
Rice Science ›› 2024, Vol. 31 ›› Issue (4): 463-474.DOI: 10.1016/j.rsci.2024.04.005
收稿日期:2023-12-26
接受日期:2024-03-29
出版日期:2024-07-28
发布日期:2024-08-08
. [J]. Rice Science, 2024, 31(4): 463-474.
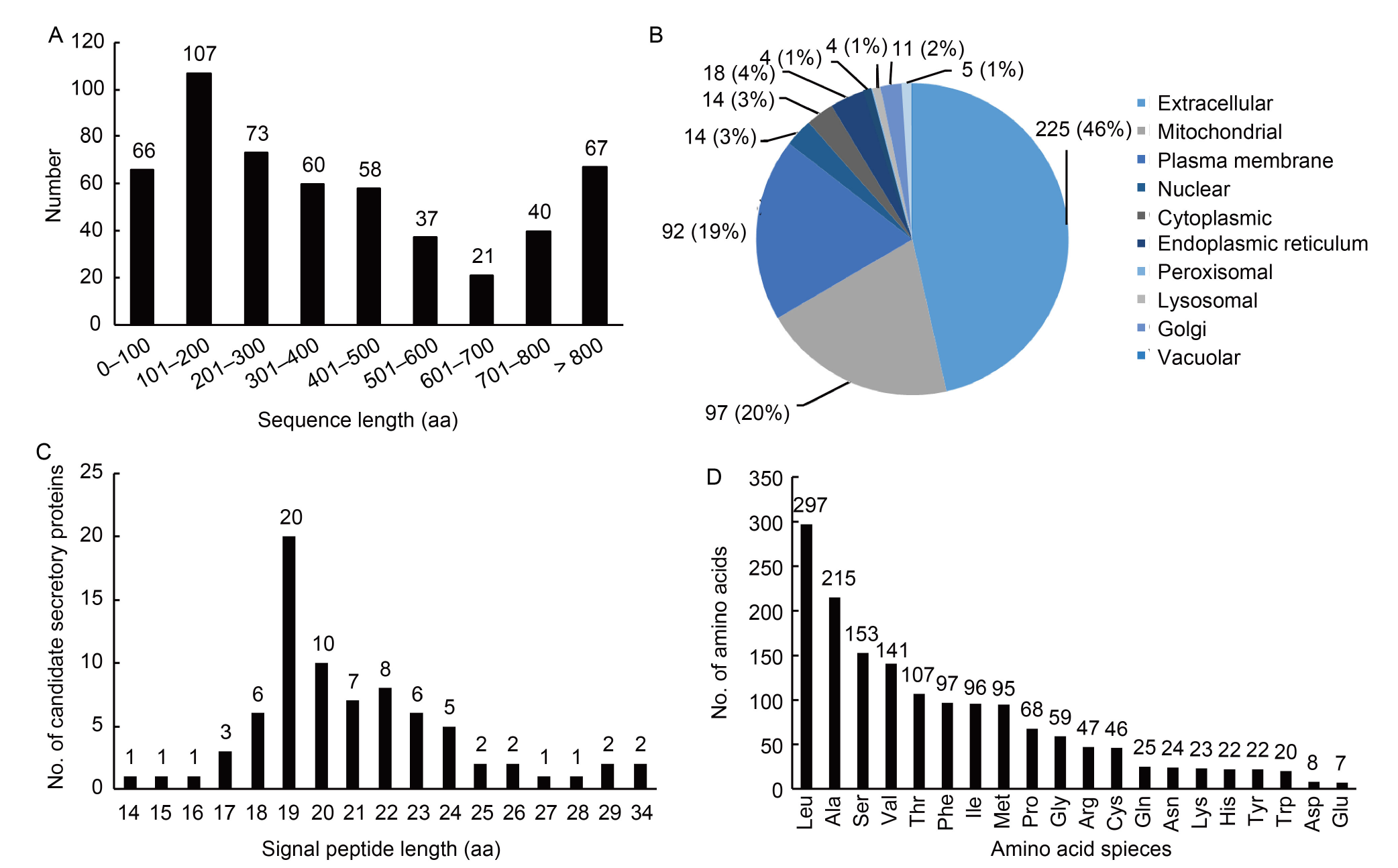
Fig. 1. Bioinformatics analysis for sequences of 10 489 proteins of Rhizoctonia solani AG1-IA. A, Length distribution of protein sequences with signal peptides in R. solani AG1-IA. B, Subcellular localizations of 484 proteins with signal peptides. C, Length distribution of candidate effector sequences with signal peptides in R. solani AG1-IA. D, Frequency of amino acids of signal peptides in R. solani AG1-IA candidate effectors.
