
Rice Science ›› 2024, Vol. 31 ›› Issue (4): 449-462.DOI: 10.1016/j.rsci.2024.04.002
收稿日期:2023-11-18
接受日期:2024-03-01
出版日期:2024-07-28
发布日期:2024-08-08
. [J]. Rice Science, 2024, 31(4): 449-462.
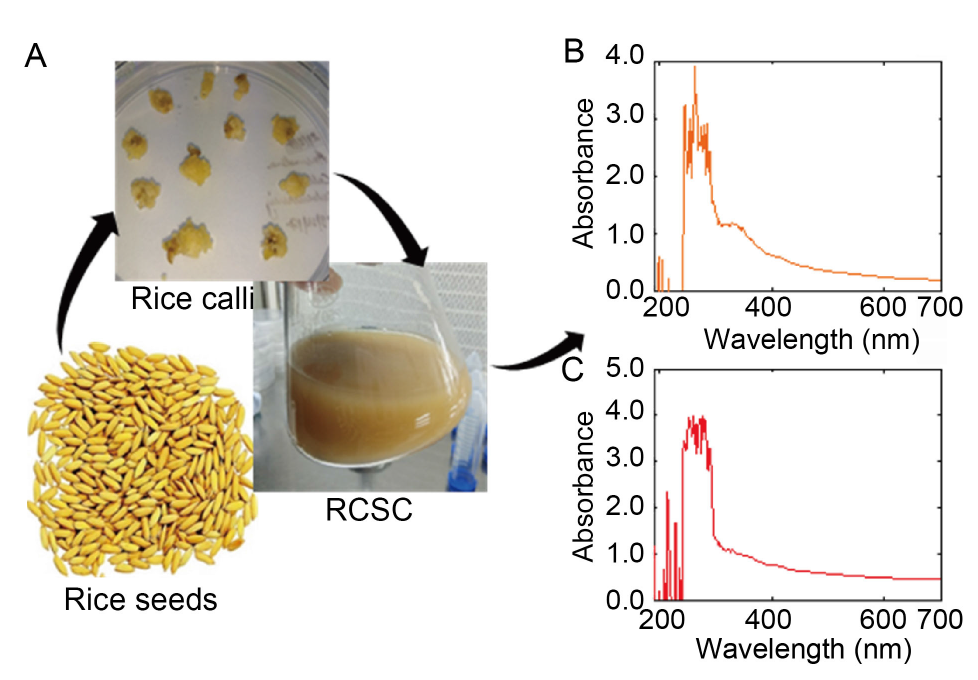
Fig. 1. Rice calli grown on Murashige and Skoog medium under sterile conditions (A), and rice callus suspension cultures (RCSC) of Basmati 386 (B) and Basmati 1121 (C) showing a characteristic absorbance pattern in the ultraviolet range.
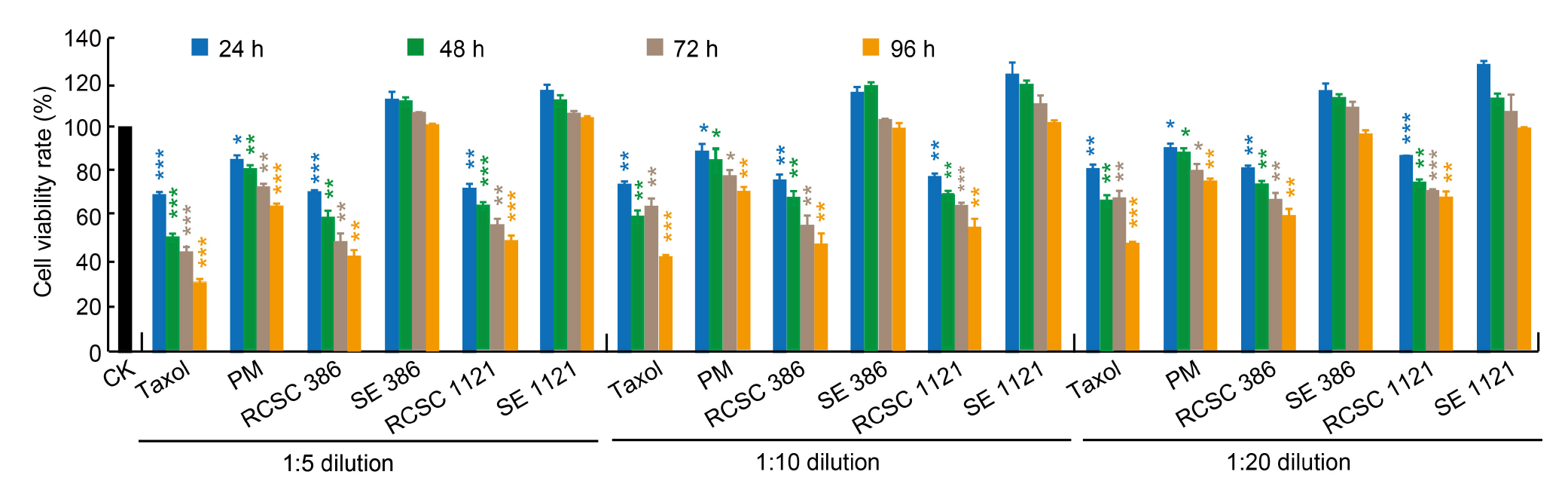
Fig. 2. Alamar Blue assay for A549 lung cancer cell line treated with different dilutions of Taxol, plant medium (PM), rice callus suspension culture (RCSC), and rice seed extract (SE) for 24 to 96 h. RCSC 386 and RCSC 1121 represent RCSC from aromatic rice varieties Basmati 386 and Basmati 1121, respectively, and SE 386 and SE 1121 represent SE from Basmati 386 and Basmati 1121, respectively. CK, PM, and Taxol represent untreated, negative, and positive controls, respectively. The means of cell viability rate relative to untreated control, along with standard deviation values, were presented. Student’s t-test was performed to assess the significant levels at P < 0.05 (*), P < 0.005 (**), and P < 0.001 (***).
