
Rice Science ›› 2025, Vol. 32 ›› Issue (4): 475-498.DOI: 10.1016/j.rsci.2025.03.003
收稿日期:2024-10-29
接受日期:2025-01-12
出版日期:2025-07-28
发布日期:2025-08-06
. [J]. Rice Science, 2025, 32(4): 475-498.

Fig. 1. Pathway of terpenoids produced by metabolic engineering in rice. 2 -Acetyl-CoA, 2-Acetyl coenzyme A; Acetoacetyl-CoA, Acetoacetyl coenzyme A; HMG-CoA, β-Hydroxy β-methylglutaryl coenzyme A; MVA, Mevalonate; MVAP, Mevalonate-5-phosphate; MVAPP, Mevalonate-5-diphosphate; IPP, Isopentenyl pyrophosphate; DMAPP, Dimethylallyl pyrophosphate; FPP, Farnesyl diphosphate; GGPP, Geranylgeranyl diphosphate; G3P, Glyceraldehyde 3-phosphate; DXP, 1-Deoxy-d-xylulose 5-phosphate; MEP, Methylerythritol phosphate; CDP-ME, 4-Diphosphocytidyl-2-C-methylerythritol; CDP-MEP, 4-Diphosphocytidyl-2-C-methyl-d-erythritol 2-phosphate; MEcPP, 2-C-Methyl-d-erythritol-2,4-cyclopyrophosphate; HMBPP, (E)-4-Hydroxy-3-methyl-but-2-enyl pyrophosphate. PDS, Phytoene desaturase; ZISO, ζ-Carotene isomerase; ZDS, ζ-Carotene desaturase; CRTISO, Carotenoid isomerase.
| Product | Transgene | Target or Source | Production yield | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Promoter::Gene | Gene origin (Scientific name) | Cultivar | Organ | ||||||||
| Terpenoids | |||||||||||
| Phytoene | Gt1::Psy 35S::Psy | Psy (Narcissus pseduonarcissus) | Taipei 309 (Japonica) | Seed | Maximum 0.74 µg/g | Burkhardt et al, | |||||
| β-Carotene | Gt1::Psy_35S::Tp:CrtI_Gt1::Lcy | Psy (N. pseduonarcissus) CrtI (Pantoea ananas) Lcy (N. pseduonarcissus) | Taipei 309 (Japonica) | Seed | 1.6 µg/g | Ye et al, | |||||
| β-Carotene | Gt-1::Psy_35S::Tp:CrtI | Psy (N. pseduonarcissus) CrtI (P. ananas) | Taipei 309 (Japonica) IR64 (Indica) | Seed | Taipei 309: 1.2 µg/g IR64: 0.4, 0.8 µg/g | Hoa et al, | |||||
| β-Carotene | Glu::Tp:SSUCrtI_Glu::ZmPsy | Psy (Zea mays) CrtI (P. ananas) | Kaybonnet (Indica) | Callus & seed | 37 µg/g | Paine et al, | |||||
| β-Carotene | Glb::Psy:2A:Tp:CrtI (PAC) Glb::Psy:IRES:Tp:CrtI (PIC) | Psy (Capsicum annuum) CrtI (P. ananas) | Nackdong (Japonica) | Seed | 1.30 µg/g (PAC) 2.25 µg/g (PAC, T8) 0.15 µg/g (PIC) | Ha et al, | |||||
| Carotenoid | LMW::ZmPSY1 (P) LMW::ZmPSY1_RP5::AtDXS (T) LMW::ZmPSY1_LMW::AtOR (M) LMW::ZmPSY1_D-hordein::Tp:PaCrtI (L) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_RP5::AtDXS (D) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_LMW::AtOR (O) LWM::ZmPSY1_D-hordein:: Tp:PaCrtI_γ-zein::Tp:sCrBKT (B) | PSY1 (Z. mays) CrtI (P. ananas) DXS (Arabidopsis thaliana) OR (A. thaliana) BKT (Chlamydomonas reinhardtii) | Not mentioned | Callus | 4.7 ± 0.9 μg/g (P) 84.8 ± 4.3 μg/g (T) 144.8 ± 19.6 μg/g (M) 164.4 ± 26.8 μg/g (L) 323.4 ± 15 μg/g (D) 355.3 ± 70.6 μg/g (O) 277.6 ± 0.6 μg/g (B) | Bai et al, | |||||
| β-Carotene | Glb::stPsy:2A:Tp:stCrtI (stPAC) | Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Hwayoung (Japonica) | Seed | 3.50 µg/g (stPAC) 4.18 µg/g (stPAC, T5) | Jeong et al, | |||||
| β-Carotene | Ubi::OsCCD1-Ri Ubi::OsCCD4a-Ri Ubi::OsCCD4b-Ri Ubi::OsCCD1-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD1-Ri × stPAC) Ubi::OsCCD4a-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD4a-Ri × stPAC) Ubi::OsCCD4b-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD4b-Ri × stPAC) | CCD1 (Oryza sativa) CCD4a (O. sativa) CCD4b (O. sativa) Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Ilmi (Japonica) | Leaf & seed | 1.4-fold higher in seed (OsCCD1-Ri × stPAC) 1.3-fold higher in leaf (OsCCD4a-Ri × stPAC) 1.6-fold higher in seed (OsCCD4b-Ri × stPAC) | Ko et al, | |||||
| β-Carotene | PGD1::OsDXS2 PGD1::OsDXR PGD1::OsDXS2_Glb::stPsy:2A: Tp:stCrtI (OsDXS2_stPAC) PGD1::OsDXR_Glb::stPsy:2A: Tp:stCrtI (OsDXR_stPAC) | DXS2 (O. sativa) DXR (O. sativa) Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Ilmi (Japonica) | Seed | 21.7 µg/g, 315.3-fold higher than WT (OsDXS2_stPAC) | You et al, | |||||
| β-Carotene | Glb::stPsy:T2A:PTp:stCrtI (stPTAC) Glb::stPsy:T2A:R3Tp:stCrtI (stPTARC) | Psy (C. annuum) CrtI (P. ananas) T2A (Thosea asigna virus) | Dongjin (Japonica) | Seed | 2.56 µg/g (stPTAC) 2.73 µg/g (stPTARC) | Lee et al, | |||||
| Carotenoid | LMW::ZmPSY1_D-hordein::Tp:PaCrtI (L) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_RP5::AtDXS (D) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_LMW::AtOR (O) | PSY1 (Z. mays) CrtI (P. ananas) DXS (A. thaliana) OR (A. thaliana) | EYI105 (Japonica) | Seed | 5.43, 5.51, 4.61 µg/g (L) 17.79, 14.94, 31.78 µg/g (D) 11.53, 18.59, 25. 83 µg/g (O) | Bai et al, | |||||
| Carotenoid | GluB1::GtHMG1_GluB1::GZmPsy1_ GluB1::Tp:GPaCrtI | HMG1 (Saccharomyces cerevisiae) Psy1 (Z. mays) CrtI (P. ananas) | Wuyun 8 (Japonica) | Seed | HPC: 14.2 µg/g | Tian et al, | |||||
| Astaxanthin | LWM::ZmPSY1_D-hordein::Tp: PaCrtI_γ-zein::Tp:sCrBKT | PSY1 (Z. mays) CrtI (P. ananas) BKT (C. reinhardtii) | EYI105 (Japonica) | Seed | 65.7%‒71.2% ketocarotenoids of the total carotenoids | Bai et al, | |||||
| β-Carotene Canthaxanthin Astaxanthin | Glb1::sZmPsy1_GluB4::Tp:sPaCrtI (GR) Glb1::sZmPsy1_GluB4::Tp:sPaCrtI_ GluC::Tp:sCrBKT (CR) Glb1::sZmPsy1_GluB4::Tp:sPaCrtI_ GluC::Tp:sCrBKT_GluB1:: Tp:sHpBHY (AR) | Psy1 (Z. mays) CrtI (P. ananas) BKT (C. reinhardtii) BHY (Haematococcus pluvialis) | Huaguang 1 (Indica) | Seed | 24.73 µg/g β-carotene (GR-H1) 25.80 µg/g canthaxanthin (CR-H2) 16.23 µg/g astaxanthin (AR-H8) | Zhu et al, | |||||
| Zeaxanthin Astaxanthin Capsanthin | Glb::CaBch_Glb::CaPsy:2A:Tp: PaCrtI (B-PAC) Glb::stBch_Glb::CaPsy:2A:Tp: PaCrtI (stB-PAC) Glb::CaBch:2A:Tp:HpBkt_Glb:: CaPsy:2A:Tp:PaCrtI (BAK-PAC) Glb::stBch:2A:Tp:stBkt_Glb::CaPsy: 2A:Tp:PaCrtI (stBAK-PAC) Glb::CaCcs (Ccs) | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) Ccs (C. annuum) | Hwayoung (Japonica) | Seed | 0.83 µg/g zeaxanthin (45% of total carotenoids) (B-PAC) 1.37 µg/g ketocarotenoids, astaxanthin, adonixanthin (77% of total carotenoids) (stBAK-PAC) 0.37 µg/g ketoxanthophylls, capsanthin, capsorubin (17% of total carotenoids) (B-PAC × Ccs) | Ha et al, | |||||
| β-Carotene Zeaxanthin Astaxanthin | Glb::stPsy:T2A:Tp:stCrtI (stPTAC) Glb::stPsy:I2A2:Tp:stCrtI (stPIAC) Glb::stPsy:T2A:Tp:stCrtI:I2A1:stBch Glb::stPsy:T2A:Tp:stCrtI:I2A1:Tp:stBkt Glb::stPsy:T2A:Tp:stCrtI:I2A1:stBch: I2A2:Tp:stBkt | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) T2A (T. asigna virus) I2A1, I2A2 (Infectious myonecrosis virus) | Ilmi (Japonica) | Seed | 0.81 µg/g β-carotene (stPTAC) 0.40 µg/g β-carotene (stPIAC) 0.60 µg/g zeaxanthin 0.41 µg/g adonixanthin 0.11 µg/g astaxanthin | Jeong et al, | |||||
| Astaxanthin Capsanthin | Glb::CaBch:2A:Tp:HpBkt_Glb::CaPsy: 2A:Tp:PaCrtI × (Glb::CaCcs × Glb:: CaBch_Glb::CaPsy:2A:Tp:PaCrtI) (BP × CB) (Glb::CaCcs × Glb:: CaBch_Glb:: CaPsy: 2A:Tp:PaCrtI) × stBAK-PAC: Glb::stBch:2A:Tp:stBkt_Glb::CaPsy: 2A:Tp:PaCrtI (CB × sBP) | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) Ccs (C. annuum) | Hwayoung (Japonica) | Seed | 1.57 ± 0.12 μg/g carotenoid with 18.5% capsanthin and capsorubin of total caroteonoid (BP × CB) 52.3% astaxanthin of total carotenoid (CB ×sBP) | Jeong et al, | |||||
