The emerging pests and phytopathogens have reduced the crop yield and quality, which has threatened the global food security. Traditional breeding methods, molecular marker-based breeding approaches and use of genetically modified crops have played a crucial role in strengthening the food security worldwide. However, their usages in crop improvement have been highly limited due to multiple caveats. Genome editing tools like transcriptional activator-like effector nucleases and clustered regularly interspaced short palindromic repeats (CRISPR)-associated endonuclease Cas9 (CRISPR/Cas9) have effectively overcome limitations of the conventional breeding methods and are being widely accepted for improvement of crops. Among the genome editing tools, the CRISPR/Cas9 system has emerged as the most powerful tool of genome editing because of its efficiency, amicability, flexibility, low cost and adaptability. Accumulated evidences indicate that genome editing has great potential in improving the disease resistance in crop plants. In this review, we offered a brief introduction to the mechanisms of different genome editing systems and then discussed recent developments in CRISPR/Cas9 system-based genome editing towards enhancement of rice disease resistance by different strategies. This review also discussed the possible applications of recently developed genome editing approaches like CRISPR/Cas12a (formerly known as Cpf1) and base editors for enhancement of rice disease resistance.
Rice (Oryza sativa L.) serves as the significant source of carbohydrate for more than two thirds of the world’ s population. The burgeoning population coupled with climatic change and emergence of new phytopathogens have a drastic effect on global rice productivity. During the last few decades, the traditional breeding approaches including mutation breeding and molecular breeding have significantly contributed to the development of effective disease resistance strategies in rice as required for strengthening global food security. However, these methods are time-consuming and labor-intensive. Gene transfer techniques by way of Agrobacterium-based transformation and other approaches have significantly improved the yield, quality and disease resistance in plants. However, the biosafety regulations, and social and ethical issues related to transgenic crops have always been a major hindrance to public acceptance of these genetically modified (GM) crops (Lusser et al, 2012). Afterwards, better strategies for high-yielding and stress-resistant rice varieties are the need of the hour for increasing rice productivity and ensuring global food security.
Plant diseases are the major constraints that have threaten the agricultural development and global food security. Crops are susceptible to a wide range of pathogens including bacteria, viruses and fungi, which cause significant economic loss (FAO, 2017). The pathogens affect the growth and development of crops, which results in huge yield loss thereby creating hurdles for sustainable agriculture. Multiple disease management strategies and risk assessment tools including the usage of disease forecasting protocols, combinatorial usage of chemicals, fungicides and biological control agents, crop rotations and host resistance breeding have been under practice for a very long time (Ul Haq and Ijaz, 2020). However, most of these strategies are inadequate to achieve successes in many pathosystems due to diversity in host range of the phytopathogens, environmental toxicity, discrepancy in the assessment of disease resistance reaction and inefficient disease forecasting systems. Therefore, understanding the molecular mechanism governing the interaction between host and the pathogen is central to the development of an effective disease resistance strategy.
Plants utilize multiple resistance responses towards preventing the colonization by pathogenic microorganisms. On one hand, plant resistance to phytopathogens is typically regulated by the resistance (R) genes encoding nucleotide-binding leucine-rich repeat (NB-LRRs) proteins, which neutralize the molecular activity of pathogen effector proteins in the plant cell (Cui et al, 2015). On the other hand, targeted mutation or knockout of susceptibility (S) gene(s) that acts as a host-pathogen compatibility factor also induces recessive immunity against the adapted phytopathogens (van Schie and Takken, 2014). As majority of plant diseases arise due to compatible interaction between host and the pathogen, altering the S-gene(s) that promotes compatibility could be highly significant in the development of broad spectrum and durable molecular breeding strategies for disease resistance (van Schie and Takken, 2014).