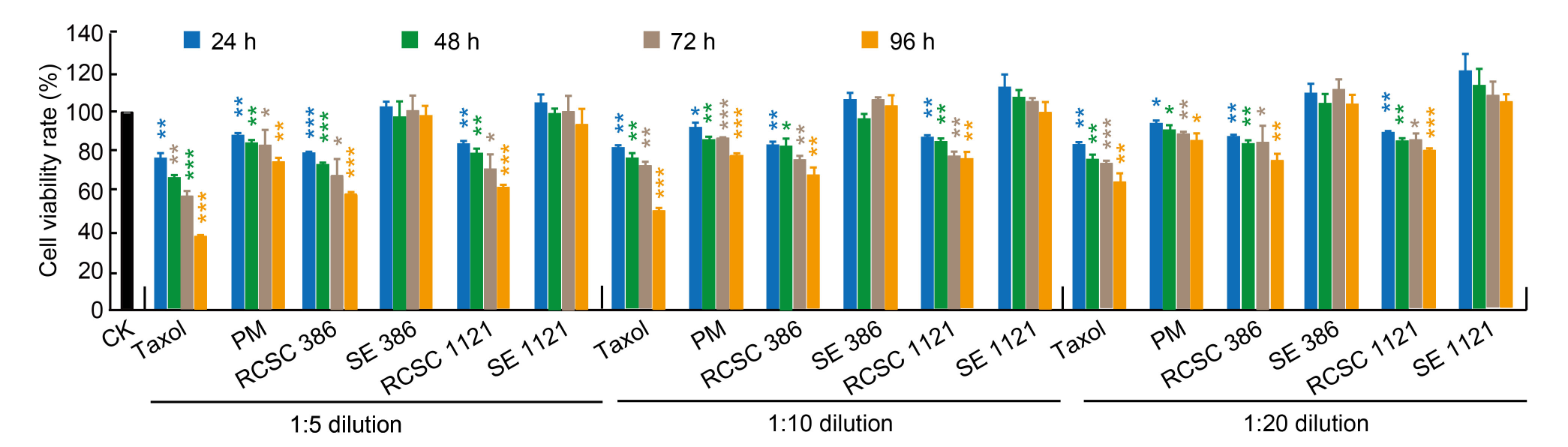
Fig. 3. Alamar Blue assay for HT-29 colon cancer cell line treated with different dilutions of Taxol, plant medium (PM), rice callus suspension culture (RCSC), and rice seed extract (SE) for 24 to 96 h. RCSC 386 and RCSC 1121 represent RCSC from aromatic rice varieties Basmati 386 and Basmati 1121, respectively, and SE 386 and SE 1121 represent SE from Basmati 386 and Basmati 1121, respectively. CK, PM, and Taxol represent untreated, negative, and positive controls, respectively. The means of cell viability rate relative to untreated control, along with standard deviation values, were presented. Student’s t-test was performed to assess the significant levels at P < 0.05 (*), P < 0.005 (**), and P < 0.001 (***).
