
Rice Science ›› 2020, Vol. 27 ›› Issue (4): 259-262.DOI: 10.1016/j.rsci.2020.05.002
• Letter • Previous Articles Next Articles
Weimin Zhou#, Menghui Ma#, Lianping Sun#, Peng Zhang, Shiqiong Lv, Zhengzheng Zhong( ), Hanhua Tong(
), Hanhua Tong( )
)
Received:2019-06-18
Accepted:2019-12-16
Online:2020-07-28
Published:2020-03-31
About author:#These authors contributed equally to this work
Weimin Zhou, Menghui Ma, Lianping Sun, Peng Zhang, Shiqiong Lv, Zhengzheng Zhong, Hanhua Tong. OsPS6 Plays Important Role in Anther Development and Microspore Formation[J]. Rice Science, 2020, 27(4): 259-262.
Add to citation manager EndNote|Ris|BibTeX
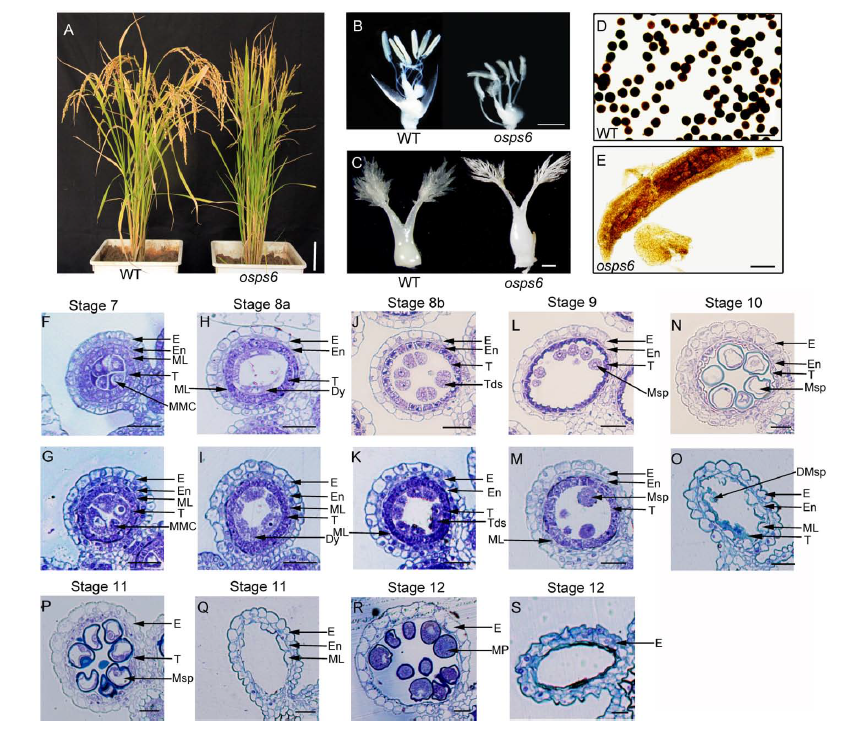
Fig. 1. Phenotype comparisons between wild type (WT) Nipponbare and osps6 mutant.A, Phenotypic differences between the WT and osps6 mutant during ripening. Scale bar, 10 cm. B, Spikelets of the WT and osps6 mutant with the palea and lemma removed. Scale bar, 2 mm. C, Stigmas and ovaries of the WT and osps6 mutant at stage 12. Scale bar, 100 µm. D and E, Pollen grains of the WT and osps6 mutant with I2-KI staining. Scale bars, 50 µm. F to S, Transverse sections of male gametogenesis in the WT and osps6 mutant. Scale bars, 15 µm. F, H, J, L, N, P and R show the wild type; G, I, K, M, O, Q and S show the osps6 mutant. F and G, Cross section of single locule at the microspore mother cell stage. H and I, Cross section of single locule at the dyads stage. J and K, Cross section of single locule at the tetrads stage. L and M, Cross section of single locule at the young microspore stage. N and O, Cross section of single locule at the vacuolated pollen stage. P and Q, Cross section of single locule at the pollen mitosis stage showing two types of pollen grains in the mutant locule. R and S, Cross section of single locule at the mature pollen stage showing two types of pollen grains in the mutant locule.DMsp, Degenerated microspores; Dy, Dyad cell; E, Epidermis; En, Endothecium; ML, Middle layer; MMC, Microspore mother cell; MP, Mature pollen; Msp, Microspores; T, Tapetal layer; Tds, Tetrads.
