
Rice Science ›› 2024, Vol. 31 ›› Issue (1): 77-86.DOI: 10.1016/j.rsci.2023.11.007
• Research Papers • Previous Articles Next Articles
Dong Xinli1,2,#, Zhou Yang1,#, Zhang Yaqi1, Rong Fuxi1, Du Jiahong1, Hong Zheyuan1, HU Peisong1,2, Lü Yusong1,2( )
)
Received:2023-07-07
Accepted:2023-11-27
Online:2024-01-28
Published:2024-02-06
Contact:
Lü Yusong (About author:First author contact:#These authors contributed equally to this work
Dong Xinli, Zhou Yang, Zhang Yaqi, Rong Fuxi, Du Jiahong, Hong Zheyuan, HU Peisong, Lü Yusong. OsbZIP01 Affects Plant Growth and Development by Regulating OsSD1 in Rice[J]. Rice Science, 2024, 31(1): 77-86.
Add to citation manager EndNote|Ris|BibTeX
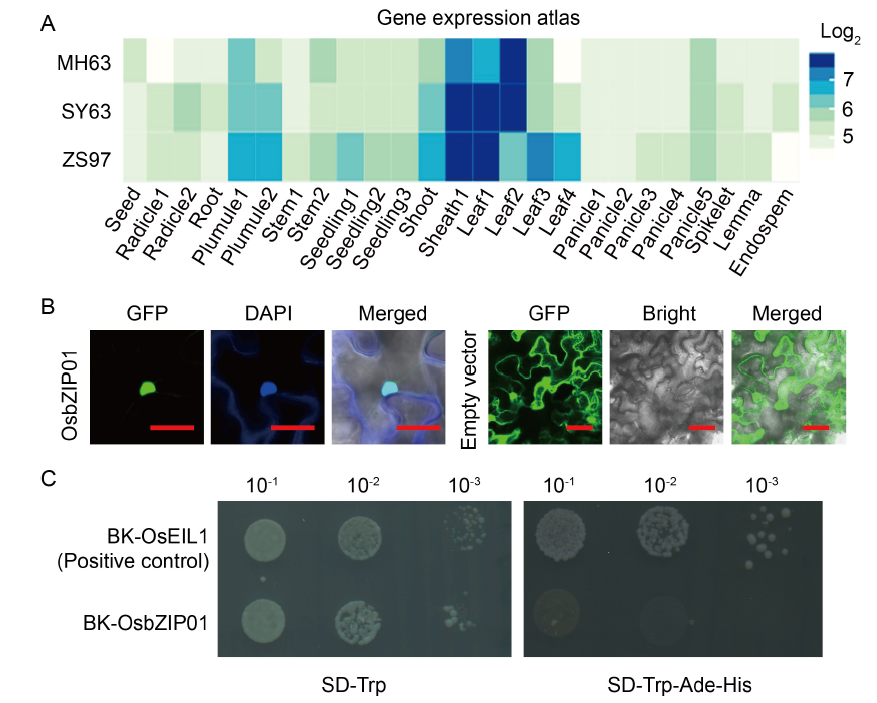
Fig. 1. Expression pattern of OsbZIP01 and its protein characterization. A, Gene expression atlas of OsbZIP01 in three representative indica varieties of China is from the CREP database (http://crep.ncpgr.cn/crep-cgi/home.pl). The expression value is log2-transformed. MH63, Minghui 63; SY63, Shanyou 63; ZS97, Zhenshan 97. B, Subcellular localization of OsbZIP01. DAPI, 4′,6-diamidino-2-phenylindole; GFP, Green fluorescent protein. Scale bars are 100 μm. C, Transactivation assay of OsbZIP01 in yeast cells. BK, pDEST-GBKT7; SD, Synthetic dropout medium; Trp, Tryptophan; Ade, Adenine; His, Histidine.