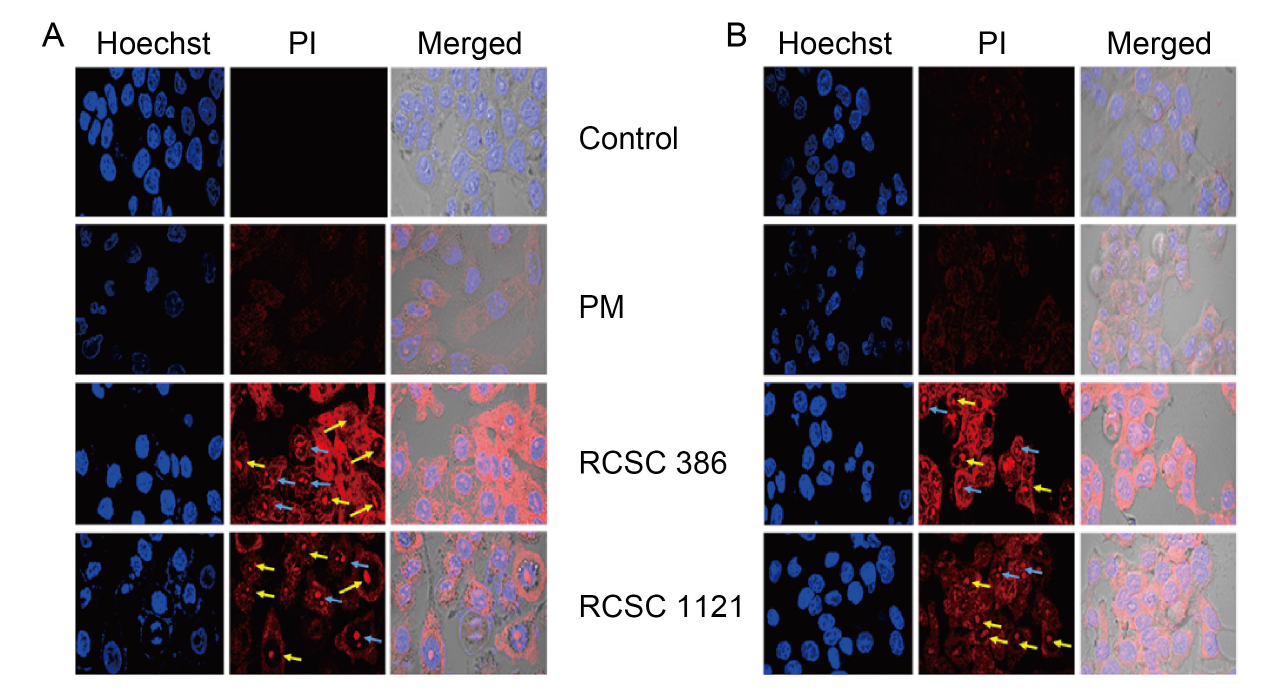
Fig. 4. Analysis of live/dead cells by Hoechst 33342 and propidium iodide (PI) staining, as visualized by a confocal laser scanning microscope after treatment with 1:5 dilution of plant medium (PM), RCSC 386, and RCSC 1121 for 72 h. A, A549 lung cancer cell line. B, HT-29 colon cancer cell line. RCSC 386 and RCSC 1121 represent rice callus suspension culture (RCSC) from aromatic rice varieties Basmati 386 and Basmati 1121, respectively. Yellow and blue arrows indicate cell shrinkage and nuclear fragmentation, respectively.
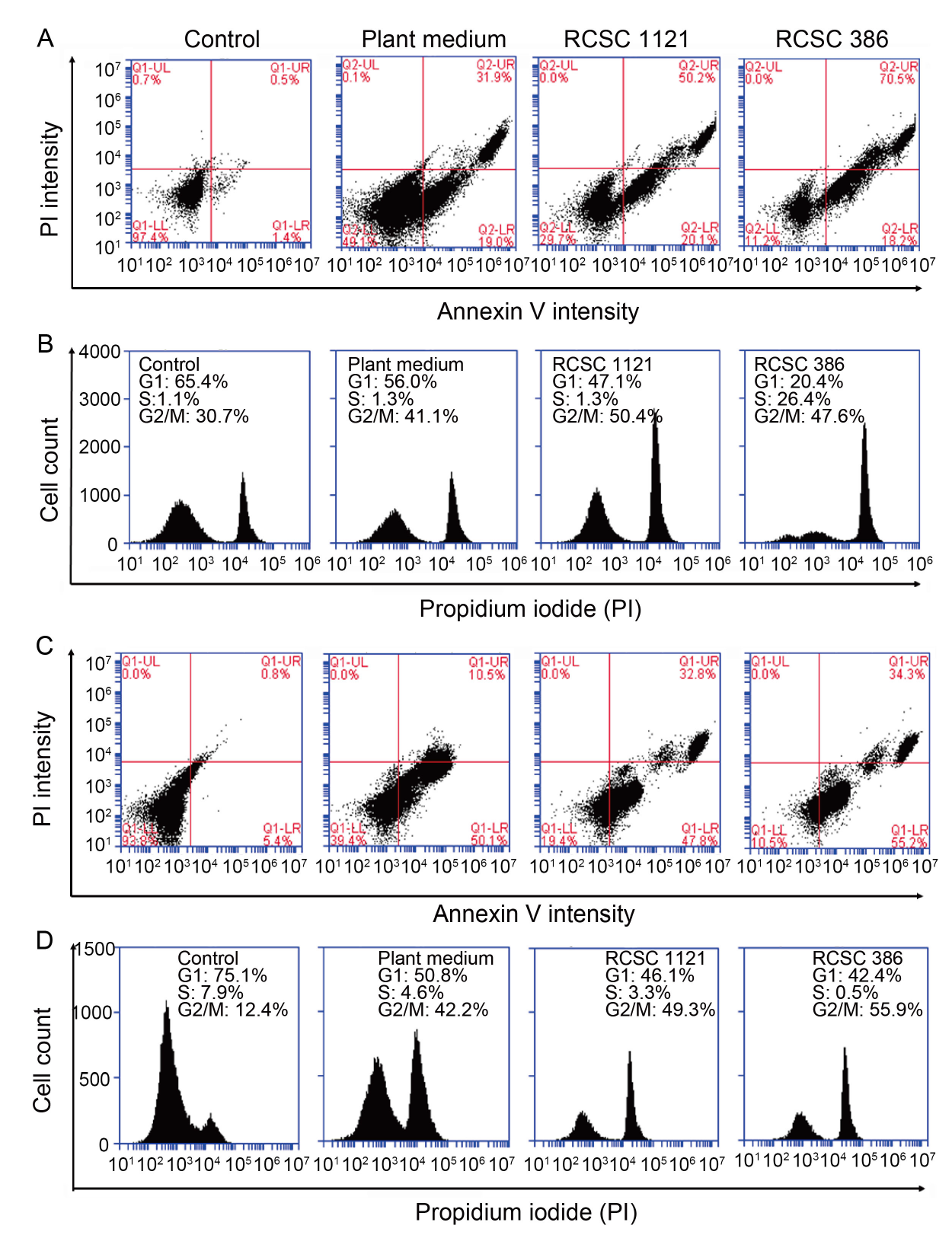
Fig. 5. Cell cycle analysis using flow cytometry for Annexin V and propidium iodide based assays. A and B, Lung cancer cell line A549 was stained with Annexin V (A) and propidium iodide (B). C and D, Colon cancer cell line HT-29 was stained with Annexin V (C) and propidium iodide (D). RCSC 386 and RCSC 1121 represent rice callus suspension culture (RCSC) from aromatic rice varieties Basmati 386 and Basmati 1121, respectively. The analysis was performed after treatment for 72 h. In A and C, the four quadrants, upper left (UL), upper right (UR), lower right (LR), and lower left (LL), represent necrotic, late apoptotic, early apoptotic, and living cells, respectively.