| Coenzyme Q10 | 35S::ddsA (no targeting) 35S::S14:ddsA (mitochondria-targeting) 35S::CTS:ddsA (Golgi-targeting) | ddsA (Gluconobacter oxydans) | Nipponbare (Japonica) | Leaf | 40‒70 µg/g in leaves (S14:ddsA T1) 12 µg/g in seeds (S14:ddsA T2) | Takahashi et al, | |||||
| Coenzyme Q10 | 35S::S14:ddsA (mitochondria-targeting) | ddsA (G. oxydans) | Haiibuki (Japonica) Chukei-toku 70 (Japonica) Nipponbare (Japonica) | Bran, germ, & seed | 11.0 ± 0.4 μg/g in seed 63.3 ± 3.6 μg/g in bran 180 ± 8.1 μg/g in germ (S14:ddsA, Nipponbare brown rice) 16.8 ± 4.5 μg/g in seed (S14:ddsA, Haiibuki) 22.1 ± 8.3 μg/g in seed (S14:ddsA, Toku 70) | Takahashi et al, | |||||
| Coenzyme Q10 | 35S::S14:ddsA (mitochondria-targeting) | ddsA (G. oxydans) | Sugary (Japonica) Shrunken (Japonica) | Seed | 34.5 ± 15.3 μg/g in seed (S14:ddsA, Sugary mutants) 28.1 ± 14.3 μg/g in seed (S14:ddsA, Shrunken mutants) | Takahashi et al, | |||||
| β-Amyrin | Ubi::AsbAS1 | AS1 (Avena strigosa) | Nipponbare (Japonica) | Root & leaf | ‒ | Inagaki et al, | |||||
| Sapogenins (oleanane-type) | Ubi::βAS | βAS (Panax japonicus) | Taijing 9 (Japonica) | Seed | 83‒115 μg/g | Huang et al, | |||||
| Sapogenins (dammarane-type) | Ubi::OPDS | DS (Panax ginseng) | Shuhui 527 (Indica) | Seed | 0.35‒0.59 mg/g dammarane-type sapogenin 20(S)-protopanaxadiol 0.23‒0.43 mg/g dammarane-type sapogenin 20(S)-protopanaxatriol | Huang et al, | |||||
| Protopanaxadiol (dammarane-type triterpenoid sapoins) | Glb::CYP716A47_Glb::PgDDS | CYP716A47 (P. ginseng) PgDDS (P. ginseng) | Dongjin (Japonica) | Seed | 16.4 µg/g protopanaxadiol 4.5 µg/g dammarenediol-II | Han et al, | |||||
| Flavonoids | |||||||||||
| Flavonoid | ProP::C1_ProP::R-S | C1 (Z. mays) R-S (Z. mays) | HwaYoung (Japonica) | Kernel | 30 times higher than wild type 6 times higher than black rice | Shin et al, | |||||
| Flavonoid | Gt1::Lc | Lc (Z. mays) | Chao2-10 (Japonica) Qingjiaozidao (Japonica) | Seed | 4.15 times higher than wild type (Chao2-10) 1.42 times higher than wild type (Qingjiaozidao) | Song et al, | |||||
| Anthocyanin | (ocs)3mas::OsANS | ANS (O. sativa) | Nootripathu (Indica) | Internode, leaf sheath, husk, & pericarp | 1.60‒2.50 µg/mg (Pericarp) 0.56‒1.32 µg/mg (Husk) 0.37‒1.01 µg/mg (Leaf sheath) 0.31‒0.79 µg/mg (Internode) | Reddy et al, | |||||
| Anthocyanin | GluC::ZmPl_Glub1::ZmLc_Glub4:: SsF3H_Glb1::SsDFR_Glub5::SsCHI_npr33::SsANS_10KDa::SsF3'H_ 16KDa::SsCHS | Pl (Z. mays) Lc (Z. mays) CHS (Solenostemon scutellarioides) CHI (S. scutellarioides) F3H (S. scutellarioides) F3'H (S. scutellarioides) DFR (S. scutellarioides) ANS (S. scutellarioides) | Zhonghua 11 (Japonica) Huaguang 1 (Indica) | Seed | ~1 mg/g | Zhu et al, | |||||
| Genistein | 35S::IFS | IFS (Glycine max) | Murasaki R86 (Japonica) | Leaf & root | _ | Sreevidya et al, | |||||
| Genistein | 35S::GmIFS | IFS (G. max) | ASD16 (Indica) | Leaf | 11.0 and 8.0 µg/g in leaf | Nayeem et al, | |||||
| Naringenin kaempferol Genistein Apigenin Tricin | GluB-1::OsPAL_GluB-1::OsCHS (Naringenin rice) 18-kDa::OsPAL_18-kDa::OsCHS (Naringenin rice) GluB-1::AtF3H_GluB-1::AtFLS_ GluB-1::OsPAL_GluB-1::OsCHS (Kaempferol rice) 18-kDa::AtF3H_18-kDa::AtFLS_ 18-kDa::OsPAL_18-kDa::OsCHS (Kaempferol rice) GluB-1::GmIFS_GluB-1::OsPAL_ GluB-1::OsCHS (Genistein rice) 18-kDa::GmIFS_18-kDa::OsPAL_ 18-kDa::OsCHS (Genistein rice) GluB-1::PoFNSI_GluB-1::GmFNSII_ GluB-1::OsPAL_GluB-1::OsCHS (Apigenin rice) 18-kDa::PoFNSI_18-kDa::GmFNSII_ 18-kDa::OsPAL_18-kDa::OsCHS (Apigenin rice) GluB-1::OsOMT_GluB-1::Viola F3'5'H_ GluB-1::PoFNSI_GluB-1::GmFNSII_ GluB-1::OsPAL_GluB-1::OsCHS (Tricin rice) | PAL (O. sativa) CHS (O. sativa) FLS (A. thaliana) F3H (A. thaliana) IFS (G. max) FNSI (Petroselinum crispum) FNSII (G. max) F3'5'H (Viola cornuta) OMT (O. sativa) | Kitaake (Japonica) | Seed | 1‒12 µg/g naringenin (Naringenin rice, GluB-1) 1‒70 µg/g naringenin (Naringenin rice, 18-kDa) 10‒60 µg/g kaempferol (Kaempferol rice, GluB-1) 10‒700 µg/g kaempferol (Kaempferol rice, 18-kDa) 10‒40 µg/g genistein (Genistein rice, GluB-1) 10‒350 µg/g genistein (Genistein rice, 18-kDa) 40‒120 µg/g apigenin (Apigenin rice, GluB-1) 5‒60 µg/g apigenin (Apigenin rice, 18-kDa) Maximum 110 µg/g tricin (Tricin rice) | Ogo et al, | |||||
| Non-flavonoid polyphenols & Betalains | |||||||||||
| Resveratrol | Ubi1::AhSTS1 | STS1 (Arachis hypogaea) | Dongjin (Japonica) | Leaf & seed | 1.9 µg/g in seeds 0-8.9 µg/g in leaves | Baek et al, | |||||
| Vanillin | 35S::VpVAN | VAN (Vanilla planifolia) | Taipei 309 (Japonica) | Callus | 544. 72 ± 102.50 µg/g in fresh calli | Arya et al, | |||||
| Betanin | GluB-1::GmeloS_GluB-1:: GBvDODA1S_GluB-1:: GBvCYP76AD1S | melo (Aspergilus oryzae) DODA1 (Bambusa vulgaris) CYP76AD1 (B. vulgaris) | Zhonghua 11 (Japonica) | Seed | 159.5 µg/g | Tian et al, | |||||
| Vitamins | |||||||||||
| Vitamin B9 (Folate) | Glb-1::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) | Nipponbare (Japonica) | Seed | 38.3 nmol/g | Storozhenko et al, 2007 | |||||
| Vitamin B9 (Folate) | Ubi::HPPK/DHPS | HPPK/DHPS (Triticum) | Jarrah | Leaf & seed | 2.1 ± 0.32 µg/g (1.2- to 2.0-fold) in leaf; 0.6 µg/g in seed | Gillies et al, | |||||
| Vitamin B9 (Folate) | Glb-1::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) | Nipponbare (Japonica) | Seed | Highest at 12 d post-anthesis at 27.76 ± 3.85 µg/g | Blancquaert et al, 2013 | |||||
| Vitamin B9 (Folate) | GluB1::mtFPGS_Glob::GTPCHI_ GluB1::ADCS GluB1::ctFPGS_Glob::GTPCHI_ GluB1::ADCS GluB4::sFBP_Glob::GTPCHI_ GluB1::ADCS GluB4::CAFBP_Glob::GTPCHI_ GluB1::ADCS GluB4::GluB4FBP_Glob::GTPCHI_ GluB1::ADCS GluB4::sFBP_GluB1::mtFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::CAFBP_GluB1::mtFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::GluB4FBP_GluB1::mtFPGS_Glob::GTPCHI_GluB1::ADCS GluB4::sFBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::CAFBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::GluB4FBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) mtFPGS (A. thaliana) ctFPGS (A. thaliana) sFBP (A. thaliana) CAFBP (A. thaliana) GluB4FBP (O. sativa) | Nipponbare (Japonica) | Seed | Intermediate level of 5 µg/g | Blancquaert et al, 2015 | |||||
| Vitamin E (α-Tocopherol) | Ubi-1::HPPD | HPPD (A. thaliana) | EYI105 (Japonica) | Seed | Increase γ to α-tocopherol shift rate | Farré et al, | |||||
| Vitamin E (α-Tocotrienol) | Ubi::AtTMT Gt1::AtTMT | TMT (A. thaliana) | Wuyujing 3 (Japonica) | Seed | 8.5‒31.5-fold increase γ to α-tocotrienol shift rate (Ubi::AtTMT) 4.0‒8.0-fold increase γ to α-tocotrienol shift rate (Gt1::AtTMT) | Zhang et al, | |||||
| Vitamin B6 (Pyridoxine) | 35S::AtPDX1.1_35S::AtPDX2 Glob::AtPDX1.1_Glob::AtPDX2 | PDX1.1 (A. thaliana) PDX2 (A. thaliana) | Taipei 309 (Japonica) | Leaf, root, & seed | 28.3-fold in leaves (35S) 12.0-fold in roots (35S) 3.1-fold in seeds (35S) Similar to 35S line (Glob) | Mangel et al, | |||||