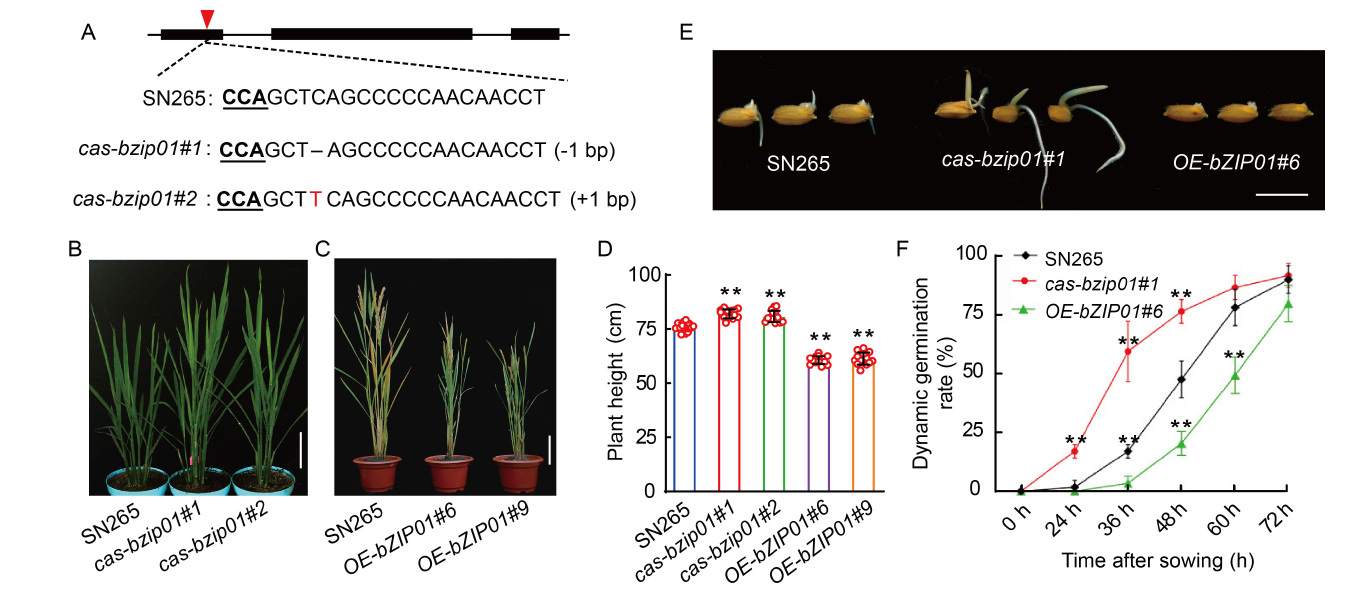
Fig. 2. Phenotypic characterization of Shennong 265 (SN265), cas-bzip01, and OE-bZIP01 transgenic plants. A, Schematic diagram of the sgRNA site of bZIP01 gene in SN265 and two mutants (cas-bzip01#1 and cas-bzip01#2) by CRISPR/Cas9 system. The red triangle indicates the sgRNA position. B, Phenotypes of SN265 and cas-bzip01 plants (cas-bzip01#1 and cas-bzip01#2) at the heading stage. Scale bar is 10 cm. C, Morphology of SN265 and overexpression lines (OE-bZIP01#6 and OE-bZIP01#9) at the maturity stage. Scale bar is 10 cm. D, Plant heights of SN265 and transgenic plants (cas-bzip01#1, cas-bzip01#2, OE-bZIP01#6, and OE-bZIP01#9) at the maturity stage. E, Seed germination phenotypes of SN265, cas-bzip01#1, and OE-bZIP01#6 plants. Sterile grains were sown on Murashige and Skoog plates, and photographs were taken at 48 h after germination at 30 ºC. Scale bar is 1 cm. F, Dynamic germination rates of SN265, cas-bzip01#1, and OE-bZIP01#6 after sown for 72 h. In D and F, Data are Mean ± SD of three independent experiments (n = 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type (SN265) at the P < 0.01 by the Student’s t-test.