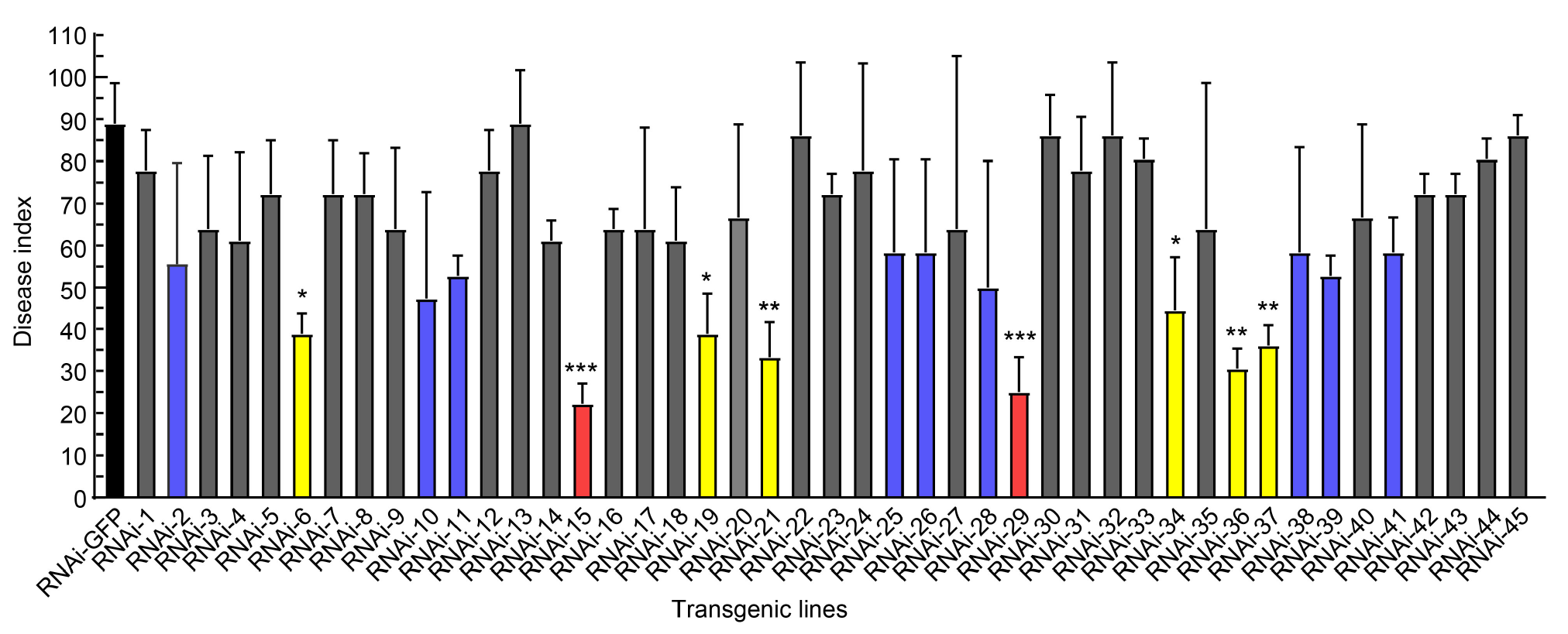
Fig. 2. Disease index for Nicotiana benthamiana infiltrated with tobacco rattle virus (TRV) vectors of 45 candidate effector genes of Rhizoctonia solani AG1-IA. The Red bar indicates disease resistance, the yellow bar indicates moderate resistance, the blue bar indicates susceptibility, and the gray bar indicates high susceptibility. RNAi-GFP was used as a negative control. The data are presented as Mean ± SE (n = 3). Statistical significance is denoted as P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***), respectively, according to one-way analysis of variance.