| Vitamin B1 (Thiamin) | Glub1::THIC (THIC) Glob::THI1_Glub1::THIC (THIC, THI1) Glub1::TH1_Glob::THI1_Glub1::THIC (THIC, THI1, TH1) | THIC (A. thaliana) THI1 (A. thaliana) TH1 (A. thaliana) | Nipponbare (Japonica) | Seed | 3 times higher than wild type (THIC) 5 times higher than wild type (THIC, THI1) 2.4‒2.6 times higher than wild type (THIC, THI1, TH1) | Strobbe et al, | |||||
| Vitamin B2 (Riboflavin) | GluB-1::rScRIB1S_GluB-1:: rScRIB7S_ GluB-1::rScRIB2S_GluB-1::rScRIB3S_ GluB-1::rScRIB4S_GluB-1::rScRIB5S | RIB1‒RIB5, RIB7 (S. cerevisiae) | Zhonghua 11 (Japonica) | Seed | 2.17 μg/g in brown seeds | Tian et al, | |||||
| Amino acids & Amino acid derivatives | |||||||||||
| Lysine | 35S::Tp:dhps GluB-1::Tp:dhps | dhps (Z. mays) | Nagdongbyeo (Japonica) | Leaf & seed | 2.5-fold in tissues (35S) 2.0-fold at seed development (GluB-1) | Lee et al, | |||||
| Lysine | 35S::Tp:AK_35S::Tp:DHPS Gt1::lkr (LKR RNAi) 35S::Tp:AK_35S::Tp:DHPS_Gt1::lkr | AK (Escherichia coli strain TOC R21) DHPS (E. coli) LKR/SDH (O. sativa) | Wuxiangjing 9 (Japonica) | Leaf & seed | ~60-fold in mature seeds than wild type; 5- to 12-fold in leaves than wild type | Long et al, | |||||
| Lysine | 35S::Tp:AK_35S::Tp:DHPS × GluB-1::Tp:AK_Gt1::Tp:DHPS_ Gt1::lkr | AK (E. coli) DHPS (E. coli) LKR/SDH (O. sativa) | Wuxiangjing 9 (Japonica) | Seed | 25-fold in seeds than wild type | Yang et al, | |||||
| Lysine Threonine | 35S::TKTKK1 35S TKTKK2 | TKTKK1 (O. sativa) TKTKK2 (O. sativa) | Nipponbare (Japonica) | Seed | 33.87% of lysine, 21.21% of threonine, and 19.43% of total amino acid increase in TKTKK1; 12.90% of lysine, 13.63% of threonine, and 14.05% of total amino acid increase in TKTKK2 | Jiang et al, | |||||
| Cysteine Methionine | Glu::S2SA | S2SA (Sesamum indicum) | TNG67 (Japonica) | Seed | 29%‒76% higher methionine 3%‒75% higher cysteine | Lee et al, | |||||
| Cysteine Methionine | Ubi::Tp:EcSAT | SAT (E. coli) | Taipei 309 (Japonica) | Leaf & seed | 2.4-fold cysteine, 2-fold glutathione, 2.7-fold free methionine in leaves 1.4-fold free methionine in seeds 4.8-fold methionine bound to seed proteins | Nguyen et al, | |||||
| Tryptophan | Ubi1::OASA1 (D323N) | OASA1 (O. sativa) | Nipponbare (Japonica) | Callus & leaf | 2 832 nmol/g in calli 12 829 nmol/g in leaves | Tozawa et al, | |||||
| Serotonin | Ubi::AK30 Ubi::AK31 Ubi::AK53 | AK30 (O. sativa)-TYDC like AK31 (O. sativa)-TDC like AK53 (O. sativa)-TDC like | Dongjin (Japonica) | Leaf & seed | 25-fold higher in leaf (TDC like) 11-fold higher in seed (TDC like) | Kang et al, | |||||
| Serotonin Tryptamine | 35S::OsTDC_Ubi::OASA1D | TDC (O. sativa) OASA1D (O. sativa) | Nipponbare (Japonica) | Callus | 302 nmol/g of serotonin 140.7 nmol/g of tryptamine | Dubouzet et al, | |||||
| Melatonin | Ubi::TDC3 | TDC3 (O. sativa) | Dongjin (Japonica) | Seed & seedling | 1.24 ng/g in seed (31-fold higher than WT) 4.5 ng/g in seedling (2-fold higher than WT) | Byeon et al, | |||||
| Serotonin | Gt1::T5H Gt1::TDC1 Gt1::TDC3 Ubi::T5H Ubi::TDC1 Ubi::TDC3 | T5H (O. sativa) TDC1 (O. sativa) TDC3 (O. sativa) | Wuxiangjing 9 (Japonica) | Seed | 1 765 ± 326 μg/g of fresh milled seeds (Gt1::TDC1) 5.48-fold higher than wild type (Gt1 promoter) | Yang et al, | |||||
| Amino acids | 35S::OsAAT1 35S::OsAAT2 35S::EcAAT | AAT1, AAT2 (O. sativa) AAT (E. coli) | Zhonghua 11 (Japonica) | Leaf & seed | 119.36 mg/g in seeds (OsAAT1) 115.36 mg/g in seeds (OsAAT2) 113.72 mg/g in seeds (EcAAT) | Zhou et al, | |||||
| Amino acids | PGD1::OsMYBR22/OsRVE1 | MYBR22/RVE1 (O. sativa) | Dongjin (Japonica) | Seed | 11.9-fold higher lysine than wild type 8.6-fold higher threonine than wild type 8.0-fold higher γ-aminobutyric acid than wild type | Jeong et al, | |||||
Table 1. Summarized information used in the metabolic engineering of rice.
| Product | Transgene | Target or Source | Production yield | Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Promoter::Gene | Gene origin (Scientific name) | Cultivar | Organ | ||||||||
| Terpenoids | |||||||||||
| Phytoene | Gt1::Psy 35S::Psy | Psy (Narcissus pseduonarcissus) | Taipei 309 (Japonica) | Seed | Maximum 0.74 µg/g | Burkhardt et al, | |||||
| β-Carotene | Gt1::Psy_35S::Tp:CrtI_Gt1::Lcy | Psy (N. pseduonarcissus) CrtI (Pantoea ananas) Lcy (N. pseduonarcissus) | Taipei 309 (Japonica) | Seed | 1.6 µg/g | Ye et al, | |||||
| β-Carotene | Gt-1::Psy_35S::Tp:CrtI | Psy (N. pseduonarcissus) CrtI (P. ananas) | Taipei 309 (Japonica) IR64 (Indica) | Seed | Taipei 309: 1.2 µg/g IR64: 0.4, 0.8 µg/g | Hoa et al, | |||||
| β-Carotene | Glu::Tp:SSUCrtI_Glu::ZmPsy | Psy (Zea mays) CrtI (P. ananas) | Kaybonnet (Indica) | Callus & seed | 37 µg/g | Paine et al, | |||||
| β-Carotene | Glb::Psy:2A:Tp:CrtI (PAC) Glb::Psy:IRES:Tp:CrtI (PIC) | Psy (Capsicum annuum) CrtI (P. ananas) | Nackdong (Japonica) | Seed | 1.30 µg/g (PAC) 2.25 µg/g (PAC, T8) 0.15 µg/g (PIC) | Ha et al, | |||||
| Carotenoid | LMW::ZmPSY1 (P) LMW::ZmPSY1_RP5::AtDXS (T) LMW::ZmPSY1_LMW::AtOR (M) LMW::ZmPSY1_D-hordein::Tp:PaCrtI (L) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_RP5::AtDXS (D) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_LMW::AtOR (O) LWM::ZmPSY1_D-hordein:: Tp:PaCrtI_γ-zein::Tp:sCrBKT (B) | PSY1 (Z. mays) CrtI (P. ananas) DXS (Arabidopsis thaliana) OR (A. thaliana) BKT (Chlamydomonas reinhardtii) | Not mentioned | Callus | 4.7 ± 0.9 μg/g (P) 84.8 ± 4.3 μg/g (T) 144.8 ± 19.6 μg/g (M) 164.4 ± 26.8 μg/g (L) 323.4 ± 15 μg/g (D) 355.3 ± 70.6 μg/g (O) 277.6 ± 0.6 μg/g (B) | Bai et al, | |||||
| β-Carotene | Glb::stPsy:2A:Tp:stCrtI (stPAC) | Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Hwayoung (Japonica) | Seed | 3.50 µg/g (stPAC) 4.18 µg/g (stPAC, T5) | Jeong et al, | |||||
| β-Carotene | Ubi::OsCCD1-Ri Ubi::OsCCD4a-Ri Ubi::OsCCD4b-Ri Ubi::OsCCD1-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD1-Ri × stPAC) Ubi::OsCCD4a-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD4a-Ri × stPAC) Ubi::OsCCD4b-Ri × Glb::stPsy:2A: Tp:stCrtI (OsCCD4b-Ri × stPAC) | CCD1 (Oryza sativa) CCD4a (O. sativa) CCD4b (O. sativa) Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Ilmi (Japonica) | Leaf & seed | 1.4-fold higher in seed (OsCCD1-Ri × stPAC) 1.3-fold higher in leaf (OsCCD4a-Ri × stPAC) 1.6-fold higher in seed (OsCCD4b-Ri × stPAC) | Ko et al, | |||||
| β-Carotene | PGD1::OsDXS2 PGD1::OsDXR PGD1::OsDXS2_Glb::stPsy:2A: Tp:stCrtI (OsDXS2_stPAC) PGD1::OsDXR_Glb::stPsy:2A: Tp:stCrtI (OsDXR_stPAC) | DXS2 (O. sativa) DXR (O. sativa) Psy (C. annuum) CrtI (P. ananas) 2A (Foot-and-mouth disease virus) | Ilmi (Japonica) | Seed | 21.7 µg/g, 315.3-fold higher than WT (OsDXS2_stPAC) | You et al, | |||||
| β-Carotene | Glb::stPsy:T2A:PTp:stCrtI (stPTAC) Glb::stPsy:T2A:R3Tp:stCrtI (stPTARC) | Psy (C. annuum) CrtI (P. ananas) T2A (Thosea asigna virus) | Dongjin (Japonica) | Seed | 2.56 µg/g (stPTAC) 2.73 µg/g (stPTARC) | Lee et al, | |||||
