
Rice Science ›› 2024, Vol. 31 ›› Issue (4): 434-448.DOI: 10.1016/j.rsci.2024.02.009
收稿日期:2023-11-16
接受日期:2024-02-23
出版日期:2024-07-28
发布日期:2024-08-08
. [J]. Rice Science, 2024, 31(4): 434-448.
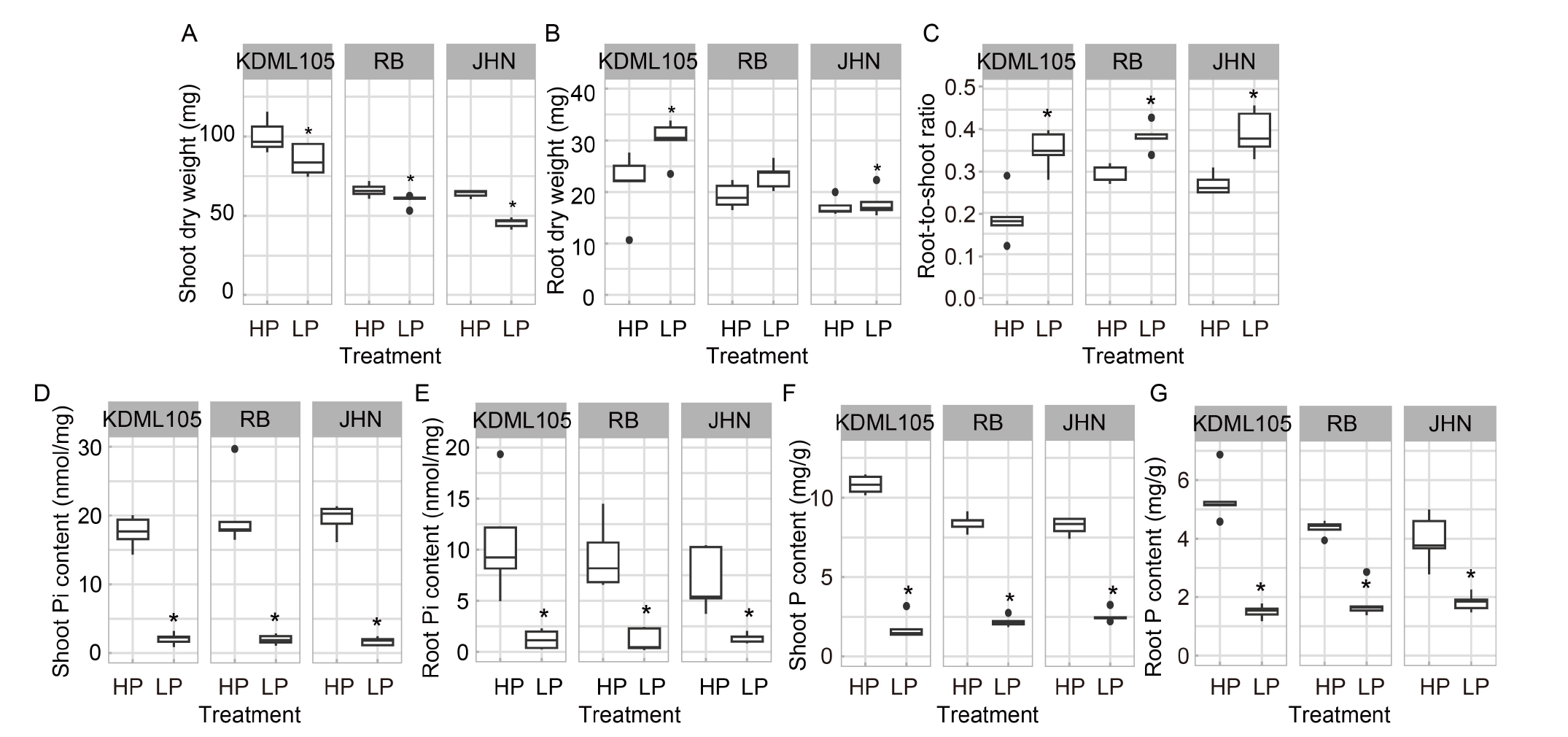
Fig. 1. Growth responses of rice seedlings to phosphorus (P) deficiency. A-G, Shoot dry weight (A), root dry weight (B), root-to-shoot ratio (C), shoot phosphate (Pi) content (D), root Pi content (E), shoot P content (F), and root P content (G) among non-pigmented Khao Dawk Mali (KDML105) and pigmented Riceberry (RB) and Jao Hom Nin (JHN) varieties under high P (HP) and low P (LP) conditions. Rice seedlings of non-pigmented KDML105 and pigmented RB and JHN varieties were hydroponically grown in HP and LP conditions for two weeks. The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Asterisks represent significant differences (P ≤ 0.05) between the HP and LP conditions by the Student’s t-test.
