
Rice Science ›› 2019, Vol. 26 ›› Issue (1): 60-68.DOI: 10.1016/j.rsci.2018.08.005
• Orginal Article • Previous Articles
Lei Sun1,2, Ling Wang2, Lianmeng Liu2, Yuxuan Hou2, Yihua Xu1,2, Mengqi Liang2, Jian Gao2, Qiqin Li1( ), Shiwen Huang1,2(
), Shiwen Huang1,2( )
)
Received:2018-06-05
Accepted:2018-08-24
Online:2019-01-29
Published:2018-10-22
Lei Sun, Ling Wang, Lianmeng Liu, Yuxuan Hou, Yihua Xu, Mengqi Liang, Jian Gao, Qiqin Li, Shiwen Huang. Infection and Colonization of Pathogenic Fungus Fusarium proliferatum in Rice Spikelet Rot Disease[J]. Rice Science, 2019, 26(1): 60-68.
Add to citation manager EndNote|Ris|BibTeX
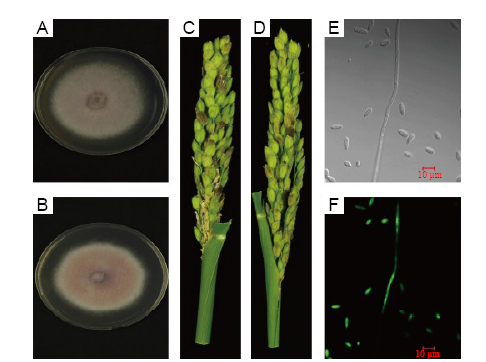
Fig. 1. Phenotype and pathogenicity of F. proliferatum labelled with green fluorescent protein (GFP).A, Wild strain 9FP. B, Mutant strain Fp-GFP11. C, Pathogenicity of the wild strain 9FP. D, Pathogenicity of the mutant strain Fp-GFP11. E, Fp-GFP11 spores and mycelium under the bright field. F, Fp-GFP11 spores and mycelium under the laser field.

Fig. 2. Anthers, pistils and ovaries infected with F. proliferatum.A, Normal anther. B, Normal pistil and ovary. C, Susceptible anther. D, Susceptible pistil and ovary.
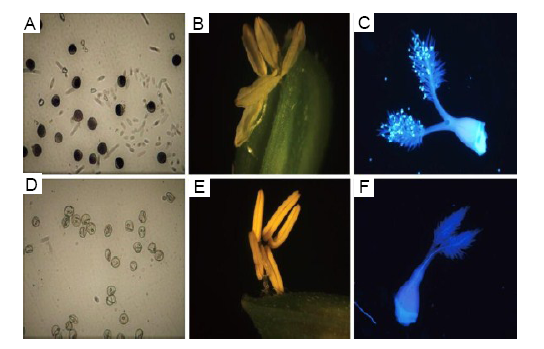
Fig. 3. Pollen fertility, anther dehiscence and pollen count on the stigma infected with F. proliferatum.A, Normal pollen. B, Normal anther. C, Normal pistil. D, Susceptible pollen. E, Susceptible anther. F, Susceptible pistil.
