim et al, 2014; Rahman et al, 2016). Root morphological adaptations in rice under drought stress are also increased by over expression of OsDREB2B, CYP735AandOsDREB1F (Kim et al, 2020). Huang et al (2018) reported DREB2-like gene OsDRAP1conferring drought tolerance in rice. Increase of grain yield in rice under drought is crucial and is achieved by transgenic approaches by introduction of some genes such as OsNAC5 (Hu et al, 2006), OsLEA3-1(Xiao et al, 2007), OsbZIP71(Liu et al, 2014), OsWRKY47 (Raineri et al, 2015), OsbZIP46(Tang et al, 2012) and OsNAC10(Jeong et al, 2010). Higher water use efficiency, accumulation of osmolytes and higher antioxidant enzyme activity and enhanced photosynthesis are noticed in transgenic rice by interrogation of genes such as EDT1/HDG11 (Yu et al, 2013), AtDREB1A(Ravikumar et al, 2014), OsMIOX(Duan et al, 2012) and OsTPS1 (Li et al, 2011). OsCPK9 improves drought tolerance through enhanced stomatal closure and better osmoregulation in transgenics (Wei et al, 2014). Overexpression of OsDREB2A enhances the survival of transgenic plants under severe drought and saline conditions (Cui et al, 2011). Several regulatory proteins, signal transduction pathway and protein kinases in rice are controlled by CDPK7andCIPK03/CIPK12(Saijo et al, 2000; Xiang et al, 2007). Reduced levels of inositol triphosphate and ROS homeostasis are carried by OsITPK2 under drought stress in rice (Du et al, 2011). The WRKY genes play important roles in plant development by responding to drought stress (Sahebi et al, 2018). Using transgenic approaches, several genes have been tested for imparting drought tolerance in rice under laboratory or glass house conditions. However, these genes should be tested under field conditions before use in molecular breeding programs.
| Table 2. List of genes and their functions associated with drought tolerance in rice. |
MicroRNAs (miRNAs) are small noncoding regulatory RNAs that modulates gene expression during abiotic stress (Singh et al, 2020). The reported miRNA- mediated gene and its function in rice subjected to drought stress are shown in Table 3. Several miRNAs also confers drought tolerance in rice by altering gene expression (Cheah et al, 2015; Faisal et al, 2017; Singh et al, 2020). The expression of miR393, miR319 and miR397 in response to drought stress was first reported in Arabidopsis (Sunkar and Zhu, 2004) and miR393 also controls tiller number increment, early flowering, hypersensitive to auxin in rice by regulating transcriptional factors OsAUX1 and OsTIR1 (Xia et al, 2012). There are 30 miRNAs identified in rice, of which 11 are down-regulated and 8 are up-regulated under drought stress (Zhou et al, 2010). Mutum et al (2016) also reported 71 novel miRNAs from Nagina 22, a drought tolerant check variety. Balyan et al (2017) reported that miR398 regulates the expression of Cu/ZnSODs that modulates the SOD activity under drought stress in rice. Early auxin response is also regulated by the expression of ARF gene under drought stress by miR160 and miR167 (Upadhyaya and Panda, 2019). Increases in stomatal closure and declines in stomatal density through ROS homeostatic genes and eventually DST-amiRNA increase drought resistance (Faisal et al, 2017). Development of roots and cell wall biogenesis and metabolism of carbohydrates are regulated by over- expression ofUDP-glucose-4-epimerasegene and mediated by Osa-miR169-3p and Osa-miR166e-3p (Cheah et al, 2015). Recently, Singh et al (2020) reported 10 miRNAs (miR531, miR827, miR8175, miR977, miR6300, miR1861, miR440, miR9773, miR3982 and miR1876) regulated by drought stress in traditional rice landraces. These miRNAs can be targeted for genetic manipulation for the drought tolerance. Therefore, many of the drought tolerance responses are regulated by miRNAs, which can potentially enrich for the development of drought tolerant rice variety.