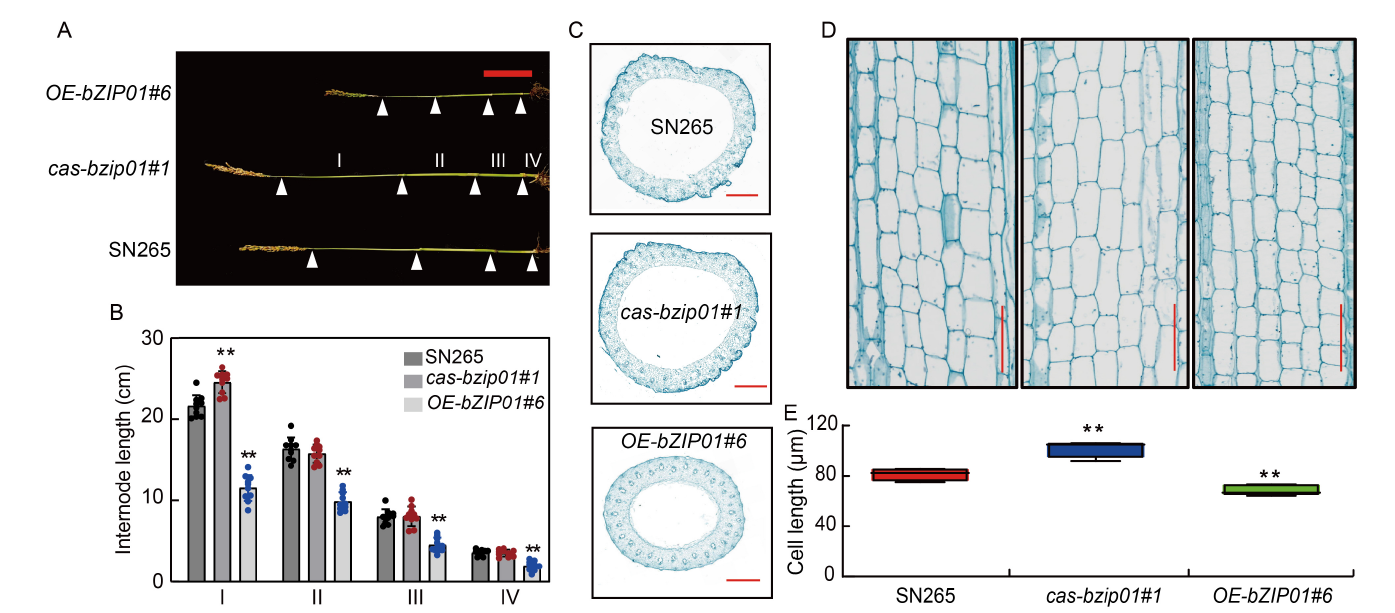
Fig. 3. Morphology comparison of cas-bzip01 and OE-bZIP01 transgenic plants. A and B, Comparison of internode lengths between wild type (Shennong 265, SN265), cas-bzip01#1, and OE-bZIP01#6 at the heading stage. Ⅰ, The first internode from the top; Ⅱ, The second internode from the top; Ⅲ, The third internode from the top; Ⅳ, The fourth internode from the top. Scale bar is 10 cm. C, Fast green-stained transverse sections of SN265, cas-bzip01#1, and OE-bZIP01#6 stems, showing differences in the diameter of the second internode from the top. Scale bars are 1 mm. D, Longitudinal sections of the first internode from the top of SN265, cas-bzip01#1, and OE-bZIP01#6. Scale bars are 120 μm. E, Comparison analysis of parenchyma cell lengths of the first internode from the top of SN265, cas-bzip01#1, and OE-bZIP01#6. In B and F, data are Mean ± SD of three independent experiments (n = 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type at the P < 0.01 by the Student’s t-test.
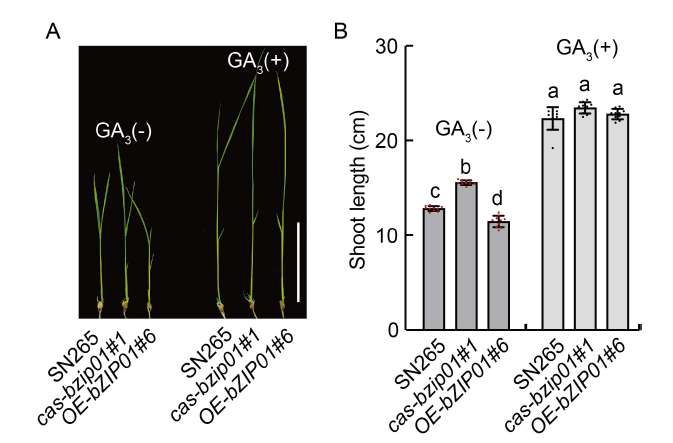
Fig. 4. Phenotype response of wild type (Shennong 265, SN265), cas-bzip01, and OE-OsbZIP01 to exogenous GA. A, Seedling phenotype of SN265 and transgenic lines (cas-bzip01#1 and OE-bZIP01#6), which were treated with exogenous 10 μmol/L GA3 for 10 d. GA3(-) represents the absence of GA3, and GA3(+) represents the application of GA3. Scale bar is 5 cm. B, Shoot lengths of SN265 and transgenic lines (cas-bzip01#1 and OE-bZIP01#6). Data are Mean ± SD of three independent experiments (n = 10 for each experiment). Different lowercase letters indicate the significant differences between transgenic plants and wild type (P < 0.01, Student’s t-test).