| Inoculation time | Sampling time (HAI) | Lemma and palea infection rate (%) | Stamen infection rate (%) | Pistil infection rate (%) |
|---|---|---|---|---|
| Booting stage | 24 | 23.33 ± 5.77 c | 0.00 ± 0.00 c | 0.00 ± 0.00 b |
| 36 | 43.33 ± 11.55 b | 6.67 ± 5.77 c | 0.00 ± 0.00 b | |
| 48 | 60.00 ± 10.00 a | 23.33 ± 5.77 b | 0.00 ± 0.00 b | |
| 60 | 63.33 ± 5.77 a | 46.67 ± 5.77 a | 23.33 ± 5.77 a | |
| 72 | 66.67 ± 5.77 a | 53.33 ± 5.77 a | 26.67 ± 5.77 a | |
| Start heading stage | 6 | 3.33 ± 5.77 b | 66.67 ± 5.77 c | 0.00 ± 0.00 c |
| 12 | 0.00 ± 0.00 b | 83.33 ± 5.77 b | 0.00 ± 0.00 c | |
| 18 | 3.33 ± 5.77 b | 96.67 ± 5.77 a | 0.00 ± 0.00 c | |
| 24 | 0.00 ± 0.00 b | 100.00 ± 0.00 a | 0.00 ± 0.00 bc | |
| 36 | 3.33 ± 5.77 b | 100.00 ± 0.00 a | 10.00 ± 0.00 b | |
| 48 | 10.00 ± 0.00 b | 100.00 ± 0.00 a | 33.33 ± 5.77 a | |
| 60 | 36.67 ± 11.55 a | 100.00 ± 0.00 a | 36.67 ± 5.77 a |
Table 1 Artificial inoculation during the booting and start heading stages (Mean ± SD, n = 3).
| Inoculation time | Sampling time (HAI) | Lemma and palea infection rate (%) | Stamen infection rate (%) | Pistil infection rate (%) |
|---|---|---|---|---|
| Booting stage | 24 | 23.33 ± 5.77 c | 0.00 ± 0.00 c | 0.00 ± 0.00 b |
| 36 | 43.33 ± 11.55 b | 6.67 ± 5.77 c | 0.00 ± 0.00 b | |
| 48 | 60.00 ± 10.00 a | 23.33 ± 5.77 b | 0.00 ± 0.00 b | |
| 60 | 63.33 ± 5.77 a | 46.67 ± 5.77 a | 23.33 ± 5.77 a | |
| 72 | 66.67 ± 5.77 a | 53.33 ± 5.77 a | 26.67 ± 5.77 a | |
| Start heading stage | 6 | 3.33 ± 5.77 b | 66.67 ± 5.77 c | 0.00 ± 0.00 c |
| 12 | 0.00 ± 0.00 b | 83.33 ± 5.77 b | 0.00 ± 0.00 c | |
| 18 | 3.33 ± 5.77 b | 96.67 ± 5.77 a | 0.00 ± 0.00 c | |
| 24 | 0.00 ± 0.00 b | 100.00 ± 0.00 a | 0.00 ± 0.00 bc | |
| 36 | 3.33 ± 5.77 b | 100.00 ± 0.00 a | 10.00 ± 0.00 b | |
| 48 | 10.00 ± 0.00 b | 100.00 ± 0.00 a | 33.33 ± 5.77 a | |
| 60 | 36.67 ± 11.55 a | 100.00 ± 0.00 a | 36.67 ± 5.77 a |
| Sampling time | Lemma/palea infection rate | Endosperm infection rate |
|---|---|---|
| (HAI) | (%) | (%) |
| 24 | 23.33 ± 5.77 a | 3.33 ± 5.77 b |
| 48 | 36.67 ± 11.55 a | 23.33 ± 5.77 a |
Table 2 Artificial inoculation during flowering stage.
| Sampling time | Lemma/palea infection rate | Endosperm infection rate |
|---|---|---|
| (HAI) | (%) | (%) |
| 24 | 23.33 ± 5.77 a | 3.33 ± 5.77 b |
| 48 | 36.67 ± 11.55 a | 23.33 ± 5.77 a |
| Different inoculation | Anther dehiscence rate | Seed-setting rate |
|---|---|---|
| (%) | (%) | |
| Sterile water | 83.63 ± 4.21 a | 90.50 ± 2.56 a |
| Spore suspension | 25.53 ± 5.04 b | 24.84 ± 3.02 b |
Table 3 Effects of different inoculation treatments on rice samples.