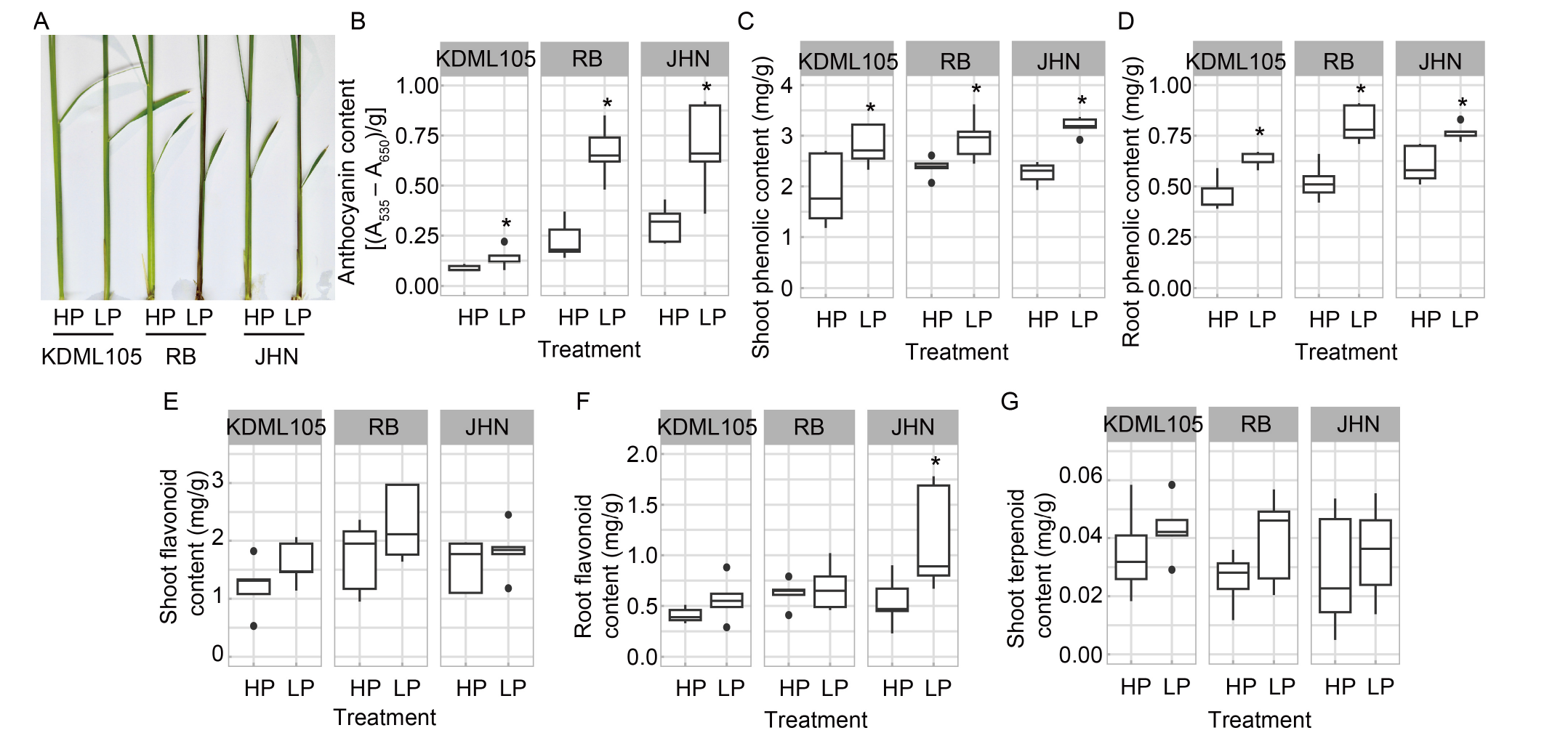
Fig. 2. Biochemical responses of rice seedlings to phosphorus (P) deficiency. A, Dark green and purple leaf sheaths were observed in the P-deficient rice seedlings. B-G, Anthocyanin content in leaf sheaths (B), phenolic content in shoots (C) and roots (D), flavonoid content in shoots (E) and roots (F), and terpenoid content in shoots (G) were compared among non-pigmented Khao Dawk Mali (KDML105) and pigmented Riceberry (RB) and Jao Hom Nin (JHN) varieties under high (HP) and low P (LP) conditions. Rice seedlings of non-pigmented KDML105 and pigmented RB and JHN varieties were hydroponically grown in HP and LP conditions for two weeks. The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Asterisks represent significant differences (P ≤ 0.05) between the HP and LP conditions by the Student’s t-test.