Fig. 6. Scratch assay displaying effect of rice callus suspension culture (RCSC) on cell migration. A, In lung cancer cell line A549. B, In colon cancer cell line HT-29. RCSC 386 and RCSC 1121 represent rice callus suspension culture (RCSC) from aromatic rice varieties Basmati 386 and Basmati 1121, respectively. Images were obtained at 24, 48, and 72 h after treatment. The gap size was determined using ImageJ software.
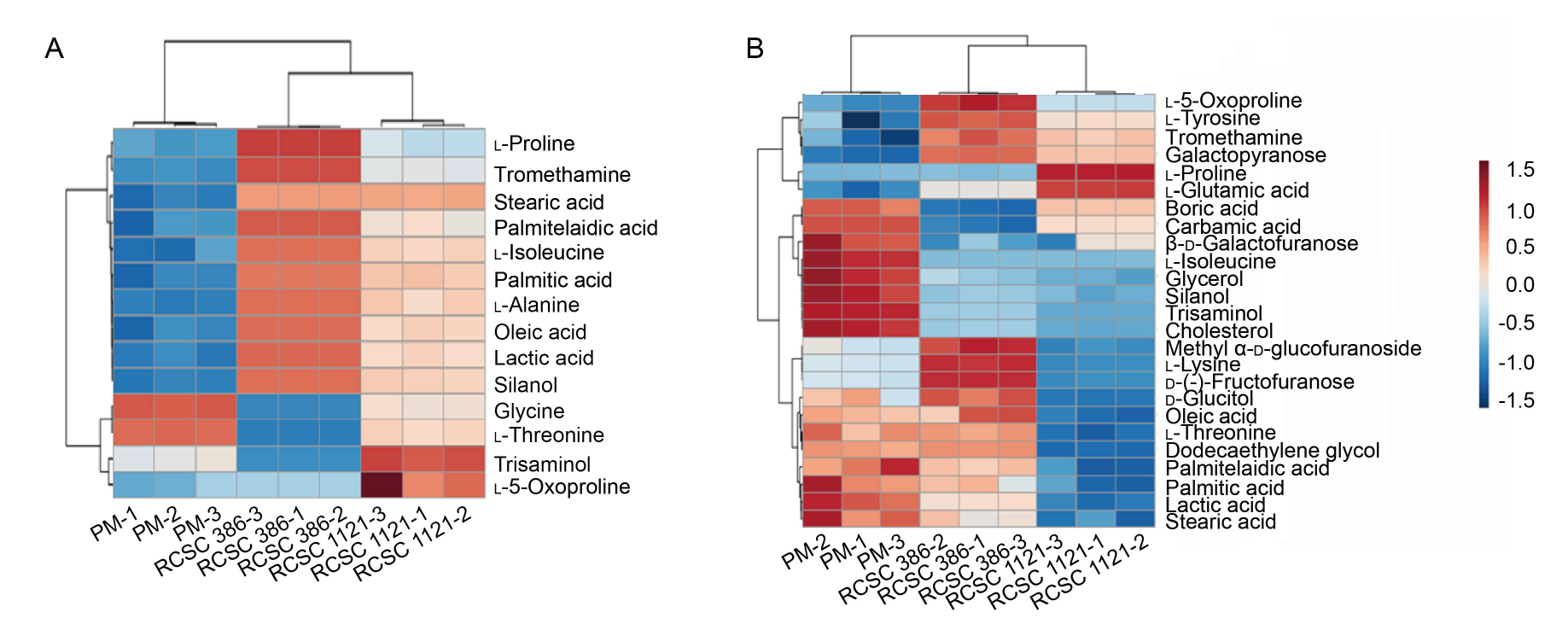
Fig. 7. Heat maps generated by hierarchical clustering analysis showing differentially expressed metabolites in RCSC 386 and RCSC 1121 treatment vs untreated control and plant medium (PM) in HT-29 cell line (A) and A549 cell line (B). Up-regulated and down-regulated metabolites are shown in red and blue colors, respectively. RCSC 386 and RCSC 1121 represent rice callus suspension culture (RCSC) from aromatic rice varieties Basmati 386 and Basmati 1121, respectively.