Recently, the emergence of multiple new breeding techniques including speed breeding platforms, high throughput genotyping and precise genome editing coupled with genetic engineering have successfully generated multiple disease-free high-yielding crop varieties (Li et al, 2020). Among them, the genome editing approaches have emerged as the revolutionary tools for crop improvement (Voytas and Gao, 2014). The genome editing tools are represented by sequence specific nucleases (SSNs) that introduce a double stranded DNA break at a specific genomic region thereby inducing the host DNA repair pathways either by homologous recombination (HR) or through non- homologous end joining (NHEJ) (Sander and Joung, 2014). While NHEJ is an error prone method that creates random mutations leading to frame shift and target gene knockout, HR pathway is much more precise resulting in gene replacement or gene knock-in when donor DNA templates are available (Baltes et al, 2015). Nevertheless, these natural processes of DNA repair result in mutation leading to alteration of specific trait. Multiple genome editing tools including zinc finger nucleases (ZFNs), transcriptional activator-like effector nucleases (TALENs) and more recently developed clustered regularly interspaced short palindromic repeats (CRISPR)-associated endonuclease Cas9 (CRISPR/Cas9) have facilitated the introduction of agronomically important traits in many plant species (Zaidi et al, 2018). Among these platforms, CRISPR/Cas9 system has greater acceptance by the scientific community for its simplicity, high specificity for target cleavage, involvement of no complex protein chemistry and universal applicability (Zaidi et al, 2018). What’ s more, CRISPR/Cas-mediated disease resistance has already been reported against multiple phytopathogens including bacteria (Peng et al, 2017), fungi (Wang et al, 2016) and viruses (Chandrasekaran et al, 2016; Zaidi et al, 2016) in different crops including rice (Sun et al, 2017; Li S Y et al, 2018; Tomlinson et al, 2019; He et al, 2020) either by target specific modification of the host genes or by precise alteration of the pathogen genomes. In this review, we summarized the different strategies associated with genome editing and their subsequent applications in the improvement of crops with focus on highlighting the advancements of the CRISPR/Cas9 system and its role in conferring disease resistance in rice.
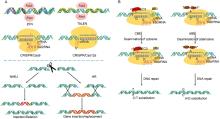
Genome editing is a novel approach wherein SSNs are used to make precise modifications in the genomic DNA. Creation of a double stranded break (DSB) activates the cell’ s DNA repair mechanism, either through NHEJ or HR method. NHEJ incorporates insertion and deletion (InDel) mutations whereas HR method results in gene insertion or replacement that is much more precise. Mega nucleases such as the I-SceI endonuclease enzyme from yeast constituted the earliest known genome editing system (Paques and Duchateau, 2007). However, these are the least efficient among the editing toolbox due to unclear communication between mega nuclease protein residues and corresponding specific target DNA sequence (Hsu et al, 2014). ZFNs are the SSNs that bind DNA through an engineered array of zinc finger motifs (Carroll, 2011). A specific zinc-finger entails about 30 amino acids in a conserved β β α configuration. The non-specific cleavage domain FokI is dimeric in nature and as such a pair of ZFNs are designed to bring the FokI monomers close to the specific DNA target for creation of a DSB (Bogdanove and Voytas, 2011) (Fig. 1-A). ZFNs have been successfully used as a genome editing tool in a wide range of crops and model plants including Arabidopsis, tobacco and maize (Cai et al, 2009; Shukla et al, 2009; Osakabe et al, 2010). Yet, their usage as an editing tool is highly limited due to several constraints including off-target binding of the zinc-finger motifs and multifaceted interactions between amino acid residues and the target sequence (Carrol, 2011; Voytas, 2013).
Unlike ZFNs, TALENs are designed by combining a FokI nuclease domain with a transcription activator- like effector (TALE) DNA binding domain (Fig. 1-A). TALEs are secretory proteins from Xanthomonasbacteria that are characterized by the presence of a C-terminal acidic activation domain and nuclear localization signal sequence, central DNA binding domain (DBD) and N-terminal translocation signal sequence (Bogdanove et al, 2010). TALEs are primarily responsible for the transcriptional activation of the disease susceptibility genes in host plants. TALENs have been well established in many plant species, including rice, wheat, tobacco and barley (Wang et al, 2014; Li T et al, 2016; Blanvillain-Baufume et al, 2017). Although TALENs are qualitatively more effective over ZFNs in terms of high target specificity and low off-target activity, the TALE DBDs are represented by extensive repeat structure which acts as a limitation for their comprehensive use in editing of multiple genomes. In contrast, the type II CRISPR/Cas9 system is the utmost innovative genome editing method that has superseded ZFNs and TALENs due to its efficiency and robustness. CRISPR/Cas9 makes use of a DNA endonuclease Cas9 along with a small RNA molecule called single guide RNA (sgRNA) that regulates the Cas9 mediated site specific DSB at specific targets (Fig. 1-A). The attachment of the Cas9-sgRNA structure to the target DNA and the ensuing cleavage depends on the presence of a protospacer adjacent motif (PAM) sequence (5′ -NGG-3′ ) at the 3′ -end of the target site. Hence, the requisite of only different spacer sequences makes CRISPR/Cas9 a very simple and highly effective editing tool that has been greatly exploited in recent years for improvement of model plants and major crops (Ma et al, 2015; Xu et al, 2016; Zhang Y et al, 2020).