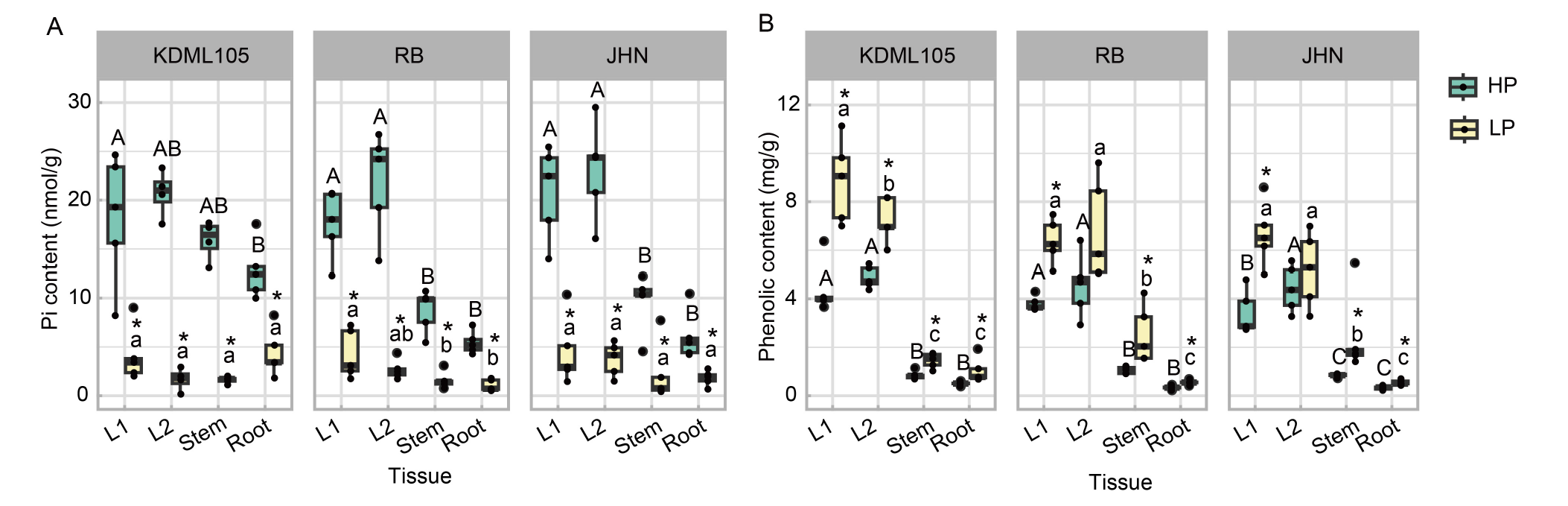
Fig. 3. Effect of phosphorus (P) deficiency on soluble phosphate (Pi) contents and accumulation of phenolic contents in leaves, stems, and roots of rice seedlings. A and B, Contents of Pi (A) and phenolics (B) are compared in the first and second fully expanded leaves (L1 and L2, respectively), stems, and roots of rice seedlings grown under high P (HP) and low P (LP) conditions. KDML105, Khao Dawk Mali (non-pigmented); RB, Riceberry (pigmented); JHN, Jao Hom Nin (pigmented). The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Comparisons of means within the same P regime were statistically analyzed by one-way analysis of variance followed by the least significant difference. Comparisons of means between the HP and LP conditions were analyzed by the Student’s t-test. Different capital letters for HP and lowercase letter for LP indicate significant differences (P ≤ 0.05) of means among various plant parts within treatments. Asterisks represent significant differences (P ≤ 0.05) of means between the HP and LP treatments.
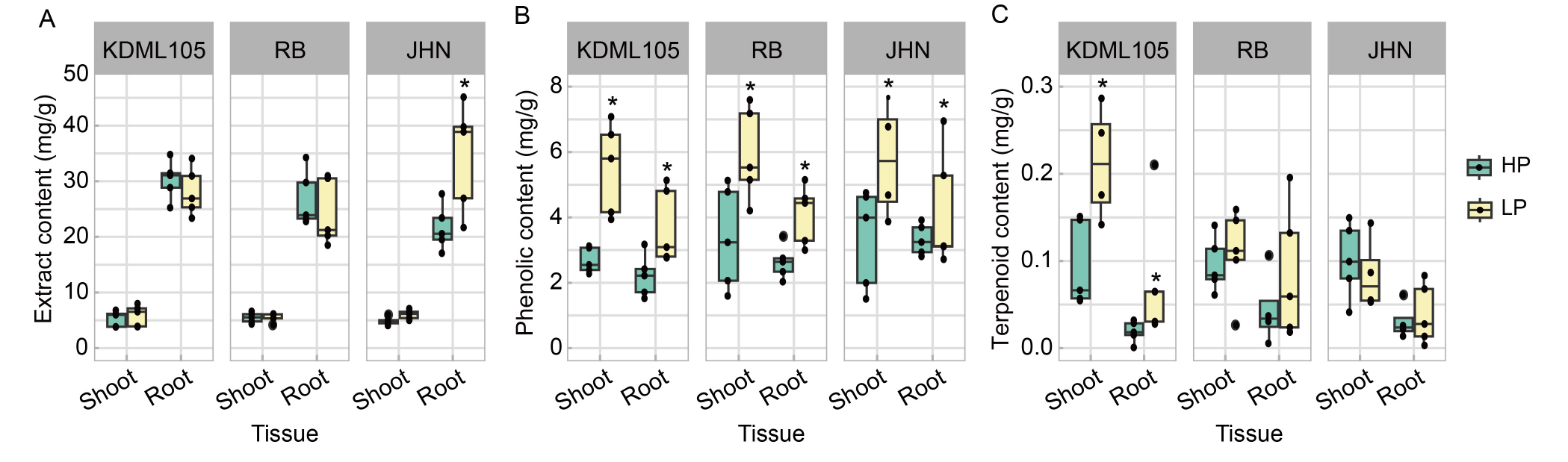
Fig. 4. Effect of phosphorus (P) deficiency on aqueous extracts and their phenolic and terpenoid contents. A-C, Extract contents (A), total phenolic contents (B), and total terpenoid contents (C) from shoots and roots of rice seedlings grown in high P (HP) and low P (LP) conditions. KDML105, Khao Dawk Mali (non-pigmented); RB, Riceberry (pigmented); JHN, Jao Hom Nin (pigmented). The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Comparisons of means within the same plant tissue of each variety in the HP and LP treatments were analyzed by the Student’s t-test. Asterisks represent significant differences (P ≤ 0.05) in means between the HP and LP treatments.