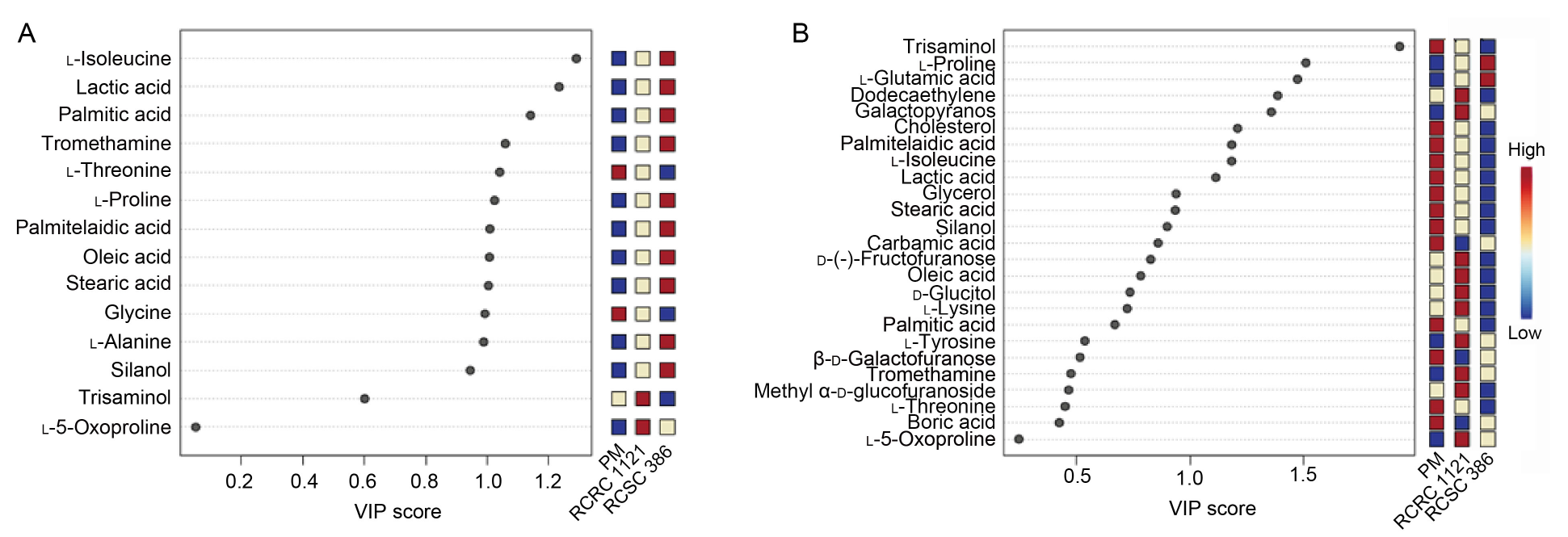
Fig. 8. Metabolites in HT-29 (A) and A549 (B) cell lines with maximal variable importance in prediction (VIP) scores identified by partial least squares discriminant analysis using MetaboAnalyst. Colored boxes indicate the relative concentrations of the metabolites in each group (PM, RCSC 1121, and RCSC 386), with up-regulation and down-regulation denoted by red and blue colors, respectively. RCSC 386 and RCSC 1121 represent rice callus suspension culture (RCSC) from aromatic rice varieties Basmati 386 and Basmati 1121, respectively. PM, Plant medium.
| Cell line | Pathway name | P-value | Impact value | Key metabolite |
|---|---|---|---|---|
| HT-29 | Glyoxylate and dicarboxylate metabolism | 1.824 × 10-4 | 0.106 | l-Glutamic acid |
| Glycine, serine and threonine metabolism | 5.161 × 10-5 | 0.246 | l-Threonine | |
| A549 | Glycerolipid metabolism | 2.396 × 10-4 | 0.237 | Glycerol |
| Arginine biosynthesis | 0.022 | 0.117 | l-Glutamic acid | |
| Alanine, aspartate and glutamate metabolism | 0.048 | 0.197 | l-Glutamic acid | |
| Arginine and proline metabolism | 0.048 | 0.164 | l-Proline, l-Glutamic acid | |
| Tyrosine metabolism | 0.048 | 0.140 | l-Tyrosine | |
| d-Glutamine and d-glutamate metabolism | 0.025 | 0.500 | l-Glutamic acid | |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 0.013 | 0.500 | l-Tyrosine |
Table 1. Impact values and key metabolites of pathways analyzed by Metaboanalyst.