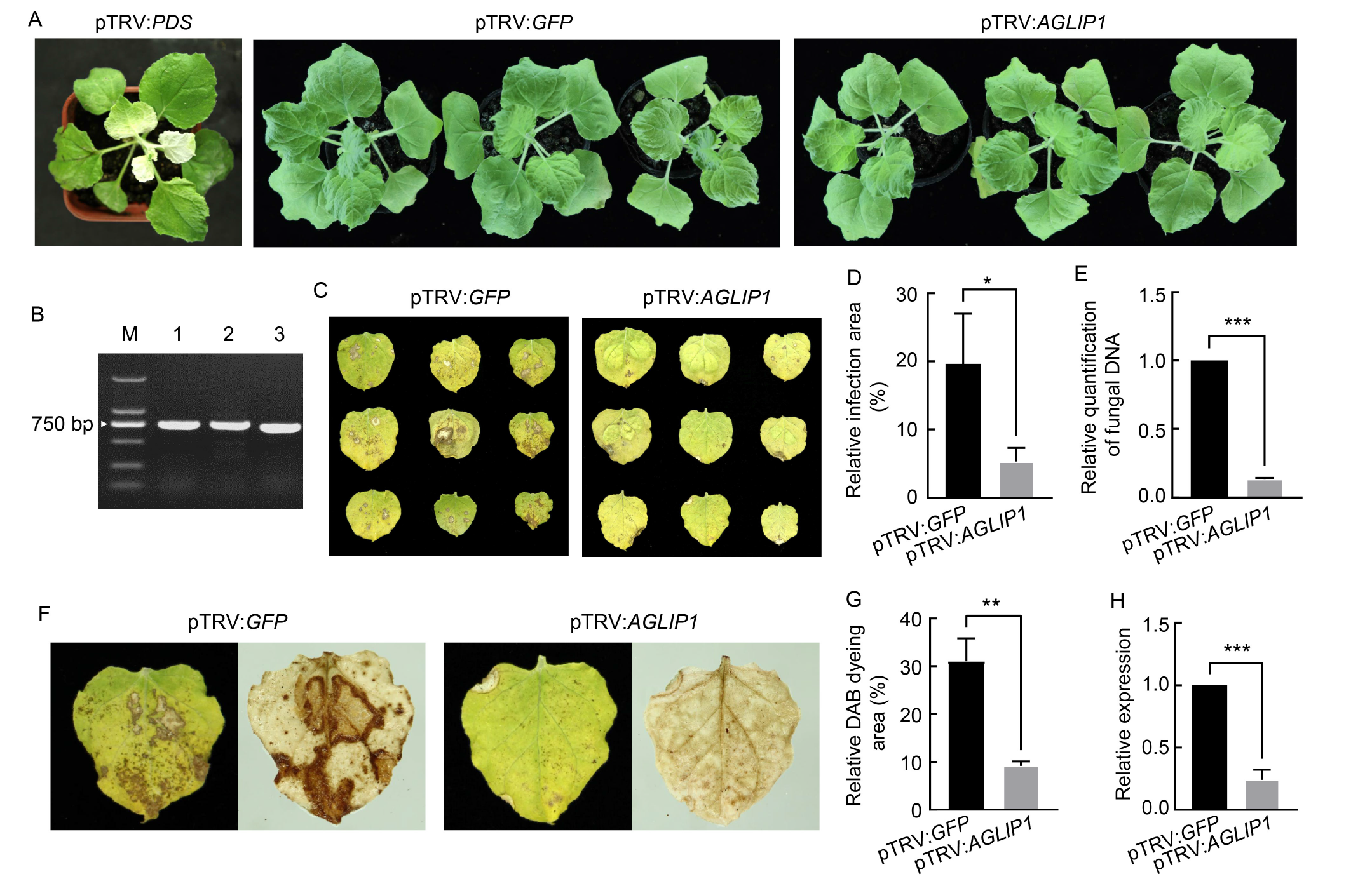
Fig. 3. Transient silencing of AGLIP1 enhanced resistance of Nicotiana benthamiana to Rhizoctonia solani AG1-IA. A, Phenotype of infiltrated N. benthamiana plants after a 14-day inoculation period. Phytoene desaturase (PDS) gene was used as a positive control for tobacco rattle virus-host induced gene silencing (TRV-HIGS) efficiency. Infiltration with pTRV:PDS caused photobleaching at 14 d post- infiltration, indicating successful gene silencing. Meanwhile, the AGLIP1-silenced tobacco plants exhibited no noticeable phenotypic differences compared with the negative control (pTRV:GFP). B, RT-PCR reflected the expression of target genes (AGLIP1, GFP, and PDS) in infiltrated N. benthamiana plants at 14 d after inoculation. M, Marker; Lanes 1, 2 and 3 represent the AGLIP1, GFP, and PDS genes, respectively. C, Symptom development on N. benthamiana leaves inoculated with GD-118 mycelial suspension at 5 d post-infection (dpi). D, Quantification of visible infected area at 5 dpi is shown as a percentage of the total N. benthamiana leaf area. E, Relative amounts of fungal DNA as determined by qRT-PCR. The samples were N. benthamiana leaves at 5 dpi and the data were normalized to the EF1α transcript level of N. benthamiana. F, 3,3ʹ-Diaminobenzidine (DAB) staining reflecting reactive oxygen species accumulation in N. benthamiana leaves. G, Quantification of visible brown spot area is shown as a percentage of the total N. benthamiana leaf area. H, Gene-specific expression levels of AGLIP1 (RNAi-15) were measured by qRT-PCR. The samples were N. benthamiana leaves at 5 dpi and the data were normalized to the GAPDH transcript level of R. solani. The data are presented as Mean ± SE (n = 3). Statistical significance is denoted as P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***), respectively, according to the Student’s t-test.