| Carotenoid | LMW::ZmPSY1_D-hordein::Tp:PaCrtI (L) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_RP5::AtDXS (D) LMW::ZmPSY1_D-hordein:: Tp:PaCrtI_LMW::AtOR (O) | PSY1 (Z. mays) CrtI (P. ananas) DXS (A. thaliana) OR (A. thaliana) | EYI105 (Japonica) | Seed | 5.43, 5.51, 4.61 µg/g (L) 17.79, 14.94, 31.78 µg/g (D) 11.53, 18.59, 25. 83 µg/g (O) | Bai et al, | |||||
| Carotenoid | GluB1::GtHMG1_GluB1::GZmPsy1_ GluB1::Tp:GPaCrtI | HMG1 (Saccharomyces cerevisiae) Psy1 (Z. mays) CrtI (P. ananas) | Wuyun 8 (Japonica) | Seed | HPC: 14.2 µg/g | Tian et al, | |||||
| Astaxanthin | LWM::ZmPSY1_D-hordein::Tp: PaCrtI_γ-zein::Tp:sCrBKT | PSY1 (Z. mays) CrtI (P. ananas) BKT (C. reinhardtii) | EYI105 (Japonica) | Seed | 65.7%‒71.2% ketocarotenoids of the total carotenoids | Bai et al, | |||||
| β-Carotene Canthaxanthin Astaxanthin | Glb1::sZmPsy1_GluB4::Tp:sPaCrtI (GR) Glb1::sZmPsy1_GluB4::Tp:sPaCrtI_ GluC::Tp:sCrBKT (CR) Glb1::sZmPsy1_GluB4::Tp:sPaCrtI_ GluC::Tp:sCrBKT_GluB1:: Tp:sHpBHY (AR) | Psy1 (Z. mays) CrtI (P. ananas) BKT (C. reinhardtii) BHY (Haematococcus pluvialis) | Huaguang 1 (Indica) | Seed | 24.73 µg/g β-carotene (GR-H1) 25.80 µg/g canthaxanthin (CR-H2) 16.23 µg/g astaxanthin (AR-H8) | Zhu et al, | |||||
| Zeaxanthin Astaxanthin Capsanthin | Glb::CaBch_Glb::CaPsy:2A:Tp: PaCrtI (B-PAC) Glb::stBch_Glb::CaPsy:2A:Tp: PaCrtI (stB-PAC) Glb::CaBch:2A:Tp:HpBkt_Glb:: CaPsy:2A:Tp:PaCrtI (BAK-PAC) Glb::stBch:2A:Tp:stBkt_Glb::CaPsy: 2A:Tp:PaCrtI (stBAK-PAC) Glb::CaCcs (Ccs) | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) Ccs (C. annuum) | Hwayoung (Japonica) | Seed | 0.83 µg/g zeaxanthin (45% of total carotenoids) (B-PAC) 1.37 µg/g ketocarotenoids, astaxanthin, adonixanthin (77% of total carotenoids) (stBAK-PAC) 0.37 µg/g ketoxanthophylls, capsanthin, capsorubin (17% of total carotenoids) (B-PAC × Ccs) | Ha et al, | |||||
| β-Carotene Zeaxanthin Astaxanthin | Glb::stPsy:T2A:Tp:stCrtI (stPTAC) Glb::stPsy:I2A2:Tp:stCrtI (stPIAC) Glb::stPsy:T2A:Tp:stCrtI:I2A1:stBch Glb::stPsy:T2A:Tp:stCrtI:I2A1:Tp:stBkt Glb::stPsy:T2A:Tp:stCrtI:I2A1:stBch: I2A2:Tp:stBkt | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) T2A (T. asigna virus) I2A1, I2A2 (Infectious myonecrosis virus) | Ilmi (Japonica) | Seed | 0.81 µg/g β-carotene (stPTAC) 0.40 µg/g β-carotene (stPIAC) 0.60 µg/g zeaxanthin 0.41 µg/g adonixanthin 0.11 µg/g astaxanthin | Jeong et al, | |||||
| Astaxanthin Capsanthin | Glb::CaBch:2A:Tp:HpBkt_Glb::CaPsy: 2A:Tp:PaCrtI × (Glb::CaCcs × Glb:: CaBch_Glb::CaPsy:2A:Tp:PaCrtI) (BP × CB) (Glb::CaCcs × Glb:: CaBch_Glb:: CaPsy: 2A:Tp:PaCrtI) × stBAK-PAC: Glb::stBch:2A:Tp:stBkt_Glb::CaPsy: 2A:Tp:PaCrtI (CB × sBP) | Psy (C. annuum) CrtI (P. ananas) Bch (C. annuum) Bkt (H. pluvialis) Ccs (C. annuum) | Hwayoung (Japonica) | Seed | 1.57 ± 0.12 μg/g carotenoid with 18.5% capsanthin and capsorubin of total caroteonoid (BP × CB) 52.3% astaxanthin of total carotenoid (CB ×sBP) | Jeong et al, | |||||
| Coenzyme Q10 | 35S::ddsA (no targeting) 35S::S14:ddsA (mitochondria-targeting) 35S::CTS:ddsA (Golgi-targeting) | ddsA (Gluconobacter oxydans) | Nipponbare (Japonica) | Leaf | 40‒70 µg/g in leaves (S14:ddsA T1) 12 µg/g in seeds (S14:ddsA T2) | Takahashi et al, | |||||
| Coenzyme Q10 | 35S::S14:ddsA (mitochondria-targeting) | ddsA (G. oxydans) | Haiibuki (Japonica) Chukei-toku 70 (Japonica) Nipponbare (Japonica) | Bran, germ, & seed | 11.0 ± 0.4 μg/g in seed 63.3 ± 3.6 μg/g in bran 180 ± 8.1 μg/g in germ (S14:ddsA, Nipponbare brown rice) 16.8 ± 4.5 μg/g in seed (S14:ddsA, Haiibuki) 22.1 ± 8.3 μg/g in seed (S14:ddsA, Toku 70) | Takahashi et al, | |||||
| Coenzyme Q10 | 35S::S14:ddsA (mitochondria-targeting) | ddsA (G. oxydans) | Sugary (Japonica) Shrunken (Japonica) | Seed | 34.5 ± 15.3 μg/g in seed (S14:ddsA, Sugary mutants) 28.1 ± 14.3 μg/g in seed (S14:ddsA, Shrunken mutants) | Takahashi et al, | |||||
| β-Amyrin | Ubi::AsbAS1 | AS1 (Avena strigosa) | Nipponbare (Japonica) | Root & leaf | ‒ | Inagaki et al, | |||||
| Sapogenins (oleanane-type) | Ubi::βAS | βAS (Panax japonicus) | Taijing 9 (Japonica) | Seed | 83‒115 μg/g | Huang et al, | |||||
| Sapogenins (dammarane-type) | Ubi::OPDS | DS (Panax ginseng) | Shuhui 527 (Indica) | Seed | 0.35‒0.59 mg/g dammarane-type sapogenin 20(S)-protopanaxadiol 0.23‒0.43 mg/g dammarane-type sapogenin 20(S)-protopanaxatriol | Huang et al, | |||||
| Protopanaxadiol (dammarane-type triterpenoid sapoins) | Glb::CYP716A47_Glb::PgDDS | CYP716A47 (P. ginseng) PgDDS (P. ginseng) | Dongjin (Japonica) | Seed | 16.4 µg/g protopanaxadiol 4.5 µg/g dammarenediol-II | Han et al, | |||||
| Flavonoids | |||||||||||
| Flavonoid | ProP::C1_ProP::R-S | C1 (Z. mays) R-S (Z. mays) | HwaYoung (Japonica) | Kernel | 30 times higher than wild type 6 times higher than black rice | Shin et al, | |||||
| Flavonoid | Gt1::Lc | Lc (Z. mays) | Chao2-10 (Japonica) Qingjiaozidao (Japonica) | Seed | 4.15 times higher than wild type (Chao2-10) 1.42 times higher than wild type (Qingjiaozidao) | Song et al, | |||||
| Anthocyanin | (ocs)3mas::OsANS | ANS (O. sativa) | Nootripathu (Indica) | Internode, leaf sheath, husk, & pericarp | 1.60‒2.50 µg/mg (Pericarp) 0.56‒1.32 µg/mg (Husk) 0.37‒1.01 µg/mg (Leaf sheath) 0.31‒0.79 µg/mg (Internode) | Reddy et al, | |||||
| Anthocyanin | GluC::ZmPl_Glub1::ZmLc_Glub4:: SsF3H_Glb1::SsDFR_Glub5::SsCHI_npr33::SsANS_10KDa::SsF3'H_ 16KDa::SsCHS | Pl (Z. mays) Lc (Z. mays) CHS (Solenostemon scutellarioides) CHI (S. scutellarioides) F3H (S. scutellarioides) F3'H (S. scutellarioides) DFR (S. scutellarioides) ANS (S. scutellarioides) | Zhonghua 11 (Japonica) Huaguang 1 (Indica) | Seed | ~1 mg/g | Zhu et al, | |||||
| Genistein | 35S::IFS | IFS (Glycine max) | Murasaki R86 (Japonica) | Leaf & root | _ | Sreevidya et al, | |||||
| Genistein | 35S::GmIFS | IFS (G. max) | ASD16 (Indica) | Leaf | 11.0 and 8.0 µg/g in leaf | Nayeem et al, | |||||
| Naringenin kaempferol Genistein Apigenin Tricin | GluB-1::OsPAL_GluB-1::OsCHS (Naringenin rice) 18-kDa::OsPAL_18-kDa::OsCHS (Naringenin rice) GluB-1::AtF3H_GluB-1::AtFLS_ GluB-1::OsPAL_GluB-1::OsCHS (Kaempferol rice) 18-kDa::AtF3H_18-kDa::AtFLS_ 18-kDa::OsPAL_18-kDa::OsCHS (Kaempferol rice) GluB-1::GmIFS_GluB-1::OsPAL_ GluB-1::OsCHS (Genistein rice) 18-kDa::GmIFS_18-kDa::OsPAL_ 18-kDa::OsCHS (Genistein rice) GluB-1::PoFNSI_GluB-1::GmFNSII_ GluB-1::OsPAL_GluB-1::OsCHS (Apigenin rice) 18-kDa::PoFNSI_18-kDa::GmFNSII_ 18-kDa::OsPAL_18-kDa::OsCHS (Apigenin rice) GluB-1::OsOMT_GluB-1::Viola F3'5'H_ GluB-1::PoFNSI_GluB-1::GmFNSII_ GluB-1::OsPAL_GluB-1::OsCHS (Tricin rice) | PAL (O. sativa) CHS (O. sativa) FLS (A. thaliana) F3H (A. thaliana) IFS (G. max) FNSI (Petroselinum crispum) FNSII (G. max) F3'5'H (Viola cornuta) OMT (O. sativa) | Kitaake (Japonica) | Seed | 1‒12 µg/g naringenin (Naringenin rice, GluB-1) 1‒70 µg/g naringenin (Naringenin rice, 18-kDa) 10‒60 µg/g kaempferol (Kaempferol rice, GluB-1) 10‒700 µg/g kaempferol (Kaempferol rice, 18-kDa) 10‒40 µg/g genistein (Genistein rice, GluB-1) 10‒350 µg/g genistein (Genistein rice, 18-kDa) 40‒120 µg/g apigenin (Apigenin rice, GluB-1) 5‒60 µg/g apigenin (Apigenin rice, 18-kDa) Maximum 110 µg/g tricin (Tricin rice) | Ogo et al, | |||||