| Different inoculation | Anther dehiscence rate | Seed-setting rate |
|---|---|---|
| (%) | (%) | |
| Sterile water | 83.63 ± 4.21 a | 90.50 ± 2.56 a |
| Spore suspension | 25.53 ± 5.04 b | 24.84 ± 3.02 b |
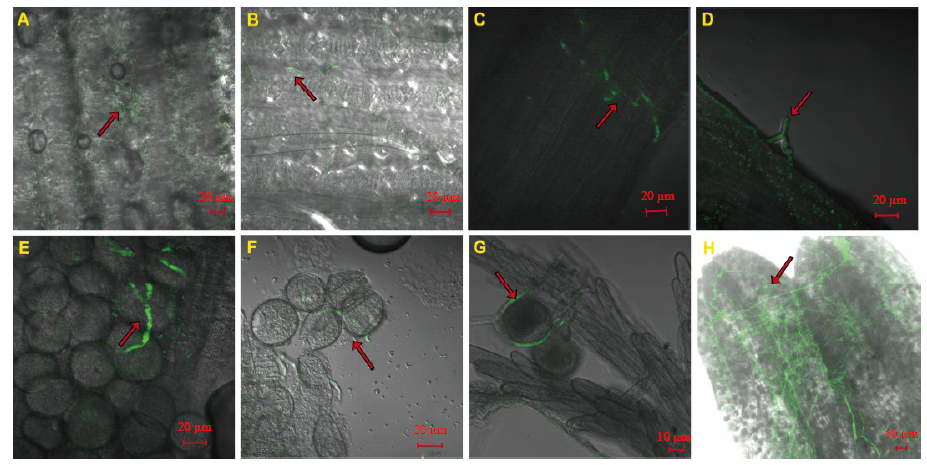
Fig. 4. Effects of Fp-GFP11 infection process on rice floral organs during the panicle initiation stage.A, 24 hours after inoculation (HAI). B and C, 36 HAI. D and E, 48 HAI. F and G, 60 HAI. H, 72 HAI. The arrows indicate the Fp-GFP11.
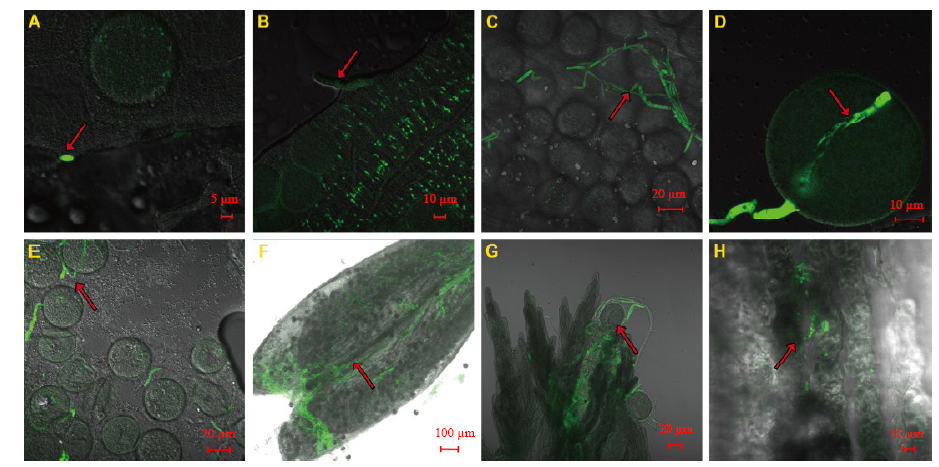
Fig. 5. Effects of Fp-GFP11 infection process on rice floral organs during the start heading stage.A, 6 hours after inoculation (HAI). B, 12 HAI. C and D, 18 to 24 HAI. E, 36 HAI. F and G, 48 HAI. H, 60 HAI. The arrows indicate the Fp-GFP11.