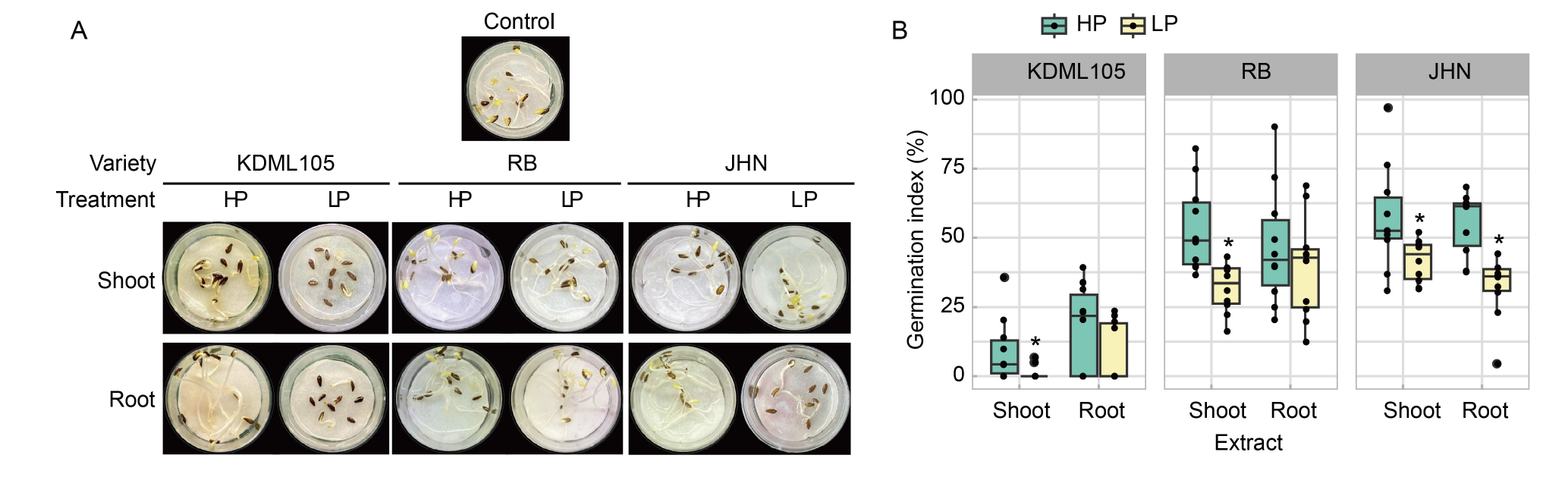
Fig. 5. Effect of phosphorus (P) deficiency on rice allelopathy in a lettuce germination assay. A, Lettuce seeds were germinated in shoot and root extracts of Khao Dawk Mali (KDML105), Riceberry (RB), and Jao Hom Nin (JHN) rice seedlings treated with high P (HP) and low P (LP) conditions. Distilled water was used as the control. B, Germination index (GI) of lettuce seeds was determined at 3 d after germination. The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). GIs associated with extracts from the same plant tissue of each variety were compared between HP and LP conditions by the Student’s t-test. Asterisks represent significant differences (P ≤ 0.05) in means between the HP and LP conditions.
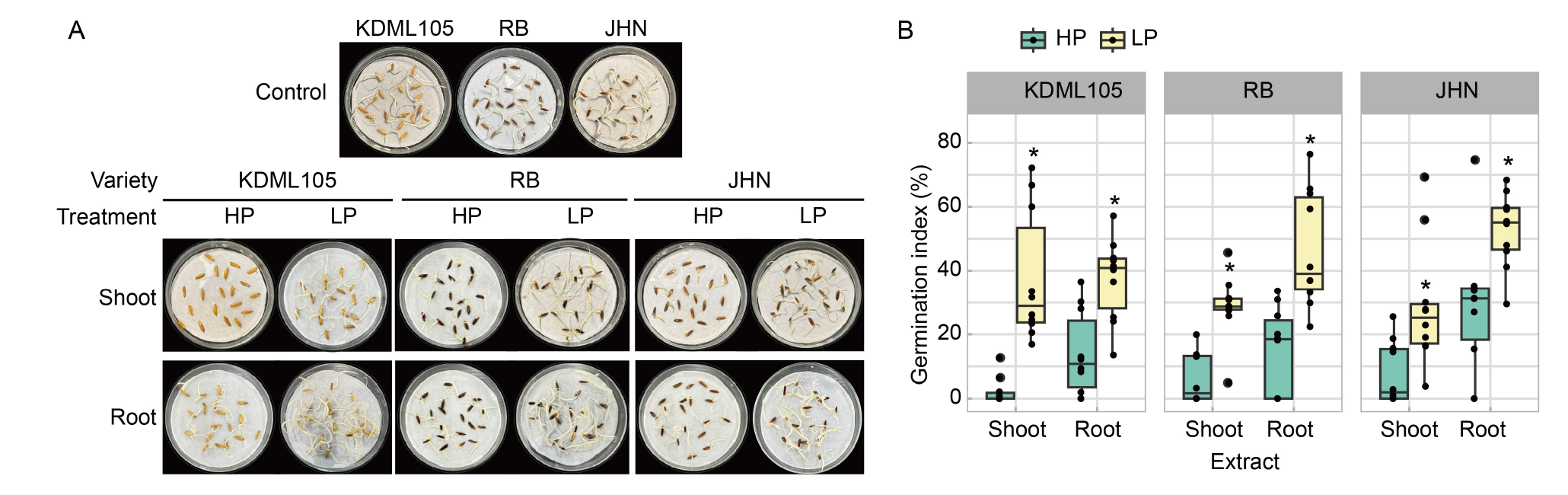
Fig. 6. Effect of phosphorus (P) deficiency on intra-specific allelopathy in rice seedlings. A, Khao Dawk Mali (KDML105), Riceberry (RB), and Jao Hom Nin (JHN) rice seeds were germinated in shoot and root extracts from seedlings of the same variety, grown in high P (HP) and low P (LP) conditions. Distilled water was used as the control. B, Germination indices were determined after 2 d after germination. The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Comparisons of means within the same plant tissue between the HP and LP treatments in each variety were analyzed by the Student’s t-test. Asterisks represent significant differences (P ≤ 0.05) of means between the HP and LP treatments.