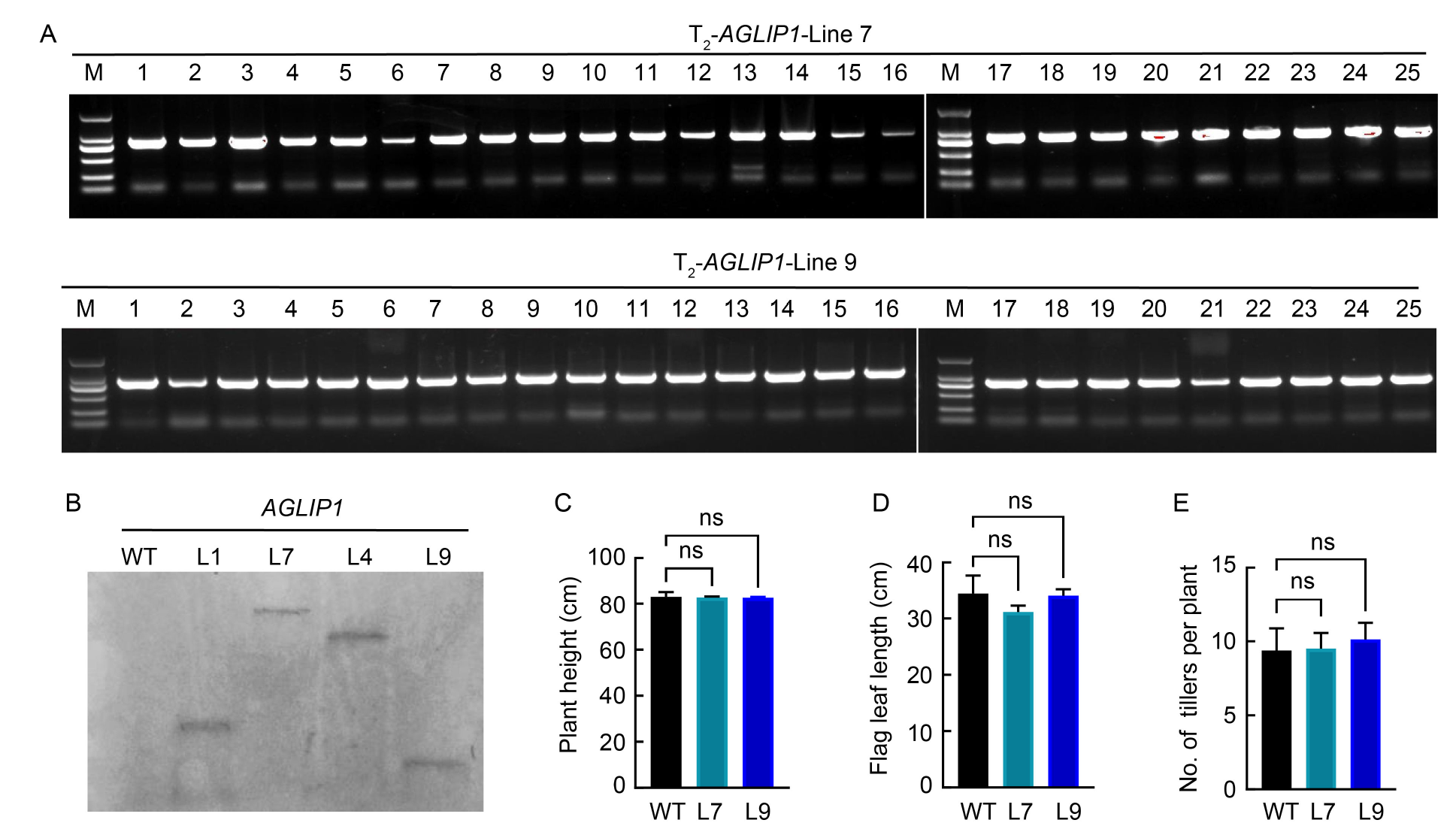
Fig. 4. Molecular analysis and morphological characteristics of homozygous T2 transgenic rice. A, PCR analysis of T2-AGLIP1-Line 7 (L7) and T2-AGLIP1-Line 9 (L9) transgenic plants using a hygromycin gene-specific primer. M, Marker; Lanes 1‒25 represent transgenic lines. B, Southern blot analysis of AGLIP1 transgenic plants. WT, Wild type; L1, L7, L4, and L9 represent T2-AGLIP1-Line 1, -Line 7, -Line 4, and -Line 9, respectively. C‒E, Comparisons of plant height (C), flag leaf length (D), and the number of effective tillers per plant (E) between the WT and RNAi homozygous T2 transgenic lines. The data are present as Mean ± SE (n = 3). ns indicates no significant changes were observed using one-way analysis of variance with Tukey’s test.