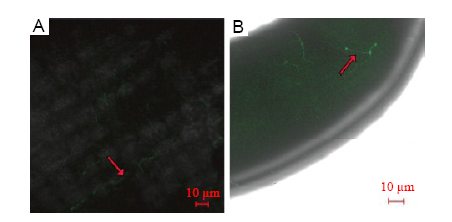
Fig. 6. Effect of Fp-GFP11 infection on rice floral organs during the flowering stage.A, 24 hours after inoculation (HAI). B, 48 HAI.The arrows indicate the Fp-GFP11.
| [1] | Boenisch M J, SchãFer W.2011. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol, 11: 110. |
| [2] | Burger H M, Lombard M J, Shephard G S, Rheeder J R, van der Westhuizen L, Gelderblom W C A.2010. Dietary fumonisin exposure in a rural population of South Africa.Food Chem Toxicol, 48: 2103-2108. |
| [3] | Carpita N C, Gibeaut D M.1993. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth.Plant J, 3(1): 1-30. |
| [4] | Chen N, Hsiang T, Goodwin P H.2003. Use of green fluorescent protein to quantify the growth of Colletotrichum during infection of tobacco. J Microbiol Method, 53(1): 113-122. |
| [5] | Cooper R M.1983. The mechanisms and significance of enzymic degradation of host cell walls. In: Callow J A. Biochemical Plant Pathology. Wiley: 101-135. |
| [6] | Cormack B.1998. Green fluorescent protein as a reporter of transcription and protein localization in fungi.Curr Opin Microbiol, 1(4): 406-410. |
| [7] | Dai G H, Zhao J, He R M, Jin S X.2005. Histochemical observation on the resistant and susceptible varieties to Ustilaginoidea virens(Cooke) Tak and the way of infection of conidia. Acta Phytophysiol Sin, 35(1): 37-42. (in Chinese with English abstract) |
| [8] | Desjardins A E, Busman M, Muhitch M, Proctor R H.2007. Complementary host-pathogen genetic analyses of the role of fumonisins in the Zea mays-Gibberella moniliformis interaction. Physiol Mol Plant Pathol, 70: 149-160. |
| [9] | Hou E Q, Zhang P S, Wang L, Liu E Y, Liu L M, Huang S W.2013. Occurrence, epidemical regularity and controlling of rice spikelet rot disease (RSRD) and pathogenicity of its pathogens.Plant Prot, 39(1): 121-127. (in Chinese with English abstract) |
| [10] | Hu M L, Luo L X, Wang S, Liu Y F, Li J Q.2014. Infection processes of Ustilaginoidea virens, during artificial inoculation of rice panicles. Eur J Plant Pathol, 139(1): 67-77. |
| [11] | Huang S W, Wang L, Liu L M, Tang S Q, Zhu D F, Savary S.2011a. Rice spikelet rot disease in China: 1. Characterization of fungi associated with the disease.Crop Prot, 30(1): 1-9. (in Chinese with English abstract) |
| [12] | Huang S W, Wang L, Liu L M, Tang S Q, Zhu D F, Savary S.2011b. Rice spikelet rot disease in China: 2. Pathogenicity tests, assessment of the importance of the disease, and preliminary evaluation of control options.Crop Prot, 30(1): 10-17. (in Chinese with English abstract) |
| [13] | Kang Z S, Buchenauer H.2000. Ultrastructural and immunocyto- chemical investigation of pathogen development and host responses in resistant and susceptible wheat spikes infected byFusarium culmorum. Physiol Mol Plant Pathol, 57(6): 255-268. |
| [14] | Kang Z S, Buchenauer H.2002. Studies on the infection process of Fusarium culmorum, in wheat spikes: Degradation of host cell wall components and localization of trichothecene toxins in infected tissue. Eur J Plant Pathol, 108(7): 653-660. |
| [15] | Kang Z S, Huang L L, Buchenauer H, Han Q M, Jiang X L.2004. Cytochemistry of cell wall component alterations in wheat spikes infected by Fusarium graminearum. Acta Phytopathol Sin, 37(6): 623-628. (in Chinese with English abstract) |
| [16] | Kang Z S, Huang L L, Han Q M, Zhang H C.2007. Cytology of infection process of Fusarium graminearum on wheat spikes. Acta Phytopathol Sin, 34(4): 329-335. (in Chinese with English abstract) |