| Non-flavonoid polyphenols & Betalains | |||||||||||
| Resveratrol | Ubi1::AhSTS1 | STS1 (Arachis hypogaea) | Dongjin (Japonica) | Leaf & seed | 1.9 µg/g in seeds 0-8.9 µg/g in leaves | Baek et al, | |||||
| Vanillin | 35S::VpVAN | VAN (Vanilla planifolia) | Taipei 309 (Japonica) | Callus | 544. 72 ± 102.50 µg/g in fresh calli | Arya et al, | |||||
| Betanin | GluB-1::GmeloS_GluB-1:: GBvDODA1S_GluB-1:: GBvCYP76AD1S | melo (Aspergilus oryzae) DODA1 (Bambusa vulgaris) CYP76AD1 (B. vulgaris) | Zhonghua 11 (Japonica) | Seed | 159.5 µg/g | Tian et al, | |||||
| Vitamins | |||||||||||
| Vitamin B9 (Folate) | Glb-1::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) | Nipponbare (Japonica) | Seed | 38.3 nmol/g | Storozhenko et al, 2007 | |||||
| Vitamin B9 (Folate) | Ubi::HPPK/DHPS | HPPK/DHPS (Triticum) | Jarrah | Leaf & seed | 2.1 ± 0.32 µg/g (1.2- to 2.0-fold) in leaf; 0.6 µg/g in seed | Gillies et al, | |||||
| Vitamin B9 (Folate) | Glb-1::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) | Nipponbare (Japonica) | Seed | Highest at 12 d post-anthesis at 27.76 ± 3.85 µg/g | Blancquaert et al, 2013 | |||||
| Vitamin B9 (Folate) | GluB1::mtFPGS_Glob::GTPCHI_ GluB1::ADCS GluB1::ctFPGS_Glob::GTPCHI_ GluB1::ADCS GluB4::sFBP_Glob::GTPCHI_ GluB1::ADCS GluB4::CAFBP_Glob::GTPCHI_ GluB1::ADCS GluB4::GluB4FBP_Glob::GTPCHI_ GluB1::ADCS GluB4::sFBP_GluB1::mtFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::CAFBP_GluB1::mtFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::GluB4FBP_GluB1::mtFPGS_Glob::GTPCHI_GluB1::ADCS GluB4::sFBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::CAFBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS GluB4::GluB4FBP_GluB1::ctFPGS_ Glob::GTPCHI_GluB1::ADCS | GTPCHI (A. thaliana) ADCS (A. thaliana) mtFPGS (A. thaliana) ctFPGS (A. thaliana) sFBP (A. thaliana) CAFBP (A. thaliana) GluB4FBP (O. sativa) | Nipponbare (Japonica) | Seed | Intermediate level of 5 µg/g | Blancquaert et al, 2015 | |||||
| Vitamin E (α-Tocopherol) | Ubi-1::HPPD | HPPD (A. thaliana) | EYI105 (Japonica) | Seed | Increase γ to α-tocopherol shift rate | Farré et al, | |||||
| Vitamin E (α-Tocotrienol) | Ubi::AtTMT Gt1::AtTMT | TMT (A. thaliana) | Wuyujing 3 (Japonica) | Seed | 8.5‒31.5-fold increase γ to α-tocotrienol shift rate (Ubi::AtTMT) 4.0‒8.0-fold increase γ to α-tocotrienol shift rate (Gt1::AtTMT) | Zhang et al, | |||||
| Vitamin B6 (Pyridoxine) | 35S::AtPDX1.1_35S::AtPDX2 Glob::AtPDX1.1_Glob::AtPDX2 | PDX1.1 (A. thaliana) PDX2 (A. thaliana) | Taipei 309 (Japonica) | Leaf, root, & seed | 28.3-fold in leaves (35S) 12.0-fold in roots (35S) 3.1-fold in seeds (35S) Similar to 35S line (Glob) | Mangel et al, | |||||
| Vitamin B1 (Thiamin) | Glub1::THIC (THIC) Glob::THI1_Glub1::THIC (THIC, THI1) Glub1::TH1_Glob::THI1_Glub1::THIC (THIC, THI1, TH1) | THIC (A. thaliana) THI1 (A. thaliana) TH1 (A. thaliana) | Nipponbare (Japonica) | Seed | 3 times higher than wild type (THIC) 5 times higher than wild type (THIC, THI1) 2.4‒2.6 times higher than wild type (THIC, THI1, TH1) | Strobbe et al, | |||||
| Vitamin B2 (Riboflavin) | GluB-1::rScRIB1S_GluB-1:: rScRIB7S_ GluB-1::rScRIB2S_GluB-1::rScRIB3S_ GluB-1::rScRIB4S_GluB-1::rScRIB5S | RIB1‒RIB5, RIB7 (S. cerevisiae) | Zhonghua 11 (Japonica) | Seed | 2.17 μg/g in brown seeds | Tian et al, | |||||
| Amino acids & Amino acid derivatives | |||||||||||
| Lysine | 35S::Tp:dhps GluB-1::Tp:dhps | dhps (Z. mays) | Nagdongbyeo (Japonica) | Leaf & seed | 2.5-fold in tissues (35S) 2.0-fold at seed development (GluB-1) | Lee et al, | |||||
| Lysine | 35S::Tp:AK_35S::Tp:DHPS Gt1::lkr (LKR RNAi) 35S::Tp:AK_35S::Tp:DHPS_Gt1::lkr | AK (Escherichia coli strain TOC R21) DHPS (E. coli) LKR/SDH (O. sativa) | Wuxiangjing 9 (Japonica) | Leaf & seed | ~60-fold in mature seeds than wild type; 5- to 12-fold in leaves than wild type | Long et al, | |||||
| Lysine | 35S::Tp:AK_35S::Tp:DHPS × GluB-1::Tp:AK_Gt1::Tp:DHPS_ Gt1::lkr | AK (E. coli) DHPS (E. coli) LKR/SDH (O. sativa) | Wuxiangjing 9 (Japonica) | Seed | 25-fold in seeds than wild type | Yang et al, | |||||
| Lysine Threonine | 35S::TKTKK1 35S TKTKK2 | TKTKK1 (O. sativa) TKTKK2 (O. sativa) | Nipponbare (Japonica) | Seed | 33.87% of lysine, 21.21% of threonine, and 19.43% of total amino acid increase in TKTKK1; 12.90% of lysine, 13.63% of threonine, and 14.05% of total amino acid increase in TKTKK2 | Jiang et al, | |||||
| Cysteine Methionine | Glu::S2SA | S2SA (Sesamum indicum) | TNG67 (Japonica) | Seed | 29%‒76% higher methionine 3%‒75% higher cysteine | Lee et al, | |||||
| Cysteine Methionine | Ubi::Tp:EcSAT | SAT (E. coli) | Taipei 309 (Japonica) | Leaf & seed | 2.4-fold cysteine, 2-fold glutathione, 2.7-fold free methionine in leaves 1.4-fold free methionine in seeds 4.8-fold methionine bound to seed proteins | Nguyen et al, | |||||
| Tryptophan | Ubi1::OASA1 (D323N) | OASA1 (O. sativa) | Nipponbare (Japonica) | Callus & leaf | 2 832 nmol/g in calli 12 829 nmol/g in leaves | Tozawa et al, | |||||
| Serotonin | Ubi::AK30 Ubi::AK31 Ubi::AK53 | AK30 (O. sativa)-TYDC like AK31 (O. sativa)-TDC like AK53 (O. sativa)-TDC like | Dongjin (Japonica) | Leaf & seed | 25-fold higher in leaf (TDC like) 11-fold higher in seed (TDC like) | Kang et al, | |||||
| Serotonin Tryptamine | 35S::OsTDC_Ubi::OASA1D | TDC (O. sativa) OASA1D (O. sativa) | Nipponbare (Japonica) | Callus | 302 nmol/g of serotonin 140.7 nmol/g of tryptamine | Dubouzet et al, | |||||
| Melatonin | Ubi::TDC3 | TDC3 (O. sativa) | Dongjin (Japonica) | Seed & seedling | 1.24 ng/g in seed (31-fold higher than WT) 4.5 ng/g in seedling (2-fold higher than WT) | Byeon et al, | |||||
| Serotonin | Gt1::T5H Gt1::TDC1 Gt1::TDC3 Ubi::T5H Ubi::TDC1 Ubi::TDC3 | T5H (O. sativa) TDC1 (O. sativa) TDC3 (O. sativa) | Wuxiangjing 9 (Japonica) | Seed | 1 765 ± 326 μg/g of fresh milled seeds (Gt1::TDC1) 5.48-fold higher than wild type (Gt1 promoter) | Yang et al, | |||||
| Amino acids | 35S::OsAAT1 35S::OsAAT2 35S::EcAAT | AAT1, AAT2 (O. sativa) AAT (E. coli) | Zhonghua 11 (Japonica) | Leaf & seed | 119.36 mg/g in seeds (OsAAT1) 115.36 mg/g in seeds (OsAAT2) 113.72 mg/g in seeds (EcAAT) | Zhou et al, | |||||
| Amino acids | PGD1::OsMYBR22/OsRVE1 | MYBR22/RVE1 (O. sativa) | Dongjin (Japonica) | Seed | 11.9-fold higher lysine than wild type 8.6-fold higher threonine than wild type 8.0-fold higher γ-aminobutyric acid than wild type | Jeong et al, | |||||
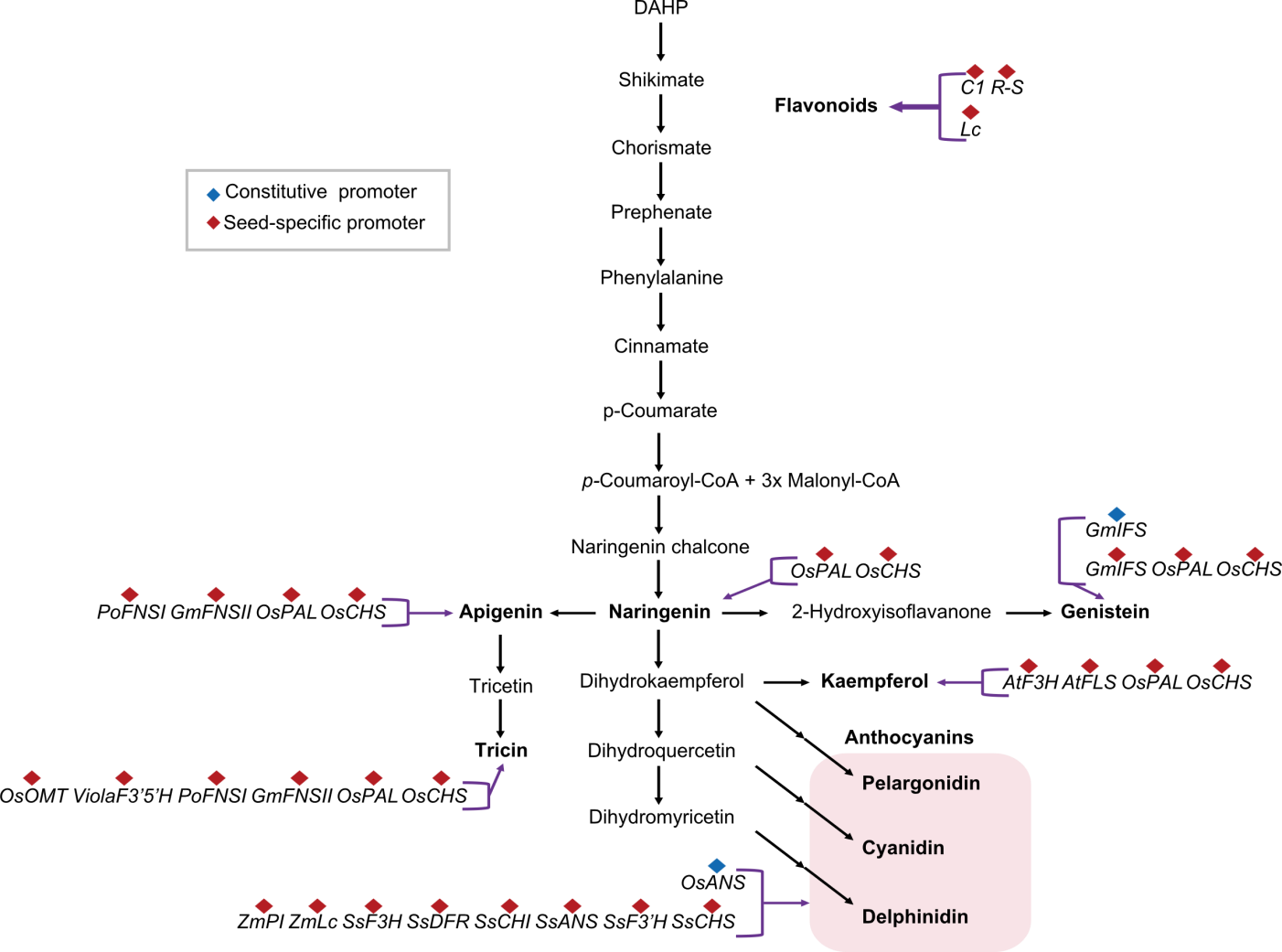
Fig. 2. Pathway of flavonoids produced by metabolic engineering in rice. DAHP, 3-Deoxy-d-arabinoheptulosonate 7-phosphate; p-Coumaroyl-CoA, p-Coumaroyl coenzyme A; Malonyl-CoA, Malonyl coenzyme A.