While trait improvement has been highly fruitful using CRISPR/Cas9, the prerequisite of an NGG PAM sequence has restricted its usage to potential target sites. Lately, several Cas9 variants and homologous proteins, such as Cas9-VRER, Cas9-VQR, Cas9-EQR, Cpf1-RVR, Cpf1-RR and SaCas9, have improved the feasibility of engineering a wide range of Cas9s with improved PAM specificities for genome editing in eukaryotic cells (Kleinstiver et al, 2015; Gao et al, 2017). Hu et al (2018) used a phage-assisted continuous evolution process to develop a SpCas9 variant, called xCas9, which has broader range of PAM compatibility including NG, GAA and GAT, and at the same time greater DNA specificity and lower genome-wide off-target activity compared to SpCas9. More recently, novel cytosine and adenine base editors with engineeredStaphylococcus aureus Cas9 (SaCas9-NG), SpCas9-NG variants, have substantially expanded the targetable sites in the rice genome (Hua et al, 2019). Additionally, SpCas9-NG variants have greatly expanded the scope of genome editing in potato and tomato by targeting the non- canonical NGA and NGT PAMs (Veillet et al, 2020). Taken together, an efficient usage of these Cas9 variants would be critical to accelerate rice improvement through enhanced resistance to multiple phytopathogens.
The advent of another class II CRISPR-associated endonuclease, CRISPR/Cas12a or Cpf1, has broadened the horizon for genome editing with greater accuracy and competence (Endo et al, 2016) (Fig. 1-A). Compared to CRISPR/Cas9 system, the CRISPR/Cas12a recognizes T-rich (5′ -TTTN-3′ and 5′ -TTN-3′ ) PAMs at the 5′ -end of the target site, resulting in high cleavage efficiency (Zetsche et al, 2015). Unlike CRISPR/Cas9 which requires a 100 nt sgRNA, the CRISPR/Cas12a complex doesn’ t require a tracrRNA and therefore can facilitate gene editing with only a crRNA of 40-45 nt consisting the repeat and the spacer. And the RuvC and nuclease domains of Cas12a cleave the target and the secondary strand of the DNA at 23 and 17 bp downstream of the PAM sequence, respectively, resulting in staggered ends with 5 bp overhangs (Zetsche et al, 2015). By itself, the development of bigger mutations using CRISPR/ Cas12a upsurges the efficiency of HR mediated donor gene insertion at the specific genomic location. Moreover, Cas12a simultaneously acts as an RNase to convert pre-crRNA to crRNA and a nuclease to cleave the dsDNA, demonstrating dual enzymatic activity (Zetsche et al, 2015). Therefore, CRISPR/Cas12a has the potential to generate multiple crRNA driven by a single promoter, making it simpler than CRISPR/Cas9 system. Moreover, the off-target cleavage by Cas12a is relatively lower than Cas9. These features make CRISPR/Cas12a more advanced over Cas9, making it a potentially important genome editing tool in the future (Zaidi et al, 2017). The Cas12a from Francisella novicida(FnCas12a) and its ortholog from Lachnospiraceae bacterium (LbCas12a) and Acedomonococcus sp. BV3L6 (AsCas12a) have been used to introduce targeted mutations in many crops (Endo et al, 2016). Emerging reports have already demonstrated the successful adaptation of CRISPR/ Cas12a system in rice improvement (Yin et al, 2017; Li S Y et al, 2018). Yin et al (2017) utilized the CRISPR- LbCpf1 to target the early developmental gene EPFL9 (Epidermal Patterning Factor like-9), a positive regulator of stomatal development in rice. The homozygous mutant plants show 8-fold reduction in the stomatal density on the abaxial leaf surface. Likewise, Li S Y et al (2018) reported that the donor repair template with only the left homologous arm is good enough for precise targeted allelic replacement in the wild type OsALS (Acetolactate synthase) gene resulting in herbicide resistant rice plants. Most recently, a comparative assessment of three Cas9 (Cas9 D10A nickase, HiFi Cas9 nuclease, and WT Cas9 nuclease) and two Cas12a nucleases (LbCas12a and AsCas12a) was carried out to determine their mutation efficiency on a single target site of the rice phytoene desaturase(PDS) gene (Banakar et al, 2020). The study showed that LbCas12a results in deletion between 2 to 20 bp without the loss of PAM site, leading to higher editing efficiency over the Cas9 variants, which suggests the potential of Cas12a to generate specific and heritable targeted mutations in rice and thus can be used as a precise genome editing tool for future rice improvement programmes including in the development of disease resistant rice varieties.