| [17] | Li C Q, Liang H S, Xia Y J, Peng M.2011. Observation of the infection process of watermelon by Fusarium oxysporum f.sp. niveum using the GFP marker. Chin J Trop Crops, 32(10): 1935-1939. (in Chinese with English abstract) |
| [18] | Li L, Liu L M, Wang G R, Wang A J, Wang L, Sun L, Li Q Q, Huang S W.2015. Research progress of spikelet rot disease and bacterial panicle blight of rice.Chin J Rice Sci, 29(2): 215-222. (in Chinese with English abstract) |
| [19] | Matsui T, Omasa K, Horie T.2001. The difference in sterility due to high temperatures during the flowering period among japonica-rice varieties. Plant Prod Sci, 4(2): 90-93. |
| [20] | Matsui T, Omasa K.2002. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: Anther characteristics. Ann Bot, 89(6): 683-687. |
| [21] | Pugh G W, Johann H, Dickson J G.1933. Factors affecting infection of wheat heads by Gibberella saubinetii. J Agric Res, 46(9): 771-797. |
| [22] | Prasad P V V, Boote K J, Jr L H A, Sheehy J E, Thomas J M G.2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress.Field Crops Res, 95(2): 398-411. |
| [23] | Rheeder J P, Wfo M, Thiel P G, Sydenham E W, Shephard G S, van Schalkwyk D J.1992. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology, 82(3): 353-357. |
| [24] | Shi L H, Bielawski J, Mu J Y, Dong H L, Teng C, Zhang J, Yang X H, Tomishige N, Hanada K, Hannun Y A, Zuo J R.2009. Involvement of sphingoid bases in mediating reactive oxygen intermediate production and programmed cell death in Arabidopsis. Plant Sign Behav, 17(6): 1030-1040. |
| [25] | Skadsen R W, Hohn T M.2004. Use of Fusarium graminearum transformed with gfp to follow infection patterns in barley and Arabidopsis. Physiol Mol Plant Pathol, 64(1): 45-53. |
| [26] | Tang Y X, Jin J, Hu D W, Yong M L, Xu Y, He L P.2013. Elucidation of the infection process of Ustilaginoidea virens(teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol, 62(1): 1-8. |
| [27] | Teng C, Dong H L, Shi L H, Deng Y, Mu J Y, Zhang J, Yang X H, Zuo J R.2008. Serine palmitoyltransferase, a key enzyme for de novo synthesis of sphingolipids, is essential for male gametophyte development in Arabidopsis. Plant Physiol, 146(3): 1322-1332. |
| [28] | Tsegaye Y, Richardson C G, Bravo J E, Mulcahy B J, Lynch D V, Markham J E, Jaworski J G, Chen M, Cahoon E B, Dunn T M.2007. Arabidopsis mutants lacking long chain base phosphate lyase are fumonisin-sensitive and accumulate trihydroxy-18:1 long chain base phosphate. J Biol Chem, 282: 28195-28206. |
| [29] | Xu Y, Liu T G, He Y Q, Chen W Q.2008. Application of green fluorescent protein to the studies on filamentous fungi.Plant Prot, 34(6): 1-6. (in Chinese with English abstract) |
| [30] | Zhang X, van de Lee T, Lu W Z, Yu D Z, Ma H X.2008. Infection of Fusarium graminearum on wheat spikes with green fluorescence protein-tagged revertants. Sci Agric Sin, 42(10): 3077-3082. (in Chinese with English abstract) |
| [1] | Jiehua Qiu, Shuai Meng, Yizhen Deng, Shiwen Huang, Yanjun Kou. Ustilaginoidea virens: A Fungus Infects Rice Flower and Threats World Rice Production [J]. Rice Science, 2019, 26(4): 199-206. |
| [2] | XU Hua-wei, MO Yi-wei, WANG Wei, WANG Hai, WANG Zhong. OsPIN1a Gene Participates in Regulating Negative Phototropism of Rice Roots [J]. RICE SCIENCE, 2014, 21(2): 83-89. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||