| Pollen fertility (%) | Spikelet fertility (%) | |
|---|---|---|
| Nipponbare | 95.18 ± 0.59 | 93.46 ± 1.05 |
| osps6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
Supplemental Table 1. Pollen fertility, spikelet fertility in the wild type Nipponbare and osps6 mutant.
| Pollen fertility (%) | Spikelet fertility (%) | |
|---|---|---|
| Nipponbare | 95.18 ± 0.59 | 93.46 ± 1.05 |
| osps6 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| Cross-combination | Total No. | F2 | Expect ratio | c2 | |
|---|---|---|---|---|---|
| No. of fertile plants | No. of sterile plants | ||||
| osps6 / Nipponbare | 795 | 606 | 189 | 3:1 | 0.60 |
Supplemental Table.2. Separation ratio of pollen fertility in F2 population (osps6 / the wild type).
| Cross-combination | Total No. | F2 | Expect ratio | c2 | |
|---|---|---|---|---|---|
| No. of fertile plants | No. of sterile plants | ||||
| osps6 / Nipponbare | 795 | 606 | 189 | 3:1 | 0.60 |
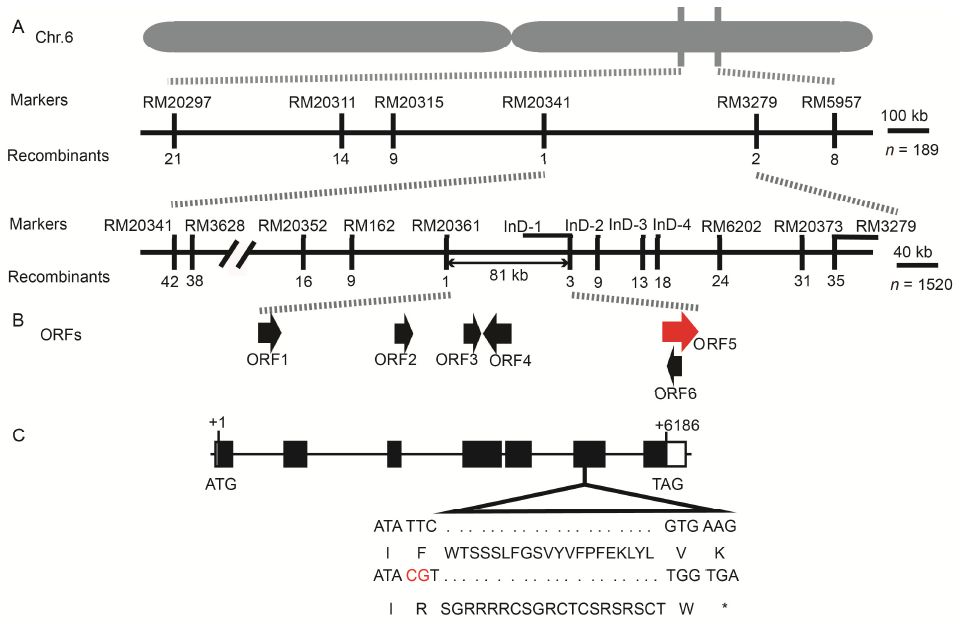
Fig. 2. Mapping-based cloning and analysis of OsPS6.A, The OsPS6 locus was mapped to a 81 kb region between the markers RM20361 and InD-1 on the long arm of chromosome 6 (Chr.6). B, Annotated open reading frames (ORFs) in the 81 kb region. C, A schematic represents the exon (solid black box), intron (black lines) and untranslated region (UTR; empty box) of OsPS6. The mutant sequence has 2 bp insertion in the sixth exon resulted in premature termination of OsPS6 protein. ATG and TAG represent the start and stop codons, respectively.