Although the HR-based gene replacement is a feasible approach with CRISPR/Cas9 and CRISPR/ Cas12a, the efficiency of template DNA delivery and target insertion is significantly low. To overcome this problem, the base editor technology has emerged as a new and advanced approach for precise nucleotide substitutions in a programmable manner without the requirement of a DSB or donor template (Fig. 1-B) (Komor et al, 2016). The base editors comprise of a catalytically inactive CRISPR/Cas9 domain (dCas9 or Cas9 nickase) and a cytosine or adenosine deaminase domain which converts one base to another. These are capable of making single-base variations or substitutions without creating a DSB in the target DNA, thereby limiting the frequency of InDels. Recently, base editing system has been efficiently used in creating targeted point mutations at multiple endogenous loci in rice and wheat (Li C et al, 2018). Most recently, rBE5 (hAID* ∆ -XTEN-Cas9n-UGI-NLS) base editor has been used to target Pi-d2, an agriculturally important rice gene that harbours a point mutation modulating the defense response to rice blast fungus (Ren et al, 2018). Cytosine and adenine base editors have also been efficiently used for precise base modification (C to T or A to G) in eukaryotic genomes (Zong et al, 2017; Hua et al, 2018; Qin et al, 2019). Base editing tool boxes have been efficiently optimized and demonstrated in several crops including rice, wheat, maize and tomato (Lu and Zhu, 2017; Zong et al, 2017; Li C et al, 2018; Tang et al, 2019). In course of time, a wide range of adenine base editors (ABEs) and cytidine base editors (CBEs) variants have been developed for efficient target specific base modification (Mishra et al, 2020). Furthermore, precise base editing in RNA has been realized using a catalytically-inactive Cas13 (dCas13) in association with adenosine deaminase acting on RNA (ADAR) to direct adenosine to inosine conversion (Cox et al, 2017). Cas13 is a type IV CRISPR-linked RNA guided RNase while ADARs mediate precise editing of the transcripts (Nishikura, 2010). Together, they have been used to develop an RNA editing system named as RNA Editing for Programmable A to I Replacement (REPAIR) that has significant applicability for research in therapeutics and biotechnology (Stafforst and Schneider, 2012). However, it is yet to be utilized in plant system.
Traditional genome editing including CRISPR/Cas9 system involves the delivery of DNA cassettes encoding editing machineries into the host genome. Often, the random integration of the editing cassette results in undesirable genetic changes and off-target effects (Zhang et al, 2018). Further, the introduction of editing cassette into the host genome invokes ethical and regulatory concerns (Jones, 2015). Therefore, scientists are increasing getting aligned to the usage of DNA-free genome editing technologies to minimize the probability of off-target mutations. DNA-free genome editing makes use of the CRISPR/Cas ribonucleoprotein (RNP) complexes that is delivered into the cell by protoplast transformation or particle bombardment leading to target DNA modification. CRISPR-RNP is an assembly of CRISPR specific guide RNA and Cas9 protein together forming an active enzyme complex (Zhang et al, 2018). The first DNA-free genome edited plants were obtained by transfecting the CRISPR/Cas RNPs into the protoplast of Arabidopsis, tobacco, lettuce and rice (Woo et al, 2015). Likewise, particle bombardment mediated DNA free editing using the CRISPR-Cas9 RNPs has been successfully demonstrated in maize (Svitashev et al, 2016), wheat (Liang et al, 2017) and rice (Toda et al, 2019). In rice, Cas9-gRNA RNPs have been directly delivered into the zygotic tissues with a mutation efficiency of 14% to 64% (Toda et al, 2019). In maize, CRISPR/Cas9 RNPs have been used to generate both knockout as well as knockin mutants (Svitashev et al, 2016).
Moreover, the CRISPR/Cas-RNPs have relatively high editing efficiency and low off-target mutations compared to CRISPR/Cas system (Liang et al, 2017; Toda et al, 2019). In another study, the base editing has been combined with the DNA-free editing system to facilitate higher frequency of C to T conversion (1.8%) in wheat (Zong et al, 2018). Overall, this transgene free precise editing system has enormous potential for the improvement of rice as well as other important crop species. Although the usage of CRISPR/Cas RNPs is still at its infancy, it can be effectively explored to accelerate rice crop breeding and the edited products can get wider acceptance in public overcoming the biosafety regulatory hurdles.