| Cell line | Pathway name | P-value | Impact value | Key metabolite |
|---|---|---|---|---|
| HT-29 | Glyoxylate and dicarboxylate metabolism | 1.824 × 10-4 | 0.106 | l-Glutamic acid |
| Glycine, serine and threonine metabolism | 5.161 × 10-5 | 0.246 | l-Threonine | |
| A549 | Glycerolipid metabolism | 2.396 × 10-4 | 0.237 | Glycerol |
| Arginine biosynthesis | 0.022 | 0.117 | l-Glutamic acid | |
| Alanine, aspartate and glutamate metabolism | 0.048 | 0.197 | l-Glutamic acid | |
| Arginine and proline metabolism | 0.048 | 0.164 | l-Proline, l-Glutamic acid | |
| Tyrosine metabolism | 0.048 | 0.140 | l-Tyrosine | |
| d-Glutamine and d-glutamate metabolism | 0.025 | 0.500 | l-Glutamic acid | |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 0.013 | 0.500 | l-Tyrosine |
| [1] | Ahmad M, Nangyal H, Imran M, Ullah F. 2016. Optimization of protocol for surface sterilization and callus induction for three rice varieties. American-Eurasian J Agric Environ Sci, 16(2): 357-361. |
| [2] | Al-Nasiry S, Geusens N, Hanssens M, Luyten C, Pijnenborg R. 2007. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum Reprod, 22(5): 1304-1309. |
| [3] | Al-Obaidi O. 2014. Aqueous and alcoholic extracts from Glycyrrhiza glabra and their activity against bacteria and Rhabdomyo sarcomas. Eur Chem Bull, 3(2): 133-137. |
| [4] | Alvarado-Kristensson M. 2018. A simple and fast method for fixation of cultured cell lines that preserves cellular structures containing gamma-tubulin. MethodsX, 5: 227-233. |
| [5] | Biter A B, Pollet J, Chen W H, Strych U, Hotez P J, Bottazzi M E. 2019. A method to probe protein structure from UV absorbance spectra. Anal Biochem, 587: 113450. |
| [6] | Bookwala M, Thipsay P, Ross S, Zhang F, Bandari S, Repka M A. 2018. Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology. Eur J Pharm Biopharm, 131: 109-119. |
| [7] | Burke L, Guterman I, Palacios Gallego R, Britton R G, Burschowsky D, Tufarelli C, Rufini A. 2020. The Janus-like role of proline metabolism in cancer. Cell Death Discov, 6: 104. |
| [8] | Butsat S, Siriamornpun S. 2010. Antioxidant capacities and phenolic compounds of the husk, bran and endosperm of Thai rice. Food Chem, 119(2): 606-613. |
| [9] | Choi J H, Yoon S K, Lee K H, Seo M S, Kim D H, Hong S B, Kim J Y, Paik H D, Kim C H. 2006. Antitumor activity of cell suspension culture of green tea seed (Camellia sinensis L.). Biotechnol Bioprocess Eng, 11(5): 396-401. |
| [10] | Chouni A, Pal A, Gopal P K, Paul S. 2021. GC-MS analysis and screening of anti-proliferative potential of methanolic extract of Garcinia cowa on different cancer cell lines. Pharmacogn J, 13(2): 347-361. |
| [11] | Cragg G M, Pezzuto J M. 2016. Natural products as a vital source for the discovery of cancer chemotherapeutic and chemopreventive agents. Med Princ Pract, 25(Suppl 2): 41-59. |
| [12] | de Campos V E B, Teixeira C A A, da Veiga V F, Ricci E, Holandino C. 2010. l-tyrosine-loaded nanoparticles increase the antitumoral activity of direct electric current in a metastatic melanoma cell model. Int J Nanomed, 5: 961-971. |
| [13] | Descôteaux C, Brasseur K, Leblanc V, Asselin E, Bérubé G. 2015. Exploring the synthesis and anticancer potential of l-tyrosine- platinum(II) hybrid molecules. Med Chem, 11(8): 717-724. |
| [14] | Deshpande A, Dhadi S R, Hager E J, Ramakrishna W. 2012. Anticancer activity of rice callus suspension culture. Phytother Res, 26(7): 1075-1081. |
| [15] | Donald S P, Sun X Y, Hu C A, Yu J, Mei J M, Valle D, Phang J M. 2001. Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res, 61(5): 1810-1815. |
| [16] | Driscoll K, Deshpande A, Chapp A, Li K F, Datta R, Ramakrishna W. 2019. Anti-inflammatory and immune-modulating effects of rice callus suspension culture (RCSC) and bioactive fractions in an in vitro inflammatory bowel disease model. Phytomedicine, 57: 364-376. |
| [17] | Efferth T. 2019. Biotechnology applications of plant callus cultures. Engineering, 5(1): 50-59. |
| [18] | El-Sayed A S A, Fathalla M, Yassin M A, Zein N, Morsy S, Sitohy M, Sitohy B. 2020. Conjugation of Aspergillus flavipes taxol with porphyrin increases the anticancer activity of taxol and ameliorates its cytotoxic effects. Molecules, 25(2): 263. |
| [19] | Evans L M, Cowey S L, Siegal G P, Hardy R W. 2009. Stearate preferentially induces apoptosis in human breast cancer cells. Nutr Cancer, 61(5): 746-753. |
| [20] | Geng P Y, Qin W S, Xu G W. 2021. Proline metabolism in cancer. Amino Acids, 53(12): 1769-1777. |