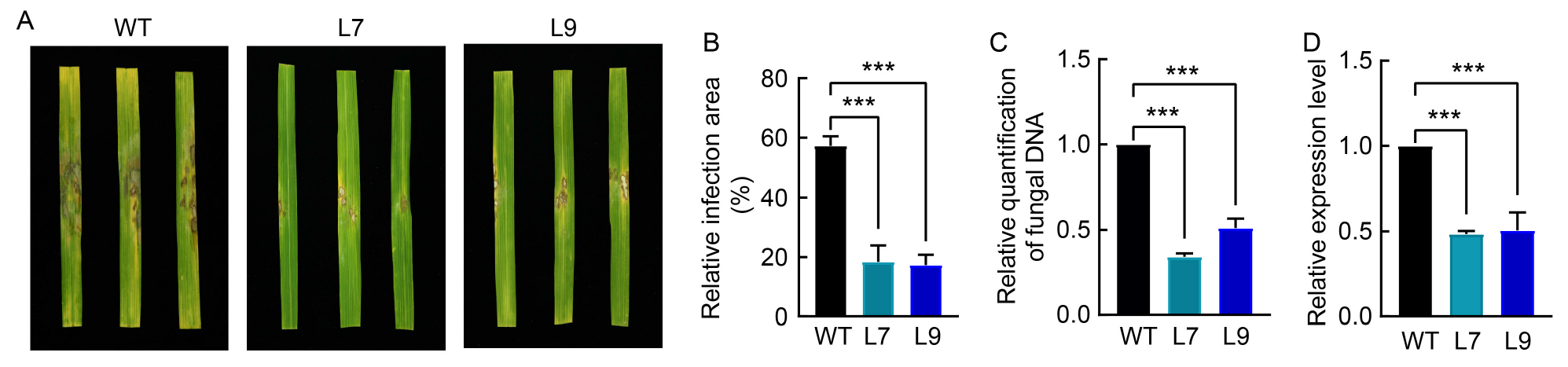
Fig. 5. Evaluation of T2-AGLIP1 lines against sheath blight disease pathogen Rhizoctonia solani AG1-IA. A, Disease symptoms in R. solani AG1-IA infected detached leaves of T2 homozygous transgenic lines at 72 h post-infection (hpi). B, Quantification of visible infected area is shown as a percentage of the total rice leaf area at 72 hpi. C, Relative amounts of fungal DNA were determined by qRT-PCR. The samples were rice leaves at 72 hpi and the data werenormalized to the 18S rRNA transcript level of rice. D, Gene-specific expression levels of AGLIP1 were measured by qRT-PCR. The samples were japonica rice leaves at 72 hpi, and the data were normalized to the GAPDH transcript level of R. solani. WT, Wild type; L7, T2-AGLIP1-Line 7 transgenic plants; L9, T2-AGLIP1-Line 9 transgenic plants. The data are presented as Mean ± SE (n = 3). Statistical significance is denoted as P < 0.001 (***) according to one-way analysis of variance with Tukey’s test.