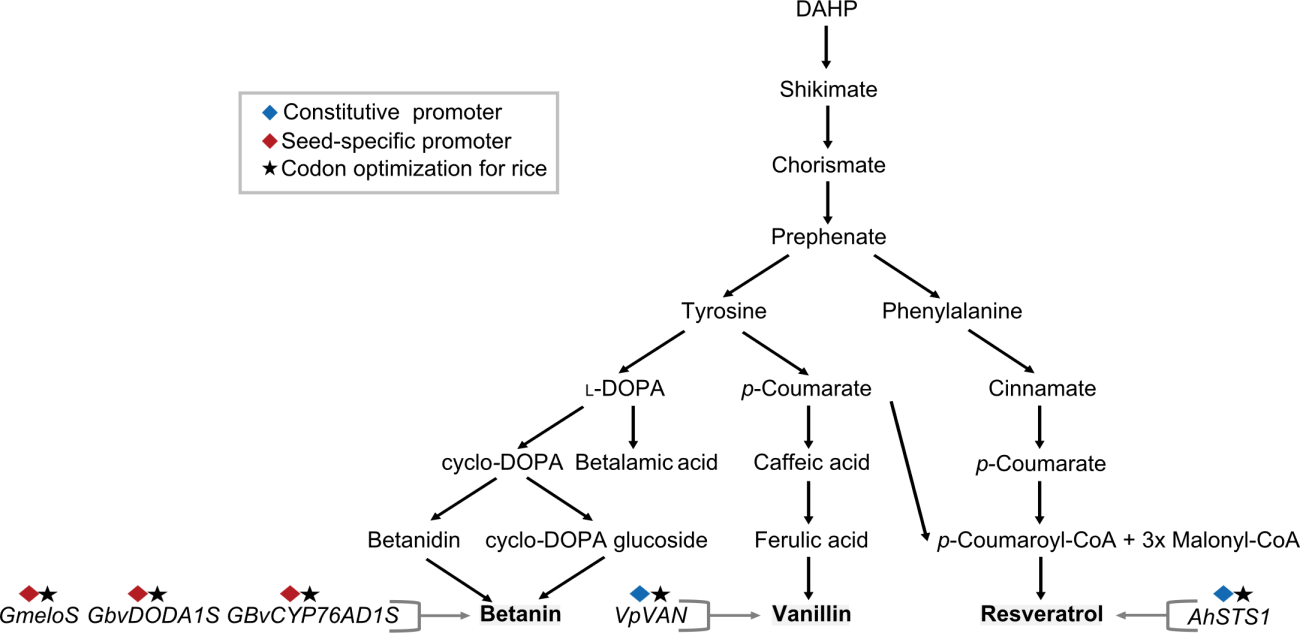
Fig. 3. Pathway of polyphenols and betalains produced by metabolic engineering in rice. DAHP, 3-Deoxy-d-arabinoheptulosonate 7-phosphate; l-DOPA, l-3,4-dihydroxyphenylalanine; cyclo-DOPA, (2S)-5,6-Dihydroxy-2,3-dihydro-1H-indole-2-carboxylic acid; p-Coumaroyl-CoA, p-Coumaroyl coenzyme A; Malonyl-CoA, Malonyl coenzyme.

Fig. 4. Pathway of vitamins produced by metabolic engineering in rice. DAHP, 3-Deoxy-d-arabinoheptulosonate 7-phosphate; ADC, Aminodeoxychorismate; p-ABA, p-Aminobenzoic acid; HPP, Hydroxyphenylpyruvate; HGA, Homogentisic acid; IPP, Isopentenyl pyrophosphate; DMAPP, Dimethylallyl pyrophosphate; GGPP, Geranylgeranyl diphosphate; PDP, Phytyl- diphosphate; DMPBQ, 2,3-Dimethyl-6-phytyl-1,4-benzoquinol; MPBQ, 2-Methyl-6-phytyl-1,4-benzoquinol; MGGBQ, 2-Methyl-6-geranylgeranyl-benzoquinol; DMGGBQ, 2-Dimethyl-6-geranylgeranylbenzoquinol; HMDHP, Hydroxymethyldihydropterine; HMDHP-PP, Hydroxymethyldihydrop- terin pyrophosphate; DHP, Dihydropteroate; DHF, Dihydrofolate; THF, Tetrahydrofolate; DHN, Dihydroneopterin; DHN-P, Dihydroneopterin phosphate; DHN-PPP, Dihydroneopterin triphosphate; GTP, Guanosine triphosphate; DARPP, 2,5-Diamino-6-ribosyl-amino-4(3H)pyrimidinedione 5′-phosphate; ARPP, 5-Amino-6-ribosyl-amino-2,4(1H,3H)pyrimidinedione 5′-phosphate; DArPP, 2,5-Diamino-6-ribityl-amino-4(3H)pyrimidinedione 5′-phosphate; ArPP, 5-Amino-6-ribityl-amino-2,4(1H,3H)pyrimidinedione 5′-phosphate; ArP, 5-Amino-6-ribityl-amino-2,4(1H,3H)pyrimidinedione; G6P, Glucose 6-phosphate; R5P, Ribose 5-phosphate; DHBP, 3,4-Dihydroxy-2-butanone-4-phosphate; DRL, 6,7-Dimethyl-8-ribityllumazine; G3P, Glyceraldehyde 3-phosphate; PNP, Pyridoxine 5′-phosphate; PLP, Pyridoxal 5′-phosphate; PMP, Pyridoxamine 5′-phosphate; PN, Pyridoxine; PL, Pyridoxal; PM, Pyridoxamine; PN-Glu, Pyridoxine glucosides; AIR, 5-Aminoimidazole ribonucleotide; SAM, S-adenosylmethionine; HMP-P, 4-Amino-2-methyl-5-hydroxymethylpyrimidine phosphate; HMP-PP, HMP-pyrophosphate; Gly, Glycine; NAD+, Nicotinamide adenine dinucleotide; HET-P, 4-Methyl-5-β-hydroxyethylthiazole phosphate; TMP, Thiamin monophosphate.

Fig. 5. Pathway of amino acids and amino acid derivatives produced by metabolic engineering in rice. DAHP, 3-Deoxy-d-arabinoheptulosonate 7-phosphate.
| [1] | Al-Karmalawy A A, Dahab M A, Metwaly A M, et al. 2021. Molecular docking and dynamics simulation revealed the potential inhibitory activity of ACEIs against SARS-CoV-2 targeting the hACE2 receptor. Front Chem, 9: 661230. |
| [2] | Alseekh S, de Souza L P, Benina M, et al. 2020. The style and substance of plant flavonoid decoration; towards defining both structure and function. Phytochemistry, 174: 112347. |
| [3] | Anand R, Mohan L, Bharadvaja N. 2022. Disease prevention and treatment using β-carotene: The ultimate provitamin A. Rev Bras Farmacogn, 32(4): 491-501. |
| [4] | Arya S S, Kumari D D, Rookes J E, et al. 2021a. Rice cell suspension culture as a model for producing high-value recombinant proteins and plant specialized metabolites. Plant Cell Tissue Organ Cult, 145(3): 463-486. |
| [5] | Arya S S, Rookes J E, Cahill D M, et al. 2021b. Vanillin: A review on the therapeutic prospects of a popular flavouring molecule. Adv Tradit Med, 21(3): 1-17. |
| [6] | Arya S S, Mahto B K, Sengar M S, et al. 2022. Metabolic engineering of rice cells with Vanillin Synthase gene (VpVAN) to produce vanillin. Mol Biotechnol, 64(8): 861-872. |
| [7] | Baek S H, Shin W C, Ryu H S, et al. 2013. Creation of resveratrol-enriched rice for the treatment of metabolic syndrome and related diseases. PLoS One, 8(3): e57930. |
| [8] | Baek S H, Chung H J, Lee H K, et al. 2014. Treatment of obesity with the resveratrol-enriched rice DJ-526. Sci Rep, 4: 3879. |
| [9] | Bai C, Rivera S M, Medina V, et al. 2014. An in vitro system for the rapid functional characterization of genes involved in carotenoid biosynthesis and accumulation. Plant J, 77(3): 464-475. |
| [10] | Bai C, Capell T, Berman J, et al. 2016. Bottlenecks in carotenoid biosynthesis and accumulation in rice endosperm are influenced by the precursor-product balance. Plant Biotechnol J, 14(1): 195-205. |
| [11] | Bai C, Berman J, Farre G, et al. 2017. Reconstruction of the astaxanthin biosynthesis pathway in rice endosperm reveals a metabolic bottleneck at the level of endogenous β-carotene hydroxylase activity. Transgenic Res, 26(1): 13-23. |
| [12] | Bai W H, Li C, Li W, et al. 2024. Machine learning assists prediction of genes responsible for plant specialized metabolite biosynthesis by integrating multi-omics data. BMC Genomics, 25(1): 418. |
| [13] | Bailey J E. 1991. Toward a science of metabolic engineering. Science, 252: 1668-1675. |
| [14] | Banerjee G, Chattopadhyay P. 2019. Vanillin biotechnology: The perspectives and future. J Sci Food Agric, 99(2): 499-506. |
| [15] | Bhullar N K, Gruissem W. 2013. Nutritional enhancement of rice for human health: The contribution of biotechnology. Biotechnol Adv, 31(1): 50-57. |
| [16] | Bin Rahman A N M R, Zhang J H. . 2023. Trends in rice research: 2030 and beyond. Food Energy Secur, 12(2): e390. |
| [17] | Biswas P S, Swamy B P M, Kader M A, et al. 2021. Development and field evaluation of near-isogenic lines of GR2-EBRRI dhan29 Golden Rice. Front Plant Sci, 12: 619739. |
| [18] | Blancquaert D, van Daele J, Storozhenko S, et al. 2013. Rice folate enhancement through metabolic engineering has an impact on rice seed metabolism, but does not affect the expression of the endogenous folate biosynthesis genes. Plant Mol Biol, 83(4/5): 329-349. |
| [19] | Blancquaert D, van Daele J, Strobbe S, et al. 2015. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat Biotechnol, 33(10): 1076-1078. |
| [20] | Burkhardt P K, Beyer P, Wünn J, et al. 1997. Transgenic rice (Oryza sativa) endosperm expressing daffodil (Narcissus pseudonarcissus) phytoene synthase accumulates phytoene, a key intermediate of provitamin A biosynthesis. Plant J, 11(5): 1071-1078. |
| [21] | Byeon Y, Park S, Lee H Y, et al. 2014. Elevated production of melatonin in transgenic rice seeds expressing rice tryptophan decarboxylase. J Pineal Res, 56(3): 275-282. |
| [22] | Cho J G, Song N Y, Nam T G, et al. 2013. Flavonoids from the grains of C1/R-S transgenic rice, the transgenic Oryza sativa spp. japonica, and their radical scavenging activities. J Agric Food Chem, 61(43): 10354-10359. |
| [23] | Choi K R, Jang W D, Yang D, et al. 2019. Systems metabolic engineering strategies: Integrating systems and synthetic biology with metabolic engineering. Trends Biotechnol, 37(8): 817-837. |
| [24] | Chopra P, Chhillar H, Kim Y J, et al. 2023. Phytochemistry of ginsenosides: Recent advancements and emerging roles. Crit Rev Food Sci Nutr, 63(5): 613-640. |
| [25] | Cohen S N, Chang A C, Boyer H W, et al. 1973. Construction of biologically functional bacterial plasmids in vitro. Proc Natl Acad Sci USA, 70(11): 3240-3244. |
| [26] | Dang Y M, Li Z X, Yu F. 2024. Recent advances in astaxanthin as an antioxidant in food applications. Antioxidants, 13(7): 879. |
| [27] | Deepasree K, Subhashree V. 2023. Molecular docking and dynamic simulation studies of terpenoid compounds against phosphatidylinositol-specific phospholipase C from Listeria monocytogenes. Inform Med Unlocked, 39: 101252. |
| [28] | Dubouzet J G, Matsuda F, Ishihara A, et al. 2013. Production of indole alkaloids by metabolic engineering of the tryptophan pathway in rice. Plant Biotechnol J, 11(9): 1103-1111. |
| [29] | Elango R. 2020. Methionine nutrition and metabolism: Insights from animal studies to inform human nutrition. J Nutr, 150(Suppl 1): 2518S-2523S. |
| [30] | Esatbeyoglu T, Wagner A E, Schini-Kerth V B, et al. 2015. Betanin: A food colorant with biological activity. Mol Nutr Food Res, 59(1): 36-47. |
| [31] | Farré G, Sudhakar D, Naqvi S, et al. 2012. Transgenic rice grains expressing a heterologous ρ-hydroxyphenylpyruvate dioxygenase shift tocopherol synthesis from the γ to the α isoform without increasing absolute tocopherol levels. Transgenic Res, 21(5): 1093-1097. |
| [32] | Freese R, Lysne V. 2023. Niacin: A scoping review for Nordic nutrition recommendations 2023. Food Nutr Res, 67: 10299. |
| [33] | Galli F, Azzi A, Birringer M, et al. 2017. Vitamin E: Emerging aspects and new directions. Free Radic Biol Med, 102: 16-36. |
| [34] | Gillies S A, McIntosh S R, Henry R J. 2008. A Cereal Crop with Enhanced Folate: Rice Transgenic for Wheat HPPK/DHPS. ComBio, Canberra, NSW, 21-25 September, 2008. |
