
Rice Science ›› 2025, Vol. 32 ›› Issue (5): 617-636.DOI: 10.1016/j.rsci.2025.06.003
• Reviews • Previous Articles Next Articles
Asif Ali1,#, Sumer Zulfiqar2,#, Asad Riaz3, Maneesh Lingwan4, Sun Lianping5( ), Wu Xianjun1(
), Wu Xianjun1( )
)
Received:2025-03-01
Accepted:2025-06-19
Online:2025-09-28
Published:2025-10-11
Contact:
Wu Xianjun (About author:#These authors contributed equally to this work
Asif Ali, Sumer Zulfiqar, Asad Riaz, Maneesh Lingwan, Sun Lianping, Wu Xianjun. Molecular and Functional Roles of Tapetum Organelles: A Nursing Staff for Pollen Development[J]. Rice Science, 2025, 32(5): 617-636.
Add to citation manager EndNote|Ris|BibTeX