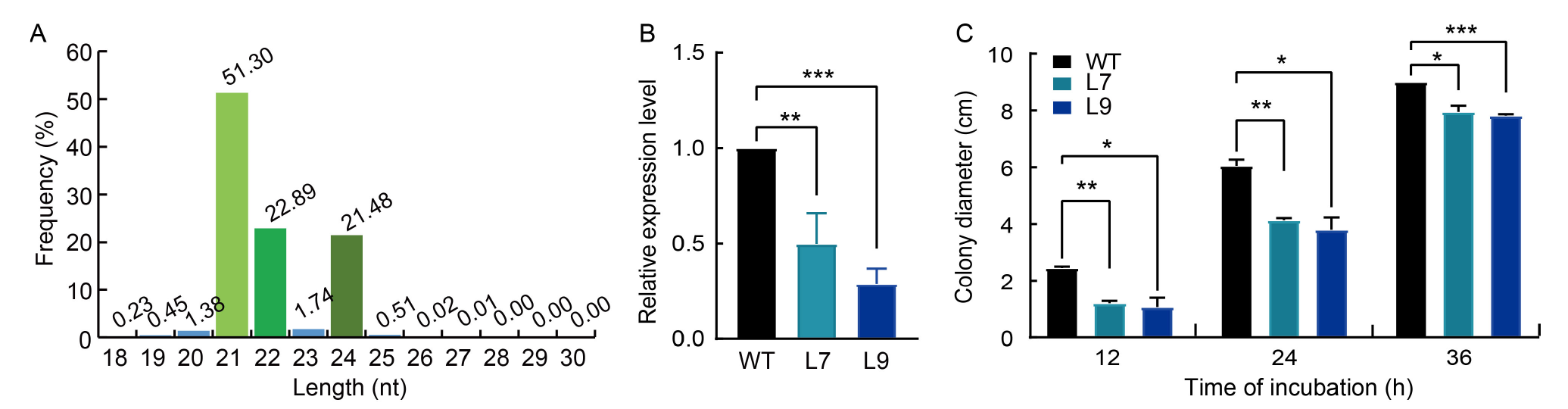
Fig. 6. AGLIP1-siRNA from rice reduced growth rate of Rhizoctonia solani AG1-IA. A, Length distribution and abundance of AGLIP1-specific siRNAs in T2-AGLIP1-Line 9. B, Relative expression levels of ALIP1 gene in R. solani-isolated rice plants. C, Colony diameter of re-isolated GD-118 from infected rice leaves. WT, Wild type; L7, T2-AGLIP1-Line 7 transgenic plant; L9, T2-AGLIP1-Line 9 transgenic plant. The data are presented as Mean ± SE (n = 3). Statistical significance is denoted as P < 0.05 (*), P < 0.01 (**), or P < 0.001 (***), respectively, one-way analysis of variance with Tukey’s test.
| [1] | Anderson J P, Hane J K, Stoll T, Pain N, Hastie M L, Kaur P, Hoogland C, Gorman J J, Singh K B. 2016. Proteomic analysis of Rhizoctonia solani identifies infection-specific, redox associated proteins and insight into adaptation to different plant hosts. Mol Cell Proteomics, 15(4): 1188-1203. |
| [2] | Blümke A, Falter C, Herrfurth C, Sode B, Bode R, Schäfer W, Feussner I, Voigt C A. 2014. Secreted fungal effector lipase releases free fatty acids to inhibit innate immunity-related callose formation during wheat head infection. Plant Physiol, 165(1): 346-358. |
| [3] | Cao W L, Zhang H M, Zhou Y, Zhao J H, Lu S B, Wang X Q, Chen X J, Yuan L M, Guan H Y, Wang G D, Shen W X, De Vleesschauwer D, Li Z Q, Shi X P, Gu J F, Guo M, Feng Z M, Chen Z X, Zhang Y F, Pan X B, Liu W D, Liang G H, Yan C J, Hu K M, Liu Q Q, Zuo S M. 2022. Suppressing chlorophyll degradation by silencing OsNYC3 improves rice resistance to Rhizoctonia solani, the causal agent of sheath blight. Plant Biotechnol J, 20(2): 335-349. |
| [4] | Charova S N, Dölfors F, Holmquist L, Moschou P N, Dixelius C, Tzelepis G. 2020. The RsRlpA effector is a protease inhibitor promoting Rhizoctonia solani virulence through suppression of the hypersensitive response. Int J Mol Sci, 21(21): 8070. |
| [5] | Chen X, Alonzo F III. 2019. Bacterial lipolysis of immune-activating ligands promotes evasion of innate defenses. Proc Natl Acad Sci USA, 116(9): 3764-3773. |
| [6] | Cota-Sánchez J H, Remarchuk K, Ubayasena K. 2006. Ready-to- use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol Biol Rep, 24(2): 161-167. |
| [7] | Eisenhaber B, Schneider G, Wildpaner M, Eisenhaber F. 2004. A sensitive predictor for potential GPI lipid modification sites in fungal protein sequences and its application to genome-wide studies for Aspergillus nidulans, Candida albicans, Neurospora crassa, Saccharomyces cerevisiae and Schizosaccharomyces pombe. J Mol Biol, 337( 2): 243-253. |
| [8] | Emanuelsson O, Nielsen H, Brunak S, von Heijne G. 2000. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol, 300(4): 1005-1016. |
| [9] | Gao F, Zhang B S, Zhao J H, Huang J F, Jia P S, Wang S, Zhang J, Zhou J M, Guo H S. 2019. Deacetylation of chitin oligomers increases virulence in soil-borne fungal pathogens. Nat Plants, 5(11): 1167-1176. |
| [10] | Hane J K, Anderson J P, Williams A H, Sperschneider J, Singh K B. 2014. Genome sequencing and comparative genomics of the broad host-range pathogen Rhizoctonia solani AG8. PLoS Genet, 10(5): e1004281. |
| [11] | Hua C L, Zhao J H, Guo H S. 2018. Trans-kingdom RNA silencing in plant-fungal pathogen interactions. Mol Plant, 11(2): 235-244. |
| [12] | Ji H M, Mao H Y, Li S J, Feng T, Zhang Z Y, Cheng L, Luo S J, Borkovich K A, Ouyang S Q. 2021. Fol-milR1, a pathogenicity factor of Fusarium oxysporum, confers tomato wilt disease resistance by impairing host immune responses. New Phytol, 232(2): 705-718. |