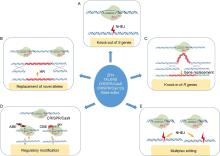
During the latter half of the 19th and early 20th centuries, host resistance breeding has played a pivotal role in the improvement of yield and other agronomic traits of rice, thereby addressing the challenges of feeding the world’ s growing population. However, traditional methods of resistance breeding are expensive and time-consuming, and sometimes the resistance allele influences plant growth and development (Miah et al, 2013). Extensive genetic and genomic studies have revealed significant molecular details about rice innate immunity, including a large number of targets for control and inhibition of pathogenic infection. While plants have evolved resistance (R) genes that can neutralize pathogen derived virulence proteins or effectors and activate effector triggered immunity, they also have susceptibility (S) genes that are essentially involved in successful pathogenic infection. In context of genome editing, the primary strategy for disease resistance involves knocking out of these Sgenes through the error prone NHEJ pathway-based repair of target DNA (Fig. 2-A). Alternatively, knocking in R gene allele to the target site of interest via the HR mechanism has the potential for resistance development in widely accepted susceptible genotypes (Fig. 2-B and -C). Besides, alleles of certain R and Sgenes vary at the single nucleotide level. Thus, precise editing by the way of targeted replacement or base editing can generate allelic variants for disease resistance (Fig. 2-B and -D). Then again, editing platforms can be used to induce disease resistance by altering the cis-regulatory regions of target genes and quantitative trait loci. More than hundred regulatory mutations have been realized in the tomato SlCLV3 promoter using a CRISPR/Cas9 system (Rodriguez-Leal et al, 2017). Such modifications can result in systematic assessment of cis-regulatory regions with resistant traits, which can enhance rice breeding (Fig. 2-D). Simultaneous editing of several Sgenes or regulatory elements via a multiplex editing platform (Fig. 2-E) can essentially result in broad spectrum disease resistance. Multiple sgRNAs driven by independent promoters can be multiplexed into single Cas9 or Cpf1/sgRNA expression vector using the Golden Gate cloning or the Gibson assembly method (Silva and Patron, 2017). Wang et al (2017) demonstrated the feasibility of multiplex gene editing using CRISPR/ Cpf1 system. The advent of multiple editing platforms has facilitated one or more of these strategies towards resistance development against biotic stress in crops particularly in rice (Mishra et al, 2018; Yin and Qiu, 2019). The application of CRISPR/Cas tools has mainly been explored in rice against viral infection, followed by efforts to improve fungal and bacterial disease resistance (Table 1). Recent studies demonstrating the power of the genome editing technology in establishing resistance to these pathogen categories are discussed below.
| Table 1. List of genes targeted by genome editing tools for rice disease resistance. |
Bacterial leaf blight (BLB), caused by γ -proteobacterium Xanthomonas oryzaepv. oryzae (Xoo), is one of the most destructive vascular diseases of rice, especially in the major rice growing regions of Southeast Asia and sub-Saharan Africa. It is singly responsible for more than 75% of yield loss with millions of hectares of rice affected annually (Varshney et al, 2019). Identification of genetically resistant rice plants and their usage in genomic-assisted breeding have been the most effective method for developing Xoo resistant varieties. However, the emergence of new and novel Xoo types has been the greatest challenge in controlling the disease. The Xoo pathogenicity in rice is primarily established through the injection of DNA-binding proteins called TALEs that bind to the effector- binding elements (EBEs) in the promoters of the S genes in rice (Cohn et al, 2014). TALEs usually target the sugar transporting SWEET gene family in rice which are primarily responsible for releasing the sugar into the apoplast as nutrition of the Xoo pathogens (Cohn et al, 2014). Multiple TALEs including AvrXa7, TalC, Tal5 and PthXo3 found in different Xoo strains all target the Os11N3 (also known as OsSWEET14) gene in rice and were therefore considered as the major susceptibility factors associated with bacterial blight infection (Antony et al, 2010; Li et al, 2012; Hutin et al, 2015). TALEN-mediated editing results in the development of desired mutations within the Os11N3promoter region containing an EBE for AvrXa7, which is overlapped with another EBE for PthXo3. The edited rice plants with desired homozygous mutation for the 4 or 9 bp deletion at the target site are highly resistant to bacterial blight (Li et al, 2012). Zhou et al (2015) also reported the identification of another sucrose transporter gene OsSWEET13 as the disease susceptibility factor for PthXo2 TALE (transcription activator-like effector) dependent Xoo strain, and also identified that the PthXo2-dependent strain induces OsSWEET13 expression specifically in indica rice IR24 due to the presence of a mysterious effector binding site, which is not present in the alleles of japonica rice Nipponbare and Kitaake. In another study, a naturally occurring 18 bp deletion in O. barthii and O. glaberrima wild rice species is predicted to prevent the binding of TALEs known to target OsSWEET14and confer resistance to BLB (Hutin et al, 2015). The allele, named asxa41(t), is resistant against half of the tested Xoo strains. More recently, Blanvillain-Baufume et al (2017) used the TALEN constructs to develop an allele library of the OsSWEET14 promoter region in rice to assess the susceptibility level of edited rice lines carrying mutations in the EBEs of AvrXa7, Tal5 and TalC. And, the rice lines with disruption of the AvrXa7 and Tal5 EBEs results in resistance to Xoostrains that are dependent on the corresponding TALEs.