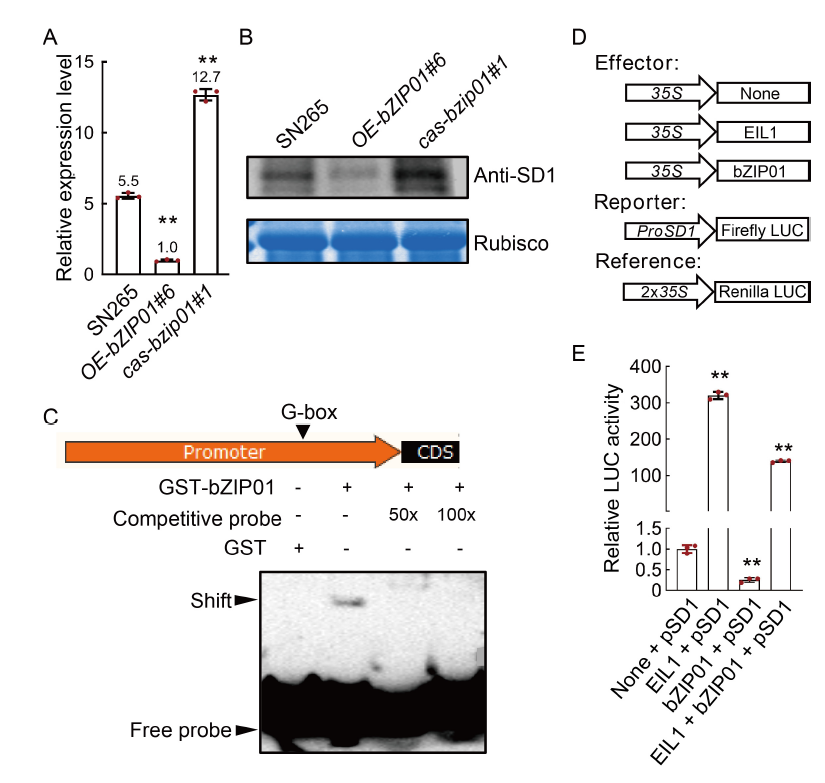
Fig. 5. OsbZIP01 directly binds to promoter region of SD1. A and B, Relative expression levels of mRNA (A) and protein (B) of SD1 in the stems of wild type (Shennong 265, SN265), cas-bzip01#1, and OE-bZIP01#6 transgenic plants. C, OsbZIP01 binds in vitro to G-box in the SD1 promoter by electrophoretic mobility shift assay. For the assay, the radiolabeled probes were incubated with OsbZIP01 protein. Competitive (unlabeled) probe (50× and 100×), G-box probe were used as indicated. GST, Glutathione S-transferase. CDS, Coding sequence. D, Schematic diagrams of the effector and reporter constructs used the dual-luciferase reporter assays. E, Transactivity assay in rice proplasts. EIL1 protein positively regulates SD1 expression, which is used as the positive control. LUC, Luciferase. Data in A and E are Mean ± SD of three independent experiments (n = 10 for each experiment). ** indicates the significant differences between transgenic plants and wild type at the P < 0.01 by the Student’s t-test.