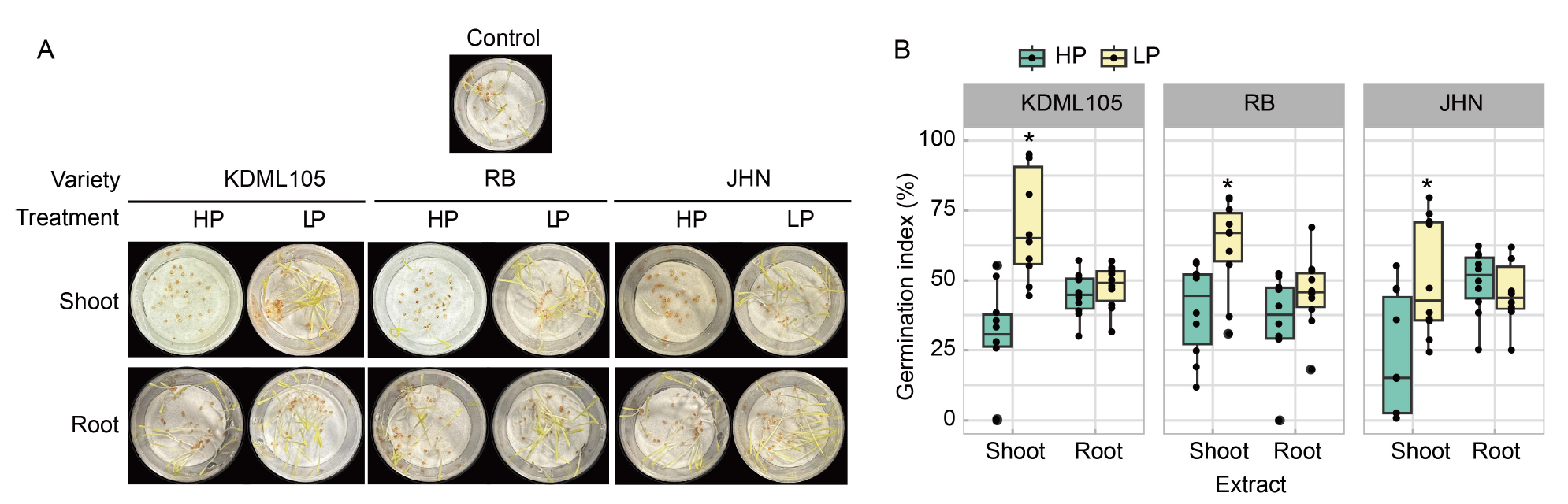
Fig. 7. Effect of phosphorus (P) deficiency on the allelopathic potential of rice seedlings in a natural rice weed Dactyloctenium aegyptium. A, Seeds of D. aegyptium were germinated in the shoot and root extracts of Khao Dawk Mali (KDML105), Riceberry (RB), and Jao Hom Nin (JHN) rice seedlings grown in high P (HP) and low P (LP) conditions. Distilled water was used as the control. B, Germination indices were determined at 2 d after germination. The box plots represent the distribution of five biological replicates with two technical replicates (n = 10). Comparisons of means within the same plant tissue between the HP and LP treatments in each variety were analyzed by the Student’s t-test. Asterisks represent significant differences (P ≤ 0.05) of means between the HP and LP treatments.