CRISPR/Cas9-mediated disruption of the TALE EBEs of two susceptibility genes, OsSWEET11 and OsSWEET14, in rice variety Kitaake was attempted to facilitate broad spectrum BLB resistance in rice (Xu et al, 2019). One of the rice mutant MS14K demonstrates broad spectrum resistance to majority of the Xoo strains. Further, two PthXo2-like TALEs are identified as major virulence factors in the compatible Xoostrains. As the PthXo2 TALEs primarily target the susceptibility gene OsSWEET13/Xa25, an analysis of EBE variants in the OsSWEET13 promoter across 3 000 rice varieties revealed the presence of at least 10 Xa25-like haplotypes. CRISPR/Cas9 strategy was further used to introduce InDels in the EBE of the OsSWEET13 promoter of the MS14K line, and a novel rice line with three edited OsSWEET EBEs resulted in broad spectrum resistance against all the tested Xoo strains (Xu et al, 2019). This suggests that genome engineering of TALE-susceptibility factor co-evolved loci is crucial for preventing effector triggered susceptibility, thereby realizing broad spectrum BLB resistance.
Recently, Kim et al (2019) employed the CRISPR/ Cas9 system to knockout rice Os8N3 (also known as OsSWEET11) that acts a susceptibility factor for the Xoo strains carrying the TAL effector PthXo1. Desired modifications in Os8N3are stably transferred into T0, T1, T2 and T3 generations without the transferred DNA (T-DNA) through genetic segregation. Not only had the homozygous mutants displayed significantly enhanced resistance to Xoo strain, the editing of Os8N3 also did not affect the agronomic traits and pollen viability in the mutant lines. Olivia et al (2019) sequenced 856 TALEs across multiple Xoo isolates from Asia and Africa distinctively targeting promoters of multiple OsSWEET genes (SWEET11, SWEET13 and SWEET14) and found the complexity involved in the development of TALE insensitive rice lines. To overcome this difficulty, a CRISPR/Cas9-mediated genome editing system is used to introduce mutations at the promoters of all the three SWEET genes at EBEs recognized by variousXoo TALEs. Sequence analyses reveal multiple TALE variants for SWEET13 and SWEET14 alleles. Introduction of as many as five SWEET promoter mutations into the rice accessions Kitaake, IR64 and Ciherang-Sub1 resulted in vigorous and broad spectrum BLB resistance. Most recently, Zhang Q W et al (2020) facilitated significant improvement in rice response to BLB pathogen through usage of a T5 exonuclease (T5exo)-Cas fusion tool box. Compared with CRISPR/ Cas9, T5exo-Cas9 or -Cas12a results in larger deletion mutations at the targeted site of UPTPthXo1(up-regulated by transcription activator-like effector PthXo1) box of the OsXa13 gene promoter in edited rice plants. Phenotypic analysis revealed that the rice plants edited by T5exo-Cas9 or -Cas12a have significant reduction in lesion length and enhancement in resistance to BLB as compared to those edited by CRISPR/Cas9 or CRISPR/Cas12a, which might be associated with the factor that T5exo-Cas9 induces larger deletions at the cis-regulatory element (Zhang Q W et al, 2020). All these studies clearly indicate that the genome editing of rice especially with the CRISPR/Cas system has significantly contributed in conferring broad spectrum BLB resistance, and these strategies can be effectively used in non-transgenic genomic assisted crop improvement programmes.
More than 30% of the plant diseases are caused by fungal phytopathogens (Giraud et al, 2010; Sharma et al, 2012). Rice blast caused by the hemibiotrophic filamentous fungus Magnaporthe oryzaeis the most widespread and devastating disease of rice. It is a recurrent difficulty for both upland and lowland rice which is highly vulnerable to this fungal phytopathogen from the seedling to the adult stage. The frequent occurrence of new races of blast pathogen has resulted in more than 30% of losses in global rice production, which is enough to feed 60 million people (Nalley et al, 2016). Utilization of blast resistant cultivars has being the most effective measure for the management of blast pathogen. As of now, more than 100 major blast R genes have been identified and 30 of them have been molecularly cloned (Xiao et al, 2019). Major blast Rgenes including Pi-ta/Pi-ta2, Pi-z, Pi-b and Pi-k/h/m/shave been deployed in combination with genomic-assisted breeding and transgenic programmes for the development of resistant genotypes across the rice producing regions (Li Y et al, 2016). For instance, the recently identified rice Pigm locus consists of cluster of genes encoding nucleotide-binding site-leucine- rich repeat (NBS-LRR) receptors that confers resistance to blast fungus M. oryzae without compromising the yield trait. The locus is characterized by a pair of epigenetically regulated antagonistic pair of NBS- LRR receptors, PigmR and PigmS. While PigmR facilitates broad spectrum resistance, PigmS prevents PigmR homodimerization to suppress resistance and in turn increases seed production (Deng et al, 2017). Likewise, a single base modification in the regulatory region of bsr-d1 induces blast resistance in rice (Li et al, 2017). However, the current challenge lies in the development of a collection of blast resistance genes that can be used against the incessantly evolving and varied strains of M. oryzae. Therefore, precise genome editing tools are the need of the hour for implementation of effective plant resistance in rice.