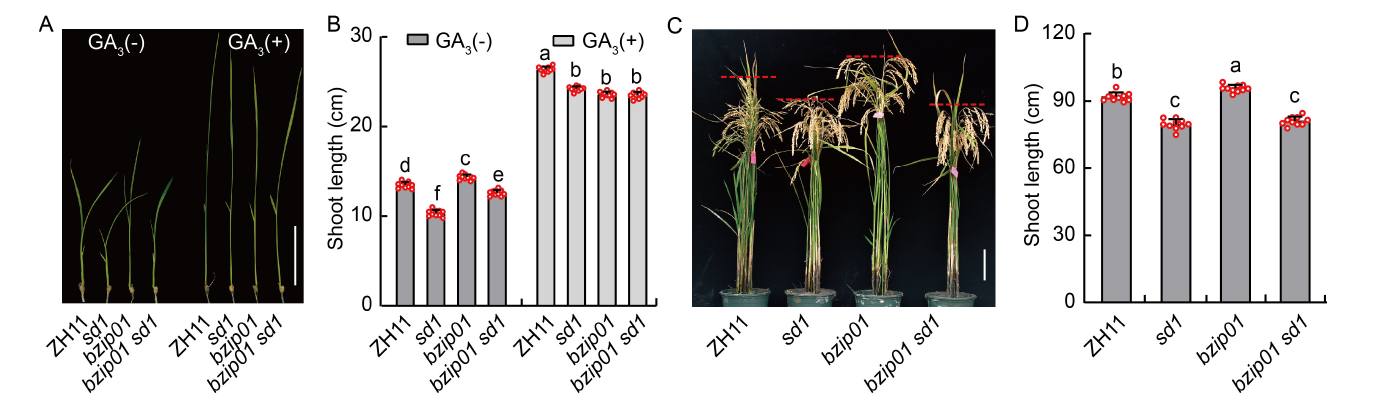
Fig. 6. Genetic relationship of OsbZIP01 and OsSD1. A and B, Shoot lengths of the single (sd1 and bzip01) and double (bzip01 sd1) mutant seedlings under Zhonghua 11 (ZH11) background with and without 10 μmol/L GA3 treatment [GA3(+) and GA3(-)] for 10 d. C and D, Phenotypic characterization (C) and shoot lengths (D) of the single (sd1 and bzip01) and double (bzip01 sd1) mutants at the maturity stage. In A and C, scale bars are 5 cm. In B and D, data are Mean ± SD of three independent experiments (n = 10 for each experiment), and different lowercase letters represent significant differences at the P < 0.05 by the Student’s t-test.
| [1] | Asano K, Yamasaki M, Takuno S, Miura K, Katagiri S, Ito T, Doi K, Wu J Z, Ebana K, Matsumoto T, Innan H, Kitano H, Ashikari M, Matsuoka M. 2011. Artificial selection for a green revolution gene during japonica rice domestication. Proc Natl Acad Sci USA, 108(27): 11034-11039. |
| [2] | Beyene G, Chauhan R D, Villmer J, Husic N, Wang N, Gebre E, Girma D, Chanyalew S, Assefa K, Tabor G, Gehan M, McGrone M, Yang M Z, Lenderts B, Schwartz C, Gao H R, Gordon- Kamm W, Taylor N J, MacKenzie D J. 2022. CRISPR/Cas9- mediated tetra-allelic mutation of the ‘Green Revolution’ SEMIDWARF-1 (SD-1) gene confers lodging resistance in tef (Eragrostis tef). Plant Biotechnol J, 20(9): 1716-1729. |
| [3] | Bhatnagar A, Burman N, Sharma E, Tyagi A, Khurana P, Khurana J P. 2023. Two splice forms of OsbZIP1, a homolog of AtHY5, function to regulate skotomorphogenesis and photomorphogenesis in rice. Plant Physiol, 193(1): 426-447. |
| [4] | Burman N, Bhatnagar A, Khurana J P. 2018. OsbZIP48, a HY5 transcription factor ortholog, exerts pleiotropic effects in light- regulated development. Plant Physiol, 176(2): 1262-1285. |
| [5] | Chen X, Lu S C, Wang Y F, Zhang X, Lv B, Luo L Q, Xi D D, Shen J B, Ma H, Ming F. 2015. OsNAC2 encoding a NAC transcription factor that affects plant height through mediating the gibberellic acid pathway in rice. Plant J, 82(2): 302-314. |
| [6] | Cho S H, Kang K, Lee S H, Lee I J, Paek N C. 2016. OsWOX3A is involved in negative feedback regulation of the gibberellic acid biosynthetic pathway in rice (Oryza sativa). J Exp Bot, 67(6): 1677-1687. |
| [7] | Chu Y L, Xu N, Wu Q, Yu B, Li X X, Chen R R, Huang J L. 2019. Rice transcription factor OsMADS57 regulates plant height by modulating gibberellin catabolism. Rice, 12(1): 38. |
| [8] | Gao S P, Chu C C. 2020. Gibberellin metabolism and signaling: Targets for improving agronomic performance of crops. Plant Cell Physiol, 61(11): 1902-1911. |
| [9] | Gao W W, Li M K, Yang S G, Gao C Z, Su Y, Zeng X, Jiao Z L, Xu W J, Zhang M Y, Xia K F. 2022. miR2105 and the kinase OsSAPK 10 co-regulate OsbZIP86 to mediate drought-induced ABA biosynthesis in rice. Plant Physiol, 189(2): 889-905. |
| [10] | Hasegawa T, Lucob-Agustin N, Yasufuku K, Kojima T, Nishiuchi S, Ogawa A, Takahashi-Nosaka M, Kano-Nakata M, Inari-Ikeda M, Sato M, Tsuji H, Wainaina C M, Yamauchi A, Inukai Y. 2021. Mutation of OUR1/OsbZIP1, which encodes a member of the basic leucine zipper transcription factor family, promotes root development in rice through repressing auxin signaling. Plant Sci, 306: 110861. |