| [35] | Giuliano G. 2017. Provitamin A biofortification of crop plants: A gold rush with many miners. Curr Opin Biotechnol, 44: 169-180. |
| [36] | Goh Y X, Jalil J, Lam K W, et al. 2022. Genistein: A review on its anti-inflammatory properties. Front Pharmacol, 13: 820969. |
| [37] | Ha S H, Liang Y S, Jung H, et al. 2010. Application of two bicistronic systems involving 2A and IRES sequences to the biosynthesis of carotenoids in rice endosperm. Plant Biotechnol J, 8(8): 928-938. |
| [38] | Ha S H, Kim J K, Jeong Y S, et al. 2019. Stepwise pathway engineering to the biosynthesis of zeaxanthin, astaxanthin and capsanthin in rice endosperm. Metab Eng, 52: 178-189. |
| [39] | Han J Y, Baek S H, Jo H J, et al. 2019. Genetically modified rice produces ginsenoside aglycone (protopanaxadiol). Planta, 250(4): 1103-1110. |
| [40] | Helmy M, Smith D, Selvarajoo K. 2020. Systems biology approaches integrated with artificial intelligence for optimized metabolic engineering. Metab Eng Commun, 11: e00149. |
| [41] | Hernández-Camacho J D, Bernier M, López-Lluch G, et al. 2018. Coenzyme Q10 supplementation in aging and disease. Front Physiol, 9: 44. |
| [42] | Hoa T T C, Al-Babili S, Schaub P, et al. 2003. Golden indica and japonica rice lines amenable to deregulation. Plant Physiol, 133(1): 161-169. |
| [43] | Huang Z W, Lin J C, Cheng Z X, et al. 2015a. Production of oleanane-type sapogenin in transgenic rice via expression of β-amyrin synthase gene from Panax japonicus C. A. Mey. BMC Biotechnol, 15: 45. |
| [44] | Huang Z W, Lin J C, Cheng Z X, et al. 2015b. Production of dammarane-type sapogenins in rice by expressing the dammarenediol-II synthase gene from Panax ginseng C. A. Mey. Plant Sci, 239: 106-114. |
| [45] | Inagaki Y S, Etherington G, Geisler K, et al. 2011. Investigation of the potential for triterpene synthesis in rice through genome mining and metabolic engineering. New Phytol, 191(2): 432-448. |
| [46] | Islam M S, Jin Y Y, Chung H J, et al. 2019. Effect of the resveratrol rice DJ526 on longevity. Nutrients, 11(8): 1804. |
| [47] | Jang W D, Kim G B, Kim Y, et al. 2022. Applications of artificial intelligence to enzyme and pathway design for metabolic engineering. Curr Opin Biotechnol, 73: 101-107. |
| [48] | Jeong Y S, Ku H K, Kim J K, et al. 2017. Effect of codon optimization on the enhancement of the β-carotene contents in rice endosperm. Plant Biotechnol Rep, 11(3): 171-179. |
| [49] | Jeong Y S, Ku H K, Jung Y J, et al. 2021a. 2A-linked Bi-, tri-, and quad-cistrons for the stepwise biosynthesis of β-carotene, zeaxanthin, and ketocarotenoids in rice endosperm. Metab Eng Commun, 12: e00166. |
| [50] | Jeong Y S, Kim J K, Baek S A, et al. 2021b. Reciprocal crosses between astaxanthin and capsanthin rice unravel effects of metabolic gene efficacy in rice endosperm. J Plant Biol, 64(4): 371-377. |
| [51] | Jeong Y S, Choi H, Kim J K, et al. 2022. Overexpression of OsMYBR22/OsRVE1 transcription factor simultaneously enhances chloroplast-dependent metabolites in rice grains. Metab Eng, 70: 89-101. |
| [52] | Jiang S Y, Ma A, Xie L, et al. 2016. Improving protein content and quality by over-expressing artificially synthetic fusion proteins with high lysine and threonine constituent in rice plants. Sci Rep, 6: 34427. |
| [53] | Jung W, Yu O, Lau S M, et al. 2000. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nat Biotechnol, 18(2): 208-212. |
| [54] | Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, et al. 2019. How important is tryptophan in human health? Crit Rev Food Sci Nutr, 59(1): 72-88. |
| [55] | Kang C K, Shin J, Cha Y, et al. 2023. Machine learning-guided prediction of potential engineering targets for microbial production of lycopene. Bioresour Technol, 369: 128455. |
| [56] | Kang S, Kang K, Lee K, et al. 2007. Characterization of rice tryptophan decarboxylases and their direct involvement in serotonin biosynthesis in transgenic rice. Planta, 227(1): 263-272. |
| [57] | Kasote D, Sreenivasulu N, Acuin C, et al. 2022. Enhancing health benefits of milled rice: Current status and future perspectives. Crit Rev Food Sci Nutr, 62(29): 8099-8119. |
| [58] | Khan M, Park S, Kim H J, et al. 2019. The resveratrol rice DJ526 callus significantly increases the lifespan of Drosophila (resveratrol rice DJ526 callus for longevity). Nutrients, 11(5): 983. |
| [59] | Ko M R, Song M H, Kim J K, et al. 2018. RNAi-mediated suppression of three carotenoid-cleavage dioxygenase genes, OsCCD1, 4a, and 4b, increases carotenoid content in rice. J Exp Bot, 69(21): 5105-5116. |
| [60] | Kołton A, Długosz-Grochowska O, Wojciechowska R, et al. 2022. Biosynthesis regulation of folates and phenols in plants. Sci Hortic, 291: 110561. |
| [61] | Lawson C E, Martí J M, Radivojevic T, et al. 2021. Machine learning for metabolic engineering: A review. Metab Eng, 63: 34-60. |
| [62] | Lee S I, Kim H U, Lee Y H, et al. 2001. Constitutive and seed-specific expression of a maize lysine-feedback-insensitive dihydrodipicolinate synthase gene leads to increased free lysine levels in rice seeds. Mol Breed, 8(1): 75-84. |
| [63] | Lee T T T, Wang M M C, Hou R C W, et al. 2003. Enhanced methionine and cysteine levels in transgenic rice seeds by the accumulation of sesame 2S albumin. Biosci Biotechnol Biochem, 67(8): 1699-1705. |
| [64] | Lee Y J, Jung Y J, Kim J H, et al. 2024. Molecular protocol to develop β-carotene-biofortified rice events via molecular optimization. Plant Physiol Biochem, 215: 109051. |
| [65] | Li D T, Wang P P, Luo Y H, et al. 2017. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit Rev Food Sci Nutr, 57(8): 1729-1741. |
| [66] | Liu X Y, Zhang P J, Zhao Q, et al. 2023. Making small molecules in plants: A chassis for synthetic biology-based production of plant natural products. J Integr Plant Biol, 65(2): 417-443. |
| [67] | Long X H, Liu Q Q, Chan M L, et al. 2013. Metabolic engineering and profiling of rice with increased lysine. Plant Biotechnol J, 11(4): 490-501. |
| [68] | Lou H C, Li S J, Shi Z H, et al. 2025. Engineering source-sink relations by prime editing confers heat-stress resilience in tomato and rice. Cell, 18(2): 530-549.e20. |
| [69] | Mallikarjuna Swamy B P, Marundan S, Samia M, et al. 2021. Development and characterization of GR2E Golden rice introgression lines. Sci Rep, 11(1): 2496. |
| [70] | Mangel N, Fudge J B, Li K T, et al. 2019. Enhancement of vitamin B6 levels in rice expressing Arabidopsis vitamin B6 biosynthesis de novo genes. Plant J, 99(6): 1047-1065. |
| [71] | Manohar C M, Kundgar S D, Doble M. 2017. Betanin immobilized LDPE as antimicrobial food wrapper. LWT, 80: 131-135. |
| [72] | Maqbool M A, Aslam M, Akbar W, et al. 2017. Biological importance of vitamins for human health: A review. J Agric Basic Sci, 2(3): 50-58. |
| [73] | Meng Q W, Li J W, Wang C S, et al. 2023. Biological function of resveratrol and its application in animal production: A review. J Anim Sci Biotechnol, 14(1): 25. |
| [74] | Meng X, Zhou J, Zhao C N, et al. 2020. Health benefits and molecular mechanisms of resveratrol: A narrative review. Foods, 9(3): 340. |
| [75] | Mrowicka M, Mrowicki J, Dragan G, et al. 2023. The importance of thiamine (vitamin B1) in humans. Biosci Rep, 43(10): BSR20230374. |
| [76] | Muthayya S, Sugimoto J D, Montgomery S, et al. 2014. An overview of global rice production, supply, trade, and consumption. Ann N Y Acad Sci, 1324: 7-14. |
| [77] | Nagegowda D A, Gupta P. 2020. Advances in biosynthesis, regulation, and metabolic engineering of plant specialized terpenoids. Plant Sci, 294: 110457. |
| [78] | Nayeem S, Sundararajan S, Rajendran V, et al. 2023. Metabolic engineering of isoflavonoid genistein in indica rice by expressing Isoflavone Synthase from Glycine max. Plant Cell Tiss Organ Cult, 155(1): 243-253. |
| [79] | Nguyen H C, Hoefgen R, Hesse H. 2012. Improving the nutritive value of rice seeds: Elevation of cysteine and methionine contents in rice plants by ectopic expression of a bacterial serine acetyltransferase. J Exp Bot, 63(16): 5991-6001. |
| [80] | Ninkuu V, Zhang L, Yan J P, et al. 2021. Biochemistry of terpenes and recent advances in plant protection. Int J Mol Sci, 22(11): 5710. |
| [81] | Nogueira A O, Oliveira Y I S, Adjafre B L, et al. 2019. Pharmacological effects of the isomeric mixture of alpha and beta amyrin from Protium heptaphyllum: A literature review. Fundam Clin Pharmacol, 33(1): 4-12. |
| [82] | Ogo Y, Ozawa K, Ishimaru T, et al. 2013. Transgenic rice seed synthesizing diverse flavonoids at high levels: A new platform for flavonoid production with associated health benefits. Plant Biotechnol J, 11(6): 734-746. |