| [13] | Jirage D, Tootle T L, Reuber T L, Frost L N, Feys B J, Parker J E, Ausubel F M, Glazebrook J. 1999. Arabidopsis thaliana PAD4 encodes a lipase-like gene that is important for salicylic acid signaling. Proc Natl Acad Sci USA, 96(23): 13583-13588. |
| [14] | Kamoun S. 2006. A catalogue of the effector secretome of plant pathogenic oomycetes. Annu Rev Phytopathol, 44: 41-60. |
| [15] | Krogh A, Larsson B, von Heijne G, Sonnhammer E L. 2001. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J Mol Biol, 305(3): 567-580. |
| [16] | Langmead B, Trapnell C, Pop M, Salzberg S L. 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol, 10(3): R25. |
| [17] | Lee W S, Hammond-Kosack K E, Kanyuka K. 2012. Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: Virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol, 160(2): 582-590. |
| [18] | Li D Y, Li S, Wei S H, Sun W X. 2021. Strategies to manage rice sheath blight: Lessons from interactions between rice and Rhizoctonia solani. Rice, 14(1): 21. |
| [19] | Li S, Peng X W, Wang Y L, Hua K Y, Xing F, Zheng Y Y, Liu W, Sun W X, Wei S H. 2019. The effector AGLIP1 in Rhizoctonia solani AG1 IA triggers cell death in plants and promotes disease development through inhibiting PAMP-triggered immunity in Arabidopsis thaliana. Front Microbiol, 10: 2228. |
| [20] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4): 402-408. |
| [21] | Meyer V. 2008. Genetic engineering of filamentous fungi: Progress, obstacles and future trends. Biotechnol Adv, 26(2): 177-185. |
| [22] | Nielsen H. 2017. Predicting secretory proteins with SignalP. Methods Mol Biol, 1611: 59-73. |
| [23] | Panwar V, McCallum B, Bakkeren G. 2013. Host-induced gene silencing of wheat leaf rust fungus Puccinia triticina pathogenicity genes mediated by the Barley stripe mosaic virus. Plant Mol Biol, 81(6): 595-608. |
| [24] | Petre B, Kamoun S. 2014. How do filamentous pathogens deliver effector proteins into plant cells? PLoS Biol, 12(2): e1001801. |
| [25] | Rao T B, Chopperla R, Methre R, Punniakotti E, Venkatesh V, Sailaja B, Reddy M R, Yugander A, Laha G S, Madhav M S, Sundaram R M, Ladhalakshmi D, Balachandran S M, Mangrauthia S K. 2019. Pectin induced transcriptome of a Rhizoctonia solani strain causing sheath blight disease in rice reveals insights on key genes and RNAi machinery for development of pathogen derived resistance. Plant Mol Biol, 100(1/2): 59-71. |
| [26] | Rao T B, Chopperla R, Prathi N B, Balakrishnan M, Prakasam V, Laha G S, Balachandran S M, Mangrauthia S K. 2020. A comprehensive gene expression profile of pectin degradation enzymes reveals the molecular events during cell wall degradation and pathogenesis of rice sheath blight pathogen Rhizoctonia solani AG1-IA. J Fungi, 6(2):71. |
| [27] | Schmidt G W, Delaney S K. 2010. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol Genet Genomics, 283(3): 233-241. |
| [28] | Senthil-Kumar M, Mysore K S. 2014. Tobacco rattle virus-based virus-induced gene silencing in Nicotiana benthamiana. Nat Protoc, 9(7): 1549-1562. |
| [29] | Southern E M. 1975. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol, 98(3): 503-517. |
| [30] | Stergiopoulos I, de Wit P J G M. 2009. Fungal effector proteins. Annu Rev Phytopathol, 47: 233-263. |
| [31] | Su X F, Rehman L, Guo H M, Li X K, Zhang R, Cheng H M. 2017. AAC as a potential target gene to control Verticillium dahliae. Genes, 8(1): 25. |
| [32] | Su X F, Rehman L, Guo H M, Li X K, Cheng H M. 2018. The oligosaccharyl transferase subunit STT3 mediates fungal development and is required for virulence in Verticillium dahliae. Curr Genet, 64(1): 235-246. |