| [11] | Hedden P. 2001. Gibberellin metabolism and its regulation. J Plant Growth Regul, 20(4): 317-318. |
| [12] | Hedden P, Phillips A L. 2000. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci, 5(12): 523-530. |
| [13] | Im J H, Lee S G, Lee E, Park S R, Ahn I, Hwang D J. 2019. OsbZIP75 positively regulates plant defense against the bacterial leaf blight pathogen Xanthomonas oryzae pv. oryzae. Plant Biotechnol Rep, 13(6): 645-651. |
| [14] | Kuroha T, Nagai K, Gamuyao R, Wang D R, Furuta T, Nakamori M, Kitaoka T, Adachi K, Minami A, Mori Y, Mashiguchi K, Seto Y, Yamaguchi S, Kojima M, Sakakibara H, Wu J Z, Ebana K, Mitsuda N, Ohme-Takagi M, Yanagisawa S, Yamasaki M, Yokoyama R, Nishitani K, Mochizuki T, Tamiya G, McCouch S R, Ashikari M. 2018. Ethylene-gibberellin signaling underlies adaptation of rice to periodic flooding. Science, 361: 181-186. |
| [15] | Li J T, Zhao Y, Chu H W, Wang L K, Fu Y R, Liu P, Upadhyaya N, Chen C L, Mou T M, Feng Y Q, Kumar P, Xu J. 2015. SHOEBOX modulates root meristem size in rice through dose-dependent effects of gibberellins on cell elongation and proliferation. PLoS Genet, 11(8): e1005464. |
| [16] | Liu C, Zheng S, Gui J S, Fu C J, Yu H S, Song D L, Shen J H, Qin P, Liu X M, Han B, Yang Y Z, Li L G. 2018. Shortened basal internodes encodes a gibberellin 2-oxidase and contributes to lodging resistance in rice. Mol Plant, 11(2): 288-299. |
| [17] | Liu F, Wang P D, Zhang X B, Li X F, Yan X H, Fu D H, Wu G. 2018. The genetic and molecular basis of crop height based on a rice model. Planta, 247(1): 1-26. |
| [18] | Lu S J, Wei H, Wang Y, Wang H M, Yang R F, Zhang X B, Tu J M. 2012. Overexpression of a transcription factor OsMADS15 modifies plant architecture and flowering time in rice (Oryza sativa L.). Plant Mol Biol Rep, 30(6): 1461-1469. |
| [19] | Ma H Z, Liu C, Li ZX, Ran Q J, Xie G N, Wang B M, Fang S, Chu J F, Zhang J R. 2018. ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol, 178(2): 753-770. |
| [20] | Monna L, Kitazawa N, Yoshino R, Suzuki J, Masuda H, Maehara Y, Tanji M S, Sato M, Nasu S, Minobe Y. 2002. Positional cloning of rice semidwarfing gene, sd-1: Rice ‘green revolution gene’ encodes a mutant enzyme involved in gibberellin synthesis. DNA Res, 9(1): 11-17. |
| [21] | Nijhawan A, Jain M, Tyagi A K, Khurana J P. 2008. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol, 146(2): 323-324. |
| [22] | Oikawa T, Koshioka M, Kojima K, Yoshida H, Kawata M. 2004. A role of OsGA20ox1, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice. Plant Mol Biol, 55: 687-700. |
| [23] | Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush G S, Kitano H, Matsuoka M. 2002. Green revolution: A mutant gibberellin-synthesis gene in rice. Nature, 416: 701-702. |
| [24] | Silverstone A L, Sun T P. 2000. Gibberellins and the green revolution. Trends Plant Sci, 5(1): 1-2. |
| [25] | Spielmeyer W, Ellis M H, Chandler P M. 2002. Semidwarf (sd-1), green revolution rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA, 99(13): 9043-9048. |