Enhanced resistance to blast disease has been demonstrated in rice by targeting the ethylene response transcription factor gene OsERF922 via CRISPR/ Cas9-targeted knockout (Wang et al, 2016). CRISPR/ Cas9 induced InDel mutations are reported in the targeted gene with a mutation frequency up to 42% in the T0 generation. The allelic mutations are stably transmitted to succeeding generations. Blast resistance screening in six homozygous mutated T2lines reveals significantly lower lesions as compared to wild type plants at both the seedling and tillering stages. Further, no significant difference is observed in the agronomic performance of the mutants, suggesting that precise editing does not alter other important traits of the plants. Furthermore, the utilization of multiple CRISPR/Cas9 constructs (Cas9/multi-target-sgRNA) for targeting several sites within OsERF922 locus results in higher number of mutants. These suggest that CRISPR/Cas9 mediated targeting of multiple sites has the potential to increase the mutation efficiency, thereby enhancing blast resistance in rice. CRISPR/Cas9 SSN has been used to disrupt OsSEC3A, a gene encoding exocyst subunit protein to explore its functional role in plant immunity (Ma et al, 2018). OsSEC3A has been previously implicated in root hair elongation, pollen germination and defense response in other plant species (Bloch et al, 2016). Two sgRNAs are designed to target the third and tenth exons of the OsSEC3A gene. CRISPR/Cas9 induced mutant displays improved immune responses coupled with up-regulated expression of pathogenesis- related proteins, salicylic acid synthesis genes, increased levels of salicylic acid, and improved resistance to the rice blast pathogen M. oryzae. However, the mutant lines also show altered growth and agronomic traits including dwarf stature, smaller seedlings, shorter main roots and decreased or impaired plant height, panicle length, tiller number, 1000-grain weight and spikelet fertility as compared to the wild type. Multiple approaches to blast resistance in rice can be fructified through precise editing of multifunctional gene(s) associated with defense signaling in rice. Recently, CRISPR/Cas9 has also been adopted to induce mutations in the proline-rich motif of Pi21 for rice resistance against M. oryzae (Li et al, 2019). CRISPR/ Cas9 vector has been designed to facilitate simultaneous targeted mutation of the thermosensitive male sterile 5 (TMS5), rice blast S gene pi21 and BLB S gene xa13 in the rice variety Pinzhan (Li et al, 2019). Three of the generated mutants with homozygous frameshift mutations at all the three genes (tms5/pi21/xa13) display characteristics of thermosensitive genic male sterility with enhanced resistance to infections of M. grisea and Xoo strain PXO99 (Li et al, 2019), thereby significantly accelerating the process of hybrid rice breeding.
Rice sheath blight, caused by Rhizoctonia solaniKuhn [Teleomorph stage, Thanatephorus cucumeris(Frank) Donk], is the second most important fungal disease of rice after blast that amounts of yield loss in the range of 8%-50% across the tropical rice growing countries of the world (Savary et al, 2000). CRISPR/ Cas9 editing has been recently attempted to modify Oryza sativa Phytochrome and Flowering Time 1 (OsPFT1) gene to understand its functional role in rice sheath blight resistance (Shah et al, 2019). Arabidopsis PFT1 is critically implicated in inducing disease susceptibility by acting as a universal adaptor between RNA polymerase II and DNA-bound transcription factors (Bä ckströ m et al, 2007It cannot be found in REFERNCE, please check.It’ s the 2nd reference in the list of REFERENCE ; Thatcher et al, 2009). CRISPR/Cas9 constructs have been mobilized into the indica rice variety ASD16 via Agrobacterium- mediated transformation that facilitated mutation in the PFT1 locus. However, the mutation efficiency and disease resistance affinity of the edited rice lines are yet to be ascertained.