| [1] | Aremu A O, Masondo N A, Rengasamy K R R, Amoo S O, Gruz J, Bíba O, Šubrtová M, Pěnčík A, Novák O, Doležal K, van Staden J. 2015. Physiological role of phenolic biostimulants isolated from brown seaweed Ecklonia maxima on plant growth and development. Planta, 241(6): 1313-1324. |
| [2] | Berendji S, Asghari J B, Matin A A. 2008. Allelopathic potential of rice (Oryza sativa) varieties on seedling growth of barnyardgrass (Echinochloa crus-galli). J Plant Interact, 3(3): 175-180. |
| [3] | Chen C Y, Wu K Q, Schmidt W. 2015. The histone deacetylase HDA19 controls root cell elongation and modulates a subset of phosphate starvation responses in Arabidopsis. Sci Rep, 5: 15708. |
| [4] | Chen R Y, Song S W, Li X C, Liu H C, Huang D F. 2013. Phosphorus deficiency restricts plant growth but induces pigment formation in the flower stalk of Chinese kale. Hortic Environ Biotechnol, 54(3): 243-248. |
| [5] | Chishaki N, Horiguchi T. 1997. Responses of secondary metabolism in plants to nutrient deficiency. In: Ando T, Fujita K, Mae T, Matsumoto H, Mori S, Sekiya J. Plant Nutrition for Sustainable Food Production and Environment. Dordrecht, the Netherlands: Springer: 78. |
| [6] | Chung I M, Kim K H, Ahn J K, Chun S C, Kim C S, Kim J T, Kim S H. 2002. Screening of allelochemicals on barnyardgrass (Echinochloa crus-galli) and identification of potentially allelopathic compounds from rice (Oryza sativa) variety hull extracts. Crop Prot, 21(10): 913-920. |
| [7] | Ciulu M, de la Luz Cádiz-Gurrea M, Segura-Carretero A. 2018. Extraction and analysis of phenolic compounds in rice: A review. Molecules, 23(11): 2890. |
| [8] | Colombo F, Cappa C, Bani C, Magni M, Biella S, Restani P, Di Lorenzo C. 2023. Characterization of color, phenolic profile, and antioxidant activity of Italian pigmented rice varieties after different technological treatments. Food Biosci, 53: 102674. |
| [9] | Cuyas L, David P, de Craieye D, Ng S, Arkoun M, Plassard C, Faharidine M, Hourcade D, Degan F, Pluchon S, Nussaume L. 2023. Identification and interest of molecular markers to monitor plant Pi status. BMC Plant Biol, 23(1): 401. |
| [10] | Daiponmak W, Theerakulpisut P, Thanonkao P, Vanavichit A, Prathepha P. 2010. Changes of anthocyanin cyanidin-3-glucoside content and antioxidant activity in Thai rice varieties under salinity stress. Scienceasia, 36(4): 286-291. |
| [11] | de Andrade S A L, Borghi A A, de Oliveira V H, de Moraes Gouveia L, Izidoro Martins A P, Mazzafera P. 2022. Phosphorus shortage induces an increase in root exudation in fifteen eucalypts species. Agronomy, 12(9): 2041. |
| [12] | de Klerk G J, Guan H Y, Huisman P, Marinova S. 2011. Effects of phenolic compounds on adventitious root formation and oxidative decarboxylation of applied indoleacetic acid in Malus ‘Jork 9’. Plant Growth Regul, 63(2): 175-185. |
| [13] | de Mendiburu F. 2015. Agricolae: Statistical procedures for agricultural research. [2023-10-22]. https://cran.r-project.org/web/packages/agricolae/agricolae.pdf. |
| [14] | de Mira N V M, Massaretto I L, Pascual C D C I, Marquez U M L. 2009. Comparative study of phenolic compounds in different Brazilian rice (Oryza sativa L.) genotypes. J Food Compos Anal, 22(5): 405-409. |
| [15] | Deikman J, Hammer P E. 1995. Induction of anthocyanin accumulation by cytokinins in Arabidopsis thaliana. Plant Physiol, 108(1): 47-57. |
| [16] | Delory B M, Delaplace P, Fauconnier M L, du Jardin P. 2016. Root- emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil, 402: 1-26. |
| [17] | Deng Y P, Men C B, Qiao S F, Wang W J, Gu J F, Liu L J, Zhang Z J, Zhang H, Wang Z Q, Yang J C. 2020. Tolerance to low phosphorus in rice varieties is conferred by regulation of root growth. Crop J, 8(4): 534-547. |
| [18] | Đorđević T, Đurović-Pejčev R, Stevanović M, Sarić-Krsmanović M, Radivojević L, Šantrić L, Gajić-Umiljendić J. 2022. Phytotoxicity and allelopathic potential of Juglans regia L. leaf extract. Front Plant Sci, 13: 986740. |
| [19] | El-Mergawi R A. 2019. Suitability of high doses of phenolic acids for controlling Corchorus olitorius and Phalaris minor weeds. Gesunde Pflanz, 71(4): 261-269. |
| [20] | Fathi E, Majdi M, Dastan D, Maroufi A. 2019. The spatio-temporal expression of some genes involved in the biosynthetic pathways of terpenes/phenylpropanoids in yarrow (Achillea millefolium). Plant Physiol Biochem, 142: 43-52. |
| [21] | Gho Y S, An G, Park H M, Jung K H. 2018. A systemic view of phosphate starvation-responsive genes in rice roots to enhance phosphate use efficiency in rice. Plant Biotechnol Rep, 12(4): 249-264. |