| [83] | Paine J A, Shipton C A, Chaggar S, et al. 2005. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol, 23(4): 482-487. |
| [84] | Parthiban S, Vijeesh T, Gayathri T, et al. 2023. Artificial intelligence-driven systems engineering for next-generation plant-derived biopharmaceuticals. Front Plant Sci, 14: 1252166. |
| [85] | Perez-Vizcaino F, Fraga C G. 2018. Research trends in flavonoids and health. Arch Biochem Biophys, 646: 107-112. |
| [86] | Pouvreau B, Vanhercke T, Singh S. 2018. From plant metabolic engineering to plant synthetic biology: The evolution of the design/build/test/learn cycle. Plant Sci, 273: 3-12. |
| [87] | Rana A, Samtiya M, Dhewa T, et al. 2022. Health benefits of polyphenols: A concise review. J Food Biochem, 46(10): e14264. |
| [88] | Reddy A M, Reddy V S, Scheffler B E, et al. 2007. Novel transgenic rice overexpressing anthocyanidin synthase accumulates a mixture of flavonoids leading to an increased antioxidant potential. Metab Eng, 9(1): 95-111. |
| [89] | Reinke R. 2021. Philippines becomes first country to approve nutrient-enriched ‘Golden Rice’ for planting. IRRI News. [2024-10-27]. https://www.irri.org/news-and-events/news/philippines-becomes-first-country-approve-nutrient-enriched-golden-rice. |
| [90] | Roell M S, Zurbriggen M D. 2020. The impact of synthetic biology for future agriculture and nutrition. Curr Opin Biotechnol, 61: 102-109. |
| [91] | Sagun J V, Yadav U P, Alonso A P. 2023. Progress in understanding and improving oil content and quality in seeds. Front Plant Sci, 14: 1116894. |
| [92] | Samal P, Babu S C, Mondal B, et al. 2022. The global rice agriculture towards 2050: An inter-continental perspective. Outlook Agric, 51(2): 164-172. |
| [93] | Sasidharan O, Gholap A, Rastogi R. 2023. A review of clinical efficacy of topical vitamin C and its derivatives. Sci Technol, 7(2): 20-26. |
| [94] | Sathish S, Venkatesh R, Safia N, et al. 2018. Studies on growth dynamics of embryogenic cell suspension cultures of commercially important Indica rice cultivars ASD16 and Pusa basmati. 3 Biotech, 8(4): 194. |
| [95] | Sears R G, Lenaghan S C, Stewart Jr C N. 2024. AI to enable plant cell metabolic engineering. Trends Plant Sci, 29(2): 126-129. |
| [96] | Sefi M, Elwej A, Chaâbane M, et al. 2019. Beneficial role of vanillin, a polyphenolic flavoring agent, on maneb-induced oxidative stress, DNA damage, and liver histological changes in Swiss albino mice. Hum Exp Toxicol, 38(6): 619-631. |
| [97] | Shin Y M, Park H J, Yim S D, et al. 2006. Transgenic rice lines expressing maize C1 and R-S regulatory genes produce various flavonoids in the endosperm. Plant Biotechnol J, 4(3): 303-315. |
| [98] | Singh R V, Sambyal K. 2022. An overview of β-carotene production: Current status and future prospects. Food Biosci, 47: 101717. |
| [99] | Song Y E, Wang X, Shen Z W, et al. 2013. Expressing the maize anthocyanin regulatory gene Lc increased flavonoid content in the seed of white pericarp rice and purple pericarp rice. Genetika, 49(11): 1292-1299. |
| [100] | Sreevidya V S, Srinivasa Rao C, Sullia S B, et al. 2006. Metabolic engineering of rice with soybean isoflavone synthase for promoting nodulation gene expression in rhizobia. J Exp Bot, 57(9): 1957-1969. |
| [101] | Stephanopoulos G. 2012. Synthetic biology and metabolic engineering. ACS Synth Biol, 1(11): 514-525. |
| [102] | Storozhenko S, de Brouwer V, Volckaert M, et al. 2007. Folate fortification of rice by metabolic engineering. Nat Biotechnol, 25(11): 1277-1279. |
| [103] | Strobbe S, Verstraete J, Stove C, et al. 2021. Metabolic engineering of rice endosperm towards higher vitamin B1 accumulation. Plant Biotechnol J, 19(6): 1253-1267. |
| [104] | Subedi L, Lee T H, Wahedi H M, et al. 2017. Resveratrol-enriched rice attenuates UVB-ROS-induced skin aging via downregulation of inflammatory cascades. Oxid Med Cell Longev, 2017: 8379539. |
| [105] | Sun T H, Yuan H, Cao H B, et al. 2018. Carotenoid metabolism in plants: The role of plastids. Mol Plant, 11(1): 58-74. |
| [106] | Takahashi S, Ogiyama Y, Kusano H, et al. 2006. Metabolic engineering of coenzyme Q by modification of isoprenoid side chain in plant. FEBS Lett, 580(3): 955-959. |
| [107] | Takahashi S, Ohtani T, Iida S, et al. 2009. Development of CoQ10-enriched rice from giant embryo lines. Breed Sci, 59(3): 321-326. |
| [108] | Takahashi S, Ohtani T, Satoh H, et al. 2010. Development of coenzyme Q10-enriched rice using sugary and shrunken mutants. Biosci Biotechnol Biochem, 74(1): 182-184. |
| [109] | Tambasco-Studart M, Titiz O, Raschle T, et al. 2005. Vitamin B6 biosynthesis in higher plants. Proc Natl Acad Sci USA, 102(38): 13687-13692. |
| [110] | Tang X, Sretenovic S, Ren Q R, et al. 2020. Plant prime editors enable precise gene editing in rice cells. Mol Plant, 13(5): 667-670. |
| [111] | Tetali S D. 2019. Terpenes and isoprenoids: A wealth of compounds for global use. Planta, 249(1): 1-8. |
| [112] | Tian Y S, Wang B, Peng R H, et al. 2019. Enhancing carotenoid biosynthesis in rice endosperm by metabolic engineering. Plant Biotechnol J, 17(5): 849-851. |
| [113] | Tian Y S, Fu X Y, Yang Z Q, et al. 2020. Metabolic engineering of rice endosperm for betanin biosynthesis. New Phytol, 225(5): 1915-1922. |
| [114] | Tian Y S, Xu J, Wang B, et al. 2021. Riboflavin fortification of rice endosperm by metabolic engineering. Plant Biotechnol J, 19(8): 1483-1485. |
| [115] | Tozawa Y, Hasegawa H, Terakawa T, et al. 2001. Characterization of rice anthranilate synthase alpha-subunit genes OASA1 and OASA2: Tryptophan accumulation in transgenic rice expressing a feedback-insensitive mutant of OASA1. Plant Physiol, 126( 4): 1493-1506. |
| [116] | van Lent P, Schmitz J, Abeel T. 2023. Simulated design-build-test-learn cycles for consistent comparison of machine learning methods in metabolic engineering. ACS Synth Biol, 12(9): 2588-2599. |
| [117] | Wright S. 1986. Recombinant DNA technology and its social transformation, 1972-1982. Osiris, 2: 303-360. |
| [118] | Wu F, Wesseler J, Zilberman D, et al. 2021. Opinion: Allow Golden Rice to save lives. Proc Natl Acad Sci USA, 118(51): e2120901118. |
| [119] | Wu G Y. 2021. Amino acids in nutrition, health, and disease. Front Biosci, 26(12): 1386-1392. |
| [120] | Yang Q Q, Zhang C Q, Chan M L, et al. 2016. Biofortification of rice with the essential amino acid lysine: Molecular characterization, nutritional evaluation, and field performance. J Exp Bot, 67(14): 4285-4296. |
| [121] | Yang Q Q, Tan Y, Ye Y, et al. 2023. Serotonin enrichment of rice endosperm by metabolic engineering. Crop J, 11(6): 1943-1948. |
| [122] | Ye X, Al-Babili S, Klöti A, et al. 2000. Engineering the provitamin A (beta-carotene) biosynthetic pathway into (carotenoid-free) rice endosperm. Science, 287: 303-305. |
| [123] | You M K, Lee Y J, Kim J K, et al. 2020. The organ-specific differential roles of rice DXS and DXR, the first two enzymes of the MEP pathway, in carotenoid metabolism in Oryza sativa leaves and seeds. BMC Plant Biol, 20(1): 167. |
| [124] | Zhang G Y, Liu R R, Xu G, et al. 2013. Increased α-tocotrienol content in seeds of transgenic rice overexpressing Arabidopsis γ-tocopherol methyltransferase. Transgenic Res, 22(1): 89-99. |
| [125] | Zheng X J, Zhang Y S, Balakrishna A, et al. 2023. Installing the neurospora carotenoid pathway in plants enables cytosolic formation of provitamin A and its sequestration in lipid droplets. Mol Plant, 16(6): 1066-1081. |
| [126] | Zhou Y, Cai H M, Xiao J H, et al. 2009. Over-expression of aspartate aminotransferase genes in rice resulted in altered nitrogen metabolism and increased amino acid content in seeds. Theor Appl Genet, 118(7): 1381-1390. |
| [127] | Zhu Q L, Yu S Z, Zeng D C, et al. 2017. Development of ‘purple endosperm rice’ by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol Plant, 10(7): 918-929. |
| [128] | Zhu Q L, Zeng D C, Yu S Z, et al. 2018. From Golden Rice to aSTARice: Bioengineering astaxanthin biosynthesis in rice endosperm. Mol Plant, 11(12): 1440-1448. |
| [129] | Zhu Q L, Wang B, Tan J T, et al. 2020. Plant synthetic metabolic engineering for enhancing crop nutritional quality. Plant Commun, 1(1): 100017. |
| [130] | Zhu Q L, Tan J T, Liu Y G. 2022. Molecular farming using transgenic rice endosperm. Trends Biotechnol, 40(10): 1248-1260. |
| [131] | Zhu X X, Liu X N, Liu T, et al. 2021. Synthetic biology of plant natural products: From pathway elucidation to engineered biosynthesis in plant cells. Plant Commun, 2(5): 100229. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||