Viral diseases also act as major global constraint in the effort to increase rice productivity. Among the different viral infections, rice tungro disease severely affects rice production in about 3.5 million hectares throughout the major rice producing Asian nations (Chancellor et al, 2006). The disease is basically caused by the interaction of two different viruses namely rice tungro spherical virus (RTSV) with a single stranded RNA genome, and rice tungro bacilliform virus (RTBV), with a double stranded DNA genome (Hull, 1996). RTBV develops the primary disease symptoms, and spreads the disease by assisting the transmission of RTBV through the green leaf hopper species such as Nephotettix virescensand N. nigropictus (Hibino and Kabauatan, 1987). Host resistance breeding programmes through the screening of huge rice germplasm collections have resulted in the identification of multiple rice varieties with specific resistance to either or both of the viruses (Khush et al, 2004). Concentrated molecular research in an indica rice variety Utri Merah demonstrated that the RTSV resistance is controlled by the presence of single-nucleotide polymorphism or deletion affecting the YVV amino acid residues in the translation initiation factor four gamma (eIF4G) gene (Lee et al, 2010). As such, it is essential to develop RTSV resistant variety to prevent the secondary spread of this disease. Recently, CRISPR/Cas9 genome editing has been successfully used to mutate eIF4G gene in the RTSV susceptible rice variety, IR64, to develop new sources of resistance to RTSV (Macovei et al, 2018). Mutation frequency ranging from 36% to 86% is realized, and induced mutations are transmitted into the subsequent generation with no detectable modifications in the closest off-target sites. Sequence analysis and pathogen infection assay revealed that in-frame mutation in the amino acid residues adjacent to the YVV residues confers enhanced RTSV resistance together with improved agronomic parameters such as plant height and grain yield. The stable mutants can be released in the rice tungro disease prone areas as alternative source of RTSV resistance for controlling the infection and improving the rice productivity.
Although genome editing approaches depend upon the targeted mutation of S genes towards introduction of disease resistance in plants, it might come with a fitness cost. Knocking out S genes, which encode proteins responsible for pre-penetration structures, defense suppression and replication machinery, may lead to phenotypic abnormalities and nutritional deficiency that can affect plant growth and development (van Schie and Takken, 2014). Whereas OsSWEET rice mutants induce resistance through restricted sugar availability for BLB pathogen, it might also lead to reduced plant stature and pollen abortion (Chu et al, 2006). This could be possibly mitigated through targeted editing of different promoter regions of the S genes has been demonstrated for SWEET genes in rice (Blanvillain-Baufume et al, 2017). Alternatively, instead of fully knocking out an Sgene, disease resistance can also be developed by introducing synthetic variants of S gene identical to the allele that naturally occurs in resistant genotypes (Bastet et al, 2017). Such new allele can induce plant resistance and at the same time demonstrate normal protein functions with no developmental cost. Besides, the recently developed base editing tools can also be used for precise introduction of single base transitions in some S genes, which vary only at the single nucleotide polymorphism level. Moreover, the availability of multiple pathogen inducible promoters and regulatory elements in plants can be targeted using a pathogen induced CRISPR system for transient switch off the S gene with no compromise in their fitness roles. Such a CRISPR vector system that can exploit the pathogen inducible promoter could be conceptualized to demonstrate their effectiveness towards disease resistance in field crops. On the contrary, the introduction of a custom design sequence into the genome will be more appropriate when specific allelic variants are involved in resistance response. A CRISPR system coupled with the HR mechanism can indefinitely expand the possibility of knocking in R gene allele into the target site of choice for developing disease resistance. Although HR is still technically challenging in plants due to low efficiency and lack of multiplexing protocols, CRISPR toolboxes need to be designed and analyzed to expand their applications in resistance breeding for rice improvement.
This research was supported by the China Priority Program-Breeding of Seven Major Crops (Grant No. 2017YFD01100100), the Innovation Program of Chinese Academy of Agricultural Sciences (Grant No. 01-ICS) and the Talented Young Scientist Program of China (Grant No. India-17-01). We thank Dr. Muktikanta Mishra, President, Centurion University of Technology and Management for his encouragement and support.
(Managing Editor: Wang Caihong)
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|
| [37] |
|
| [38] |
|
| [39] |
|
| [40] |
|
| [41] |
|
| [42] |
|
| [43] |
|
| [44] |
|
| [45] |
|
| [46] |
|
| [47] |
|
| [48] |
|
| [49] |
|
| [50] |
|
| [51] |
|
| [52] |
|
| [53] |
|
| [54] |
|
| [55] |
|
| [56] |
|
| [57] |
|
| [58] |
|
| [59] |
|
| [60] |
|
| [61] |
|
| [62] |
|
| [63] |
|
| [64] |
|
| [65] |
|
| [66] |
|
| [67] |
|
| [68] |
|
| [69] |
|
| [70] |
|
| [71] |
|
| [72] |
|
| [73] |
|
| [74] |
|
| [75] |
|
| [76] |
|
| [77] |
|
| [78] |
|
| [79] |
|
| [80] |
|
| [81] |
|
| [82] |
|
| [83] |
|
| [84] |
|
| [85] |
|
| [86] |
|
| [87] |
|
| [88] |
|
| [89] |
|
| [90] |
|
| [91] |
|
| [92] |
|
| [93] |
|
| [94] |
|
| [95] |
|
| [96] |
|
| [97] |
|
| [98] |
|
| [99] |
|
| [100] |
|
| [101] |
|