| [21] | Gueven A, Knorr D. 2011. Isoflavonoid production by soy plant callus suspension culture. J Food Eng, 103(3): 237-243. |
| [22] | Hamid R, Rotshteyn Y, Rabadi L, Parikh R, Bullock P. 2004. Comparison of Alamar Blue and MTT assays for high through- put screening. Toxicol Vitro, 18(5): 703-710. |
| [23] | Hazekawa M, Nishinakagawa T, Kawakubo-Yasukochi T, Nakashima M. 2019. Evaluation of IC50 levels immediately after treatment with anticancer reagents using a real-time cell monitoring device. Exp Ther Med, 18(4): 3197-3205. |
| [24] | Hu X, Chao M, Wu H. 2017. Central role of lactate and proton in cancer cell resistance to glucose deprivation and its clinical translation. Signal Transduct Target Ther, 2(1): 1-8. |
| [25] | Huynh T Y L, Zareba I, Baszanowska W, Lewoniewska S, Palka J. 2020. Understanding the role of key amino acids in regulation of proline dehydrogenase/proline oxidase (prodh/pox)-dependent apoptosis/autophagy as an approach to targeted cancer therapy. Mol Cell Biochem, 466(1/2): 35-44. |
| [26] | Khan A A, Alanazi A M, Jabeen M, Chauhan A, Abdelhameed A S. 2013. Design, synthesis and in vitro anticancer evaluation of a stearic acid-based ester conjugate. Anticancer Res, 33(6): 2517-2524. |
| [27] | Khan S A, Khan H, Ahmad S, Rehman F U, Khan A A, Khan M A. 2022. GCMS characterization and biological potential of the seeds and aerial part of Galium tricorne Stokes. Braz J Biol, 84: e256920. |
| [28] | Khong H, Sharma M, Dai Z M, Singh M, Hailemichael Y, Overwijk W. 2015. l-tyrosine is a promising cancer vaccine adjuvant. J Immunother Cancer, 3(Suppl 2): P440. |
| [29] | Lieu E L, Nguyen T, Rhyne S, Kim J. 2020. Amino acids in cancer. Exp Mol Med, 52(1): 15-30. |
| [30] | Lima A R, Araújo A M, Pinto J, Jerónimo C, Henrique R, Bastos M L, Carvalho M, Guedes de Pinho P. 2018. GC-MS-based endometabolome analysis differentiates prostate cancer from normal prostate cells. Metabolites, 8(1): 23. |
| [31] | Liu Y, Borchert G L, Surazynski A, Phang J M. 2008. Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene, 27: 6729-6737. |
| [32] | Lobb R J, Becker M, Wen S W, Wong C S F, Wiegmans A P, Leimgruber A, Möller A. 2015. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J Extracell Vesicles, 4: 27031. |
| [33] | Longhin E M, El Yamani N, Rundén-Pran E, Dusinska M. 2022. The Alamar Blue assay in the context of safety testing of nanomaterials. Front Toxicol, 4: 981701. |
| [34] | Luzzio F A, Mayorov A V, Figg W D. 2000. Thalidomide metabolites: Part 1. Derivatives of (+)-2-(N-phthalimido)-γ-hydroxyglutamic acid. Tetrahedron Lett, 41(14): 2275-2278. |
| [35] | Mendoza D, Arias J P, Cuaspud O, Arias M. 2020. Phytochemical screening of callus and cell suspensions cultures of Thevetia peruviana. Braz Arch Biol Technol, 63: e20180735. |
| [36] | Mergny J L, Li J, Lacroix L, Amrane S, Chaires J B. 2005. Thermal difference spectra: A specific signature for nucleic acid structures. Nucleic Acids Res, 33(16): e138. |
| [37] | Mu Y M, Yanase T, Nishi Y, Tanaka A, Saito M, Jin C H, Mukasa C, Okabe T, Nomura M, Goto K, Nawata H. 2001. Saturated FFAs, palmitic acid and stearic acid, induce apoptosis in human granulosa cells. Endocrinology, 142(8): 3590-3597. |
| [38] | Murata K, Moriyama M. 2007. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res, 67(7): 3263-3268. |
| [39] | Nong S Q, Han X Y, Xiang Y, Qian Y R, Wei Y H, Zhang T Y, Tian K Y, Shen K, Yang J, Ma X L. 2023. Metabolic reprogramming in cancer: Mechanisms and therapeutics. MedComm, 4(2): e218. |
| [40] | Patel P, Patel V, Modi A, Kumar S, Shukla Y M. 2022. Phyto- factories of anti-cancer compounds: A tissue culture perspective. Beni-Suef Univ J Basic Appl Sci, 11(1): 43. |
| [41] | Patel S. 2012. Cereal bran: The next super food with significant antioxidant and anticancer potential. Mediterr J Nutr Metab, 5(2): 91-104. |
| [42] | Peanparkdee M, Iwamoto S. 2019. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci Technol, 86: 109-117. |
| [43] | Phang J M, Pandhare J, Liu Y M. 2008. The metabolism of proline as microenvironmental stress substrate. J Nutr, 138(10): 2008S-2015S. |
| [44] | Pilon-Thomas S, Kodumudi K N, El-Kenawi A E, Russell S, Weber A M, Luddy K, Damaghi M, Wojtkowial J W, Mule J J, Ibrahim- Hashim A, Gillies R J. 2016. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Can Res, 76(6): 1381-1390. |
| [45] | Rahman N, Dhadi S R, Deshpande A, Ramakrishna W. 2016. Rice callus suspension culture inhibits growth of cell lines of multiple cancer types and induces apoptosis in lung cancer cell line. BMC Complement Altern Med, 16(1): 427. |