| Primer name | Forward primer (5′-3′) | Reverse primer (5′-3′) | Purpose |
|---|---|---|---|
| InD-1 | GAGGGCCTTTGGAACACAGA | TGAGTGGTGGTTGCTTCTCC | Fine mapping |
| InD-2 | ACCGCAATACGCAGATGGTC | GAACCCCATCCGTCCGATTT | Fine mapping |
| InD-3 | GGGATGCAAGTGGGTAGTCT | CTAGTGCGAGTAACCCCACC | Fine mapping |
| InD-4 | CCGATCTTCACCTTCCGCTT | GACATGGCTGGTCTCTCTCG | Fine mapping |
Supplemental Table. 3. New developed InDel markers.
| Primer name | Forward primer (5′-3′) | Reverse primer (5′-3′) | Purpose |
|---|---|---|---|
| InD-1 | GAGGGCCTTTGGAACACAGA | TGAGTGGTGGTTGCTTCTCC | Fine mapping |
| InD-2 | ACCGCAATACGCAGATGGTC | GAACCCCATCCGTCCGATTT | Fine mapping |
| InD-3 | GGGATGCAAGTGGGTAGTCT | CTAGTGCGAGTAACCCCACC | Fine mapping |
| InD-4 | CCGATCTTCACCTTCCGCTT | GACATGGCTGGTCTCTCTCG | Fine mapping |
| ORFs | Gene number | Putative function |
|---|---|---|
| ORF1 | Os06g0606900 | Conserved hypothetical protein |
| ORF2 | Os06g0607000 | Similar to Beta-1,3-glucanase |
| ORF3 | Os06g0607100 | Similar to phosphatidic acid phosphatase-related/PAP2-related |
| ORF4 | Os06g0607200 | Similar to Cellular retinaldehyde binding/alpha-tocopherol transport |
| ORF5 | Os06g0607700 | ATP-binding cassette (ABC) transporter |
| ORF6 | Os06g0607750 | Hypothetical protein |
Supplemental Table.4. Gene annotation of candidate genes.
| ORFs | Gene number | Putative function |
|---|---|---|
| ORF1 | Os06g0606900 | Conserved hypothetical protein |
| ORF2 | Os06g0607000 | Similar to Beta-1,3-glucanase |
| ORF3 | Os06g0607100 | Similar to phosphatidic acid phosphatase-related/PAP2-related |
| ORF4 | Os06g0607200 | Similar to Cellular retinaldehyde binding/alpha-tocopherol transport |
| ORF5 | Os06g0607700 | ATP-binding cassette (ABC) transporter |
| ORF6 | Os06g0607750 | Hypothetical protein |
| [1] | Aya K, Ueguchi-Tanaka M, Kondo M, Hamada K, Yano K, Nishimura M, Matsuoka M. 2009. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell, 21(5): 1453-1472. |
| [2] | Chen Z S, Liu X F, Wang D H, Chen R, Zhang X L, Xu Z H, Bai S N. 2018. Transcription factor OsTGA10 is a target of the MADS protein OsMADS8 and is required for tapetum development. Plant Physiol, 176(1): 819-835. |
| [3] | Du X, Fei Y Y, Wang F Q, Xu Y, Wang J, Li W Q, Zhao L, Chen Z H, Liang G H, Zhou Y, Yang J. 2019. Thermo-sensitive male sterile line created by editing TMS5 gene in japonica rice. Chin J Rice Sci, 33(5): 429-435. (in Chinese with English abstract) |
| [4] | Fu Z Z, Yu J, Cheng X W, Zong X, Xu J, Chen M J, Li Z Y, Zhang D B, Liang W Q. 2014. The rice basic helix-loop-helix transcription factor TDR INTERACTING PROTEIN2 is a central switch in early anther development. Plant Cell, 26(4): 1512-1524. |
| [5] | Jung K H, Han M J, Lee Y S, Kim Y W, Hwang I, Kim M J, Kim Y K, Nahm B H, An G. 2005. Rice undeveloped tapetum1 is a major regulator of early tapetum development. Plant Cell, 17: 2705-2722. |
| [6] | Ko S S, Li M J, Sun-Ben K M, Ho Y C, Lin Y J, Chuang M H, Hsing H X, Lien Y C, Yang H T, Chang H C, Chan M T. 2014. The bHLH142 transcription factor coordinates with TDR1 to modulate the expression of EAT1 and regulate pollen development in rice. Plant Cell, 26(6): 2486-2504. |