| [33] | Su X F, Lu G Q, Li X K, Rehman L, Liu W D, Sun G Q, Guo H M, Wang G L, Cheng H M. 2020. Host-induced gene silencing of an adenylate kinase gene involved in fungal energy metabolism improves plant resistance to Verticillium dahliae. Biomolecules, 10(1): 127. |
| [34] | Taguchi-Shiobara F, Ozaki H, Sato H, Maeda H, Kojima Y, Ebitani T, Yano M. 2013. Mapping and validation of QTLs for rice sheath blight resistance. Breed Sci, 63(3): 301-308. |
| [35] | Thines E, Weber R W, Talbot N J. 2000. MAP kinase and protein kinase A-dependent mobilization of triacylglycerol and glycogen during appressorium turgor generation by Magnaporthe grisea. Plant Cell, 12(9): 1703-1718. |
| [36] | Tiwari I M, Jesuraj A, Kamboj R, Devanna B N, Botella J R, Sharma T R. 2017. Host delivered RNAi, an efficient approach to increase rice resistance to sheath blight pathogen (Rhizoctonia solani). Sci Rep, 7: 7521. |
| [37] | Toro K S, Brachmann A. 2016. The effector candidate repertoire of the arbuscular mycorrhizal fungus Rhizophagus clarus. BMC Genomics, 17: 101. |
| [38] | Tzelepis G, Dölfors F, Holmquist L, Dixelius C. 2021. Plant mitochondria and chloroplasts are targeted by the Rhizoctonia solani RsCRP1 effector. Biochem Biophys Res Commun, 544: 86-90. |
| [39] | Tzima A K, Paplomatas E J, Tsitsigiannis D I, Kang S. 2012. The G protein β subunit controls virulence and multiple growth- and development-related traits in Verticillium dahliae. Fungal Genet Biol, 49(4): 271-283. |
| [40] | Voigt C A, Schäfer W, Salomon S. 2005. A secreted lipase of Fusarium graminearum is a virulence factor required for infection of cereals. Plant J, 42(3): 364-375. |
| [41] | Wang A J, Shu X Y, Jing X, Jiao C Z, Chen L, Zhang J F, Ma L, Jiang Y Q, Yamamoto N, Li S C, Deng Q M, Wang S Q, Zhu J, Liang Y Y, Zou T, Liu H N, Wang L X, Huang Y B, Li P, Zheng A P. 2021. Identification of rice (Oryza sativa L.) genes involved in sheath blight resistance via a genome-wide association study. Plant Biotechnol J, 19(8): 1553-1566. |
| [42] | Wei M M, Wang A J, Liu Y, Ma L, Niu X Y, Zheng A P. 2020. Identification of the novel effector RsIA_NP8 in Rhizoctonia solani AG1 IA that induces cell death and triggers defense responses in non-host plants. Front Microbiol, 11: 1115. |
| [43] | Yang Y Q, Yang M, Li M H, Zhou E X. 2012. Cloning and functional analysis of an endo-PG-encoding gene Rrspg1 of Rhizoctonia solani, the causal agent of rice sheath blight. Can J Plant Pathol, 34(3): 436-447. |
| [44] | Yin C T, Jurgenson J E, Hulbert S H. 2011. Development of a host- induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol Plant Microbe Interact, 24(5): 554-561. |
| [45] | Zhang T, Jin Y, Zhao J H, Gao F, Zhou B J, Fang Y Y, Guo H S. 2016. Host-induced gene silencing of the target gene in fungal cells confers effective resistance to the cotton wilt disease pathogen Verticillium dahliae. Mol Plant, 9(6): 939-942. |
| [46] | Zhao M, Wang C J Z, Wan J, Li Z F, Liu D L, Yamamoto N, Zhou E X, Shu C W. 2021. Functional validation of pathogenicity genes in rice sheath blight pathogen Rhizoctonia solani by a novel host-induced gene silencing system. Mol Plant Pathol, 22(12): 1587-1598. |
| [47] | Zheng A P, Lin R M, Zhang D H, Qin P G, Xu L Z, Ai P, Ding L, Wang Y R, Chen Y, Liu Y, Sun Z G, Feng H T, Liang X X, Fu R T, Tang C Q, Li Q, Zhang J, Xie Z L, Deng Q M, Li S C, Wang S Q, Zhu J, Wang L X, Liu H N, Li P. 2013. The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat Commun, 4: 1424. |
| [48] | Zimmermann D, Gomez-Barrera J A, Pasule C, Brack-Frick U B, Sieferer E, Nicholson T M, Pfannstiel J, Stintzi A, Schaller A. 2016. Cell death control by matrix metalloproteinases. Plant Physiol, 171(2): 1456-1469. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||