| [26] | Su S, Hong J, Chen X F, Zhang C Q, Chen M J, Luo Z J, Chang S W, Bai S X, Liang W Q, Liu Q Q, Zhang D B. 2021. Gibberellins orchestrate panicle architecture mediated by DELLA-KNOX signalling in rice. Plant Biotechnol J, 19(11): 2304-2318. |
| [27] | Tang L Q, Xu H Y, Wang Y F, Wang H M, Li Z Y, Liu X X, Shu Y Z, Li G, Liu W N, Ying J Z, Tong X H, Yao J L, Xiao W F, Tang S Q, Ni S, Zhang J. 2021. OsABF1 represses gibberellin biosynthesis to regulate plant height and seed germination in rice (Oryza sativa L.). Int J Mol Sci, 22(22): 12220. |
| [28] | Wang Q, Lin Q B, Wu T, Duan E C, Huang Y S, Yang C Y, Mou C L, Lan J, Zhou C L, Xie K, Liu X, Zhang X, Guo X P, Wang J, Jiang L, Wan J M. 2020. OsDOG1L-3 regulates seed dormancy through the abscisic acid pathway in rice. Plant Sci, 298: 110570. |
| [29] | Wang Y, Li J. 2008. Molecular basis of plant architecture. Annu Rev Plant Biol, 59: 253-279. |
| [30] | Yamaguchi S. 2008. Gibberellin metabolism and its regulation. Annu Rev Plant Biol, 59: 225-251. |
| [31] | Yang C, Ma Y M, Li J X. 2016. The rice YABBY4 gene regulates plant growth and development through modulating the gibberellin pathway. J Exp Bot, 67(18): 5545-5556. |
| [32] | Yang X C, Hwa C M. 2008. Genetic modification of plant architecture and variety improvement in rice. Heredity, 101(5): 396-404. |
| [33] | Ye H, Feng J H, Zhang L H, Zhang J F, Mispan M S, Cao Z Q, Beighley D H, Yang J C, Gu X Y. 2015. Map-based cloning of seed dormancy1-2 identified a gibberellin synthesis gene regulating the development of endosperm-imposed dormancy in rice. Plant Physiol, 169(3): 2152-2165. |
| [34] | Yin X M, Liu X, Xu B X, Lu P Y, Dong T, Yang D, Ye T T, Feng Y Q, Wu Y. 2019. OsMADS18, a membrane-bound MADS-box transcription factor, modulates plant architecture and the abscisic acid response in rice. J Exp Bot, 70(15): 3895-3909. |
| [35] | Zhang W, Cochet F, Ponnaiah M, Lebreton S, Matheron L, Pionneau C, Boudsocq M, Resentini F, Huguet S, Blázquez M Á, Bailly C, Puyaubert J, Baudouin E. 2019. The MPK8-TCP14 pathway promotes seed germination in Arabidopsis. Plant J, 100(4): 677-692. |
| [36] | Zhang Y, Su J B, Duan S, Ao Y, Dai J R, Liu J, Wang P, Li Y G, Liu B, Feng D R, Wang J F, Wang H B. 2011. A highly efficient rice green tissue protoplast system for transient gene expression and studying light/chloroplast-related processes. Plant Methods, 7(1): 30. |
| [37] | Zhou J P, Liu G Q, Zhao Y X, Zhang R, Tang X, Li L, Jia X Y, Guo Y C, Wu Y C, Han Y S, Bao Y, He Y, Han Q Q, Yang H, Zheng X L, Qi Y P, Zhang T, Zhang Y. 2023. An efficient CRISPR- Cas12a promoter editing system for crop improvement. Nat Plants, 9(4): 588-604. |
| [38] | Zhou W, Malabanan P B, Abrigo E. 2015. OsHox4 regulates GA signaling by interacting with DELLA-like genes and GA oxidase genes in rice. Euphytica, 201(1): 97-107. |
| [39] | Zong W, Tang N, Yang J, Peng L, Ma S Q, Xu Y, Li G L, Xiong L Z. 2016. Feedback regulation of ABA signaling and biosynthesis by a bZIP transcription factor targets drought-resistance-related genes. Plant Physiol, 171(4): 2810-2825. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||