| [7] | Li N, Zhang D S, Liu H S, Yin C S, Li X X, Liang W Q, Yuan Z, Xu B, Chu H W, Wang J, Wen T Q, Huang H, Luo D, Ma H, Zhang D B. 2006. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell, 18(11): 2999-3014. |
| [8] | Ma H. 2005. Molecular genetics analysis of microsporogenesis and microgametogenesis in flowering plants. Annu Rev Plant Biol, 56: 393-434. |
| [9] | Niu B X, He F R, He M, Ren D, Chen L T, Liu Y G. 2013. The ATP-binding cassette transporter OsABCG15 is required for anther development and pollen fertility in rice. J Integr Plant Biol, 55(8): 710-720. |
| [10] | Nonomura K I, Miyoshi K, Eiguchi M, Suzuki T, Miyao A, Hirochika H, Kurata N. 2003. The MSP1 gene is necessary to restrict the number of cells entering into male and female sporogenesis and to initiate anther wall formation in rice. Plant Cell, 15(8): 1728-1739. |
| [11] | Parish R W, Li S F. 2010. Death of a tapetum: A programme of developmental altruism. Plant Sci, 178(2): 73-89. |
| [12] | Qin P, Tu B, Wang Y P, Deng L C, Quilichini T D, Li T, Wang H, Ma B T, Li S G. 2013. ABCG15 encodes an ABC transporter protein, and is essential for post-meiotic anther and pollen exine development in rice. Plant Cell Physiol, 54(1): 138-154. |
| [13] | Ranjan R, Khurana R, Malik N, Badoni S, Parida S K, Kapoor S, Tyagi A K. 2017. bHLH142 regulates various metabolic pathway-related genes to affect pollen development and anther dehiscence in rice. Sci Rep, 7: 43397. |
| [14] | Scott R J, Spielman M, Dickinson H G. 2004. Stamen structure and function. Plant Cell, 16: S46-S60. |
| [15] | Song W Y, Yamaki T, Yamaji N, Ko D, Jung K H, Fujii-Kashino M, An G, Martinoia E, Lee Y, Ma J F. 2014. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc Natl Acad Sci USA, 111: 15699-15704. |
| [16] | Walbot V, Egger R L. 2016. Pre-meiotic anther development: Cell fate specification and differentiation. Annu Rev Plant Biol, 67: 365-395. |
| [17] | Wilson Z A, Zhang D B. 2009. From Arabidopsis to rice: Pathways in pollen development. J Exp Bot, 60(5): 1479-1492. |
| [18] | Wu L, Guan Y S, Wu Z G, Yang K, Lv J, Converse R, Huang Y X, Mao J X, Zhao Y, Wang Z W, Min H Q, Kan D Y, Zhang Y. 2014. OsABCG15 encodes a membrane protein that plays an important role in anther cuticle and pollen exine formation in rice. Plant Cell Rep, 33(11): 1881-1899. |
| [19] | Zhang D, Wilson Z A. 2009. Stamen specification and anther development in rice. Chin Sci Bull, 54: 2342-2353. |
| [20] | Zhao D Z, Wang G F, Speal B, Ma H. 2002. The EXCESS MICROSPOROCYTES1 gene encodes a putative leucine-rich repeat receptor protein kinase that controls somatic and reproductive cell fates in the Arabidopsis anther. Genes Dev, 16(15): 2021-2031. |
| [21] | Zhao G C, Shi J X, Liang W Q, Xue F Y, Luo Q, Zhu L, Qu G R, Chen M J, Schreiber L, Zhang D B. 2015. Two ATP binding cassette G transporters, rice ATP binding cassette G26 and ATP binding cassette G15, collaboratively regulate rice male reproduction. Plant Physiol, 169(3): 2064-2079. |
| [22] | Zhu L, Shi J X, Zhao G C, Zhang D B, Liang W Q. 2013. Post- meiotic deficient anther1 (PDA1) encodes an ABC transporter required for the development of anther cuticle and pollen exine in rice. J Plant Biol, 56: 59-68. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||