| [22] | Gho Y S, Kim S J, Jung K H. 2020. Phenylalanine ammonia-lyase family is closely associated with response to phosphate deficiency in rice. Genes Genomics, 42(1): 67-76. |
| [23] | Gómez‐Rubio V. 2017. ggplot2: Elegant graphics for data analysis (2nd edition). J Stat Softw, 77: 1-3. |
| [24] | Hayes P E, Adem G D, Pariasca-Tanaka J, Wissuwa M. 2022. Leaf phosphorus fractionation in rice to understand internal phosphorus- use efficiency. Ann Bot, 129(3): 287-302. |
| [25] | He H Q, Liang Y Y, Chen L J, Zhuang C G, Ke Y Q, Liang K J, Lin W X. 2006. Allelopathic potential and physiological mechanism of Oryza sativa L. under phosphorus deficiency stress. J Appl Ecol, 17(11): 2070-2074. (in Chinese with English Abstract) |
| [26] | Hu H Q, Tang C X, Rengel Z. 2005a. Influence of phenolic acids on phosphorus mobilisation in acidic and calcareous soils. Plant Soil, 268(1): 173-180. |
| [27] | Hu H Q, Tang C X, Rengel Z. 2005b. Role of phenolics and organic acids in phosphorus mobilization in calcareous and acidic soils. J Plant Nutr, 28(8): 1427-1439. |
| [28] | Javaid A, Shafique S, Bajwa R, Shafique S. 2006. Effect of aqueous extracts of allelopathic crops on germination and growth of Parthenium hysterophorus L. S Afr N J Bot, 72(4): 609-612. |
| [29] | Jiang X L, Liu Y J, Li W W, Zhao L, Meng F, Wang Y S, Tan H R, Yang H, Wei C L, Wan X C, Gao L P, Xia T. 2013. Tissue- specific, development-dependent phenolic compounds accumulation profile and gene expression pattern in tea plant [Camellia sinensis]. PLoS One, 8(4): e62315. |
| [30] | Julia C C, Rose T J, Pariasca-Tanaka J, Jeong K, Matsuda T, Wissuwa M. 2018. Phosphorus uptake commences at the earliest stages of seedling development in rice. J Exp Bot, 69(21): 5233-5240. |
| [31] | Jumrus S, Yamuangmorn S, Veeradittakit J, Kamthai S, Lordkaew S, Suwan T, Jamjod S, Prom-u-thai C. 2022. Variation of anthocyanin, phenol, and antioxidant capacity in straw among rice varieties and growing locations as a potential source of natural bioactive compounds. Plants, 11(21): 2903. |
| [32] | Juszczuk I M, Wiktorowska A, Malusá E, Rychter A M. 2004. Changes in the concentration of phenolic compounds and exudation induced by phosphate deficiency in bean plants (Phaseolus vulgaris L.). Plant Soil, 267(1): 41-49. |
| [33] | Klinnawee L, Kaewchumnong K, Nualtem K. 2023. Effect of phosphorus deficiency on allelopathic activity of lowland indica rice. Scienceasia, 49(2): 184. |
| [34] | Kunnam J, Pinta W, Ruttanaprasert R, Bunphan D, Thabthimtho T, Aninbon C. 2023. Stability of phenols, antioxidant capacity and grain yield of six rice genotypes. Plants, 12(15): 2787. |
| [35] | Li J Y, Lin S X, Zhang Q X, Zhang Q, Hu W W, He H B. 2019. Fine-root traits of allelopathic rice at the seedling stage and their relationship with allelopathic potential. PeerJ, 7: e7006. |
| [36] | Liu L, Yang D F, Liang T Y, Zhang H H, He Z G, Liang Z S. 2016. Phosphate starvation promoted the accumulation of phenolic acids by inducing the key enzyme genes in Salvia miltiorrhiza hairy roots. Plant Cell Rep, 35(9): 1933-1942. |
| [37] | Lu S S, Ye J, Li H, He F Y, Qi Y, Wang T, Wang W J, Zheng L Q. 2023. The splicing factor OsSCL26 regulates phosphorus homeostasis in rice. Plants, 12(12): 2326. |
| [38] | Lu Y, Wassmann R, Neue H U, Huang C. 1999. Impact of phosphorus supply on root exudation, aerenchyma formation and methane emission of rice plants. Biogeochemistry, 47(2): 203-218. |
| [39] | Łukowski A, Jagiełło R, Robakowski P, Adamczyk D, Karolewski P. 2022. Adaptation of a simple method to determine the total terpenoid content in needles of coniferous trees. Plant Sci, 314: 111090. |
| [40] | Luo J J, Liu Y X, Zhang H K, Wang J P, Chen Z J, Luo L J, Liu G D, Liu P D. 2020. Metabolic alterations provide insights into Stylosanthes roots responding to phosphorus deficiency. BMC Plant Biol, 20(1): 85. |
| [41] | Nayeem S, Venkidasamy B, Sundararajan S, Kuppuraj S P, Ramalingam S. 2021. Differential expression of flavonoid biosynthesis genes and biochemical composition in different tissues of pigmented and non-pigmented rice. J Food Sci Technol, 58(3): 884-893. |
| [42] | Pantigoso H A, Yuan J, He Y H, Guo Q G, Vollmer C, Vivanco J M. 2020. Role of root exudates on assimilation of phosphorus in young and old Arabidopsis thaliana plants. PLoS One, 15(6): e0234216. |