| Table 3. MicroRNA-mediated gene and its function in rice subjected to drought stress. |
The natural rice genotypic variation can be explored to identify the novel genotypes with drought tolerant traits of interest and associated genes/loci. These novel genotypes can be exploited in conventional breeding programmes through marker-assisted selection for development of drought tolerant rice variety. Breeding program is meant for producing high yield lines with improved quality parameters and further to launch the cultivars for farming. Breeding of drought tolerant rice genotypes was studied in past (Singh et al, 2016; Vikram et al, 2016; Dixit et al, 2017; Kumar et al, 2017; Barik et al, 2019), but the success rate has been far below the expectation because of the difficulty in finding fitting donors with a higher tolerant level along with its environment specific nature. Most of the marker-assisted breeding approaches for the development of drought tolerant rice variety were carried out in the last decade at International Rice Research Institute (Kumar et al, 2016; Sandhu and Kumar, 2017). By using marker-assisted breeding approaches, several QTLs for drought tolerance in rice is incorporated into leading cultivars (Singh et al, 2016). They have successfully incorporated QTLs such as qDTY9.1, qDTY2.2, qDTY10.1 and qDTY4.1in high-yielding variety IR64 by marker-assisted backcrossing approach (Singh et al, 2016). Shamsudin et al (2016) successfully developed drought-tolerant elite Malaysian rice cultivar MR219 with pyramiding of three QTLs, qDTY2.2, qDTY3.1 and qDTY12.1. Dixit et al (2017) have developed a rice variety TDK1 for high yield under drought by incorporation of three QTLs (qDTY3.1, qDTY6.1 and qDTY6.2). Drought has gained importance only as a constraint and no effective measures have yet proved to be successful to develop drought tolerant rice varieties. Majority of high-yielding varieties such as Swarna, Samba- mahsuri and IR36, which are earlier recommended for cultivation in irrigated fields, have been used for drought breeding program. When these varieties are grown in rainfed ecosystem by the farmers during the frequent drought spell, significant loss in rice production is recorded because the aforesaid high- yielding varieties cannot succumb frequent droughts (Hao et al, 2018). Thus, more attention is needed for improvement of special rice varieties with high yield under drought and adaptation to wide range of adverse climatic conditions in future.
Development of drought tolerance in rice is a thought-provoking task that requires a comprehensive thoughtful of the various morphological, biochemical, physiological and molecular characters. Although remarkable progresses have been achieved through marker-assisted breeding, we still have several critical problems to overcome molecular breeding of drought tolerance in rice. Moreover, the complex nature and multigenic control of drought tolerant traits would be a major bottleneck for the current and coming future research in the area. Maintenance of yield in rice under drought conditions is a multifaceted phenomenon controlled by the cumulative effects of several traits. Transgenic approaches play a pivotal role in improving agronomic traits and yield characteristics of rice and it would be an efficient way to boost the rice breeding program for drought tolerance. Several genes have been tested for imparting drought tolerance in rice under laboratory condition, thus it is also necessary for understanding the responses of these genes under drought in field condition. Though extensive basic research is under progress, our understanding of the whole-plant stress response mechanism is still very limited. Therefore, we need to investigate stress responses in differentiated cells, tissues and organs and to connect the data relevantly. Advancement in the emerging technologies pertaining to crop physiology, molecular genetics and breeding approaches can be used as an integrated manner in crop breeding, so that it not only improves our understanding of drought tolerance but also facilitates the genetic improvement of drought tolerant rice varieties. The summarized information of this review will facilitate further investigations of signalling mechanisms of drought tolerance in rice and development of high-yielding drought tolerant genotypes.
(Managing Editor: Li Guan)
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|
| [102] |
|
| [103] |
|
| [104] |
|
| [105] |
|
| [106] |
|
| [107] |
|
| [108] |
|
| [109] |
|
| [110] |
|
| [111] |
|
| [112] |
|
| [113] |
|
| [114] |
|
| [115] |
|
| [116] |
|
| [117] |
|
| [118] |
|
| [119] |
|
| [120] |
|
| [121] |
|
| [122] |
|
| [123] |
|