| [46] | Rampersad S N. 2012. Multiple applications of Alamar Blue as an indicator of metabolic function and cellular health in cell viability bioassays. Sensors, 12(9): 12347-12360. |
| [47] | Ranjbary A G, Bagherzadeh A, Sabbaghi S S, Faghihi A, Karimi D N, Naji S, Kardani M. 2023. Chlorogenic acid induces apoptosis and cell-cycle arrest in colorectal cancer cells. Mol Biol Rep, 50(12): 9845-9857. |
| [48] | Riccardi C, Nicoletti I. 2006. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc, 1(3): 1458-1461. |
| [49] | Rieger A M, Nelson K L, Konowalchuk J D, Barreda D R. 2011. Modified annexin V/propidium iodide apoptosis assay for accurate assessment of cell death. J Vis Exp, 50: 2597. |
| [50] | Rivera A, Maxwell S A. 2005. The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem, 280(32): 29346-29354. |
| [51] | Rodrigues D, Pinto J, Araújo A M, Jerónimo C, Henrique R, Bastos M L, Guedes de Pinho P, Carvalho M. 2019. GC-MS metabolomics reveals distinct profiles of low- and high-grade bladder cancer cultured cells. Metabolites, 9(1): 18. |
| [52] | Saal C, Becker A. 2013. Pharmaceutical salts: A summary on doses of salt formers from the Orange Book. Eur J Pharm Sci, 49(4): 614-623. |
| [53] | Sánchez-Ramos M, Bahena S M, Romero-Estrada A, Bernabé- Antonio A, Cruz-Sosa F, Gonzálesssz-Christen J, Acevedo- Fernández J J, Perea-Arango I, Alvarez L. 2018. Establishment and phytochemical analysis of a callus culture from Ageratina pichinchensis (Asteraceae) and its anti-inflammatory activity. Molecules, 23(6): 1258. |
| [54] | Schiliro C, Firestein B L. 2021. Mechanisms of metabolic reprogramming in cancer cells supporting enhanced growth and proliferation. Cells, 10: 1056. |
| [55] | Schneider C A, Rasband W S, Eliceiri K W. 2012. NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9(7): 671-675. |
| [56] | Shen M C, Zhao X M, Siegal G P, Desmond R, Hardy R W. 2014. Dietary stearic acid leads to a reduction of visceral adipose tissue in athymic nude mice. PLoS One, 9(9): e104083. |
| [57] | Shevchenko A, Tomas H, Havlis J, Olsen J V, Mann M. 2006. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc, 1(6): 2856-2860. |
| [58] | Sivanand S, Vander Heiden M G. 2020. Emerging roles for branched- chain amino acid metabolism in cancer. Cancer Cell, 37(2): 147-156. |
| [59] | Song H, Peng J S, Yao D S, Yang Z L, Liu H L, Zeng Y K, Shi X P, Lu B Y. 2012. Serum metabolic profiling of human gastric cancer based on gas chromatography/mass spectrometry. Brazilian J Med Biol Res, 45(1): 78-85. |
| [60] | Songserm P, Klanrit P, Klanrit P, Phetcharaburanin J, Thanonkeo P, Apiraksakorn J, Phomphrai K, Klanrit P. 2022. Antioxidant and anticancer potential of bioactive compounds from Rhinacanthus nasutus cell suspension culture. Plants, 11(15): 1994. |
| [61] | Srikanth K, Kumar C A, Ghosh B, Jha T. 2002. Synthesis, screening and quantitative structure-activity relationship (QSAR) studies of some glutamine analogues for possible anticancer activity. Bioorg Med Chem, 10(7): 2119-2131. |
| [62] | Tan B L, Norhaizan M E. 2017. Scientific evidence of rice by-products for cancer prevention: Chemopreventive properties of waste products from rice milling on carcinogenesis in vitro and in vivo. Biomed Res Int, 2017: 9017902. |
| [63] | Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. 1995. A novel assay for apoptosis: Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled annexin V. J Immunol Methods, 184(1): 39-51. |
| [64] | Wang H Q, Chen S, Kang W H, Ding B J, Cui S L, Zhou L, Zhang N, Luo H Y, Wang M J, Zhang F, Zhao Z Z, Guo Z H, Wang C, Li L, Wang Z Z, Chen X T, Wang Y H. 2023. High dose isoleucine stabilizes nuclear PTEN to suppress the proliferation of lung cancer. Discov Oncol, 14(1): 25. |
| [65] | Wishart D S. 2016. Emerging applications of metabolomics in drug discovery and precision medicine. Nat Rev Drug Discov, 15(7): 473-484. |
| [66] | Xu H, Jiang M, Li H, Lu D Q, Ouyang P K. 2005. Efficient production of poly(γ-glutamic acid) by newly isolated Bacillus subtilis NX-2. Process Biochem, 40( 2): 519-523. |
| [67] | Yarrow J C, Perlman Z E, Westwood N J, Mitchison T J. 2004. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC Biotechnol, 4: 21. |
| [68] | Yu Y L, Yu J, Ge S F, Su Y, Fan X Q. 2023. Novel insight into metabolic reprogramming in cancer radioresistance: A promising therapeutic target in radiotherapy. Int J Biol Sci, 19(3): 811-828. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||