| [43] | Pardo-Muras M, Puig C G, Souto X C, Pedrol N. 2020. Water- soluble phenolic acids and flavonoids involved in the bioherbicidal potential of Ulex europaeus and Cytisus scoparius. S Afr N J Bot, 133: 201-211. |
| [44] | Peanparkdee M, Patrawart J, Iwamoto S. 2020. Physicochemical stability and in vitro bioaccessibility of phenolic compounds and anthocyanins from Thai rice bran extracts. Food Chem, 329: 127157. |
| [45] | Pei L M, Liu J J, Zhou Y Y, Jiang Y H, Li H. 2021. Transcriptomic and metabolomic profiling reveals the protective role of anthocyanins in alleviating low phosphate stress in maize. Physiol Mol Biol Plants, 27(5): 889-905. |
| [46] | Pinit S, Chadchawan S, Chaiwanon J. 2020. A simple high- throughput protocol for the extraction and quantification of inorganic phosphate in rice leaves. Appl Plant Sci, 8(10): e11395. |
| [47] | Pinit S, Ruengchaijatuporn N, Sriswasdi S, Buaboocha T, Chadchawan S, Chaiwanon J. 2022. Hyperspectral and genome-wide association analyses of leaf phosphorus status in local Thai indica rice. PLoS One, 17(4): e0267304. |
| [48] | Pongprayoon W, Tisarum R, Theerawittaya C, Cha-Um S. 2019. Evaluation and clustering on salt-tolerant ability in rice genotypes (Oryza sativa L. subsp. indica) using multivariate physiological indices. Physiol Mol Biol Plants, 25(2): 473-483. |
| [49] | Pontigo S, Ulloa M, Godoy K, Nikolic N, Nikolic M, de la Luz Mora M, Cartes P. 2018. Phosphorus efficiency modulates phenol metabolism in wheat genotypes. J Soil Sci Plant Nutr, 18(3): 904-920. |
| [50] | Pudełko K, Majchrzak L, Narożna D. 2014. Allelopathic effect of fibre hemp (Cannabis sativa L.) on monocot and dicot plant species. Ind Crops Prod, 56: 191-199. |
| [51] | R Core Team. 2021. R: A language and environment for statistical computing. [2023-10-22]. R Foundation for Statistical Computing. https://api.semanticscholar.org/CorpusID:215755663. |
| [52] | Rahaman F, Shukor Juraimi A, Rafii M Y, Uddin K, Hassan L, Chowdhury A K, Karim S M R, Yusuf Rini B, Yusuff O, Bashar H M K, Hossain A. 2022. Allelopathic potential in rice: A biochemical tool for plant defence against weeds. Front Plant Sci, 13: 1072723. |
| [53] | Ramos-Escudero F, Muñoz A M, Alvarado-Ortíz C, Alvarado Á, Yáñez J A. 2012. Purple corn (Zea mays L.) phenolic compounds profile and its assessment as an agent against oxidative stress in isolated mouse organs. J Med Food, 15(2): 206-215. |
| [54] | Saengwilai P J, Bootti P, Klinnawee L. 2023. Responses of rubber tree seedlings (Hevea brasiliensis) to phosphorus deficient soils. Soil Sci Plant Nutr, 69(2): 78-87. |
| [55] | Sun G F, Luan M D, Wen J S, Wang B, Lan W Z. 2023. Genetically controlling VACUOLAR PHOSPHATE TRANSPORTER 1 contributes to low-phosphorus seeds in Arabidopsis. Plant Signal Behav, 18(1): 2186641. |
| [56] | Tawaraya K, Horie R, Wagatsuma T, Saito K, Oikawa A. 2018. Metabolite profiling of shoot extract, root extract, and root exudate of rice under nitrogen and phosphorus deficiency. Soil Sci Plant Nutr, 64(3): 312-322. |
| [57] | Thiébaut G, Thouvenot L, Rodríguez-Pérez H. 2018. Allelopathic effect of the invasive Ludwigia hexapetala on growth of three macrophyte species. Front Plant Sci, 9: 1835. |
| [58] | Wang H B, He H B, Ye C Y, Lu J C, Chen R S, Liu C H, Guo X K, Lin W X. 2010. Molecular physiological mechanism of increased weed suppression ability of allelopathic rice mediated by low phosphorus stress. Allelopathy J, 25(1): 239-248. |
| [59] | Wang K L, Wang T, Ren C, Dou P P, Miao Z Z, Liu X Q, Huang D, Wang K. 2022. Aqueous extracts of three herbs allelopathically inhibit lettuce germination but promote seedling growth at low concentrations. Plants, 11(4): 486. |
| [60] | Wang Q, Hillwig M L, Peters R J. 2011. CYP99A3: Functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J, 65(1): 87-95. |
| [61] | Wu H, Haig T, Pratley J, Lemerle D, An M. 2001. Allelochemicals in wheat (Triticum aestivum L.): Cultivar difference in the exudation of phenolic acids. J Agric Food Chem, 49(8): 3742-3745. |
| [62] | Xu Y, Chen X, Ding L, Kong C H. 2023. Allelopathy and allelochemicals in grasslands and forests. Forests, 14(3): 562. |
| [63] | Zhang M W, Zhang R F, Zhang F X, Liu R H. 2010. Phenolic profiles and antioxidant activity of black rice bran of different commercially available varieties. J Agric Food Chem, 58(13): 7580-7587. |
| [64] | Zhao J C, Yang Z Y, Zou J Q, Li Q. 2022. Allelopathic effects of sesame extracts on seed germination of moso bamboo and identification of potential allelochemicals. Sci Rep, 12(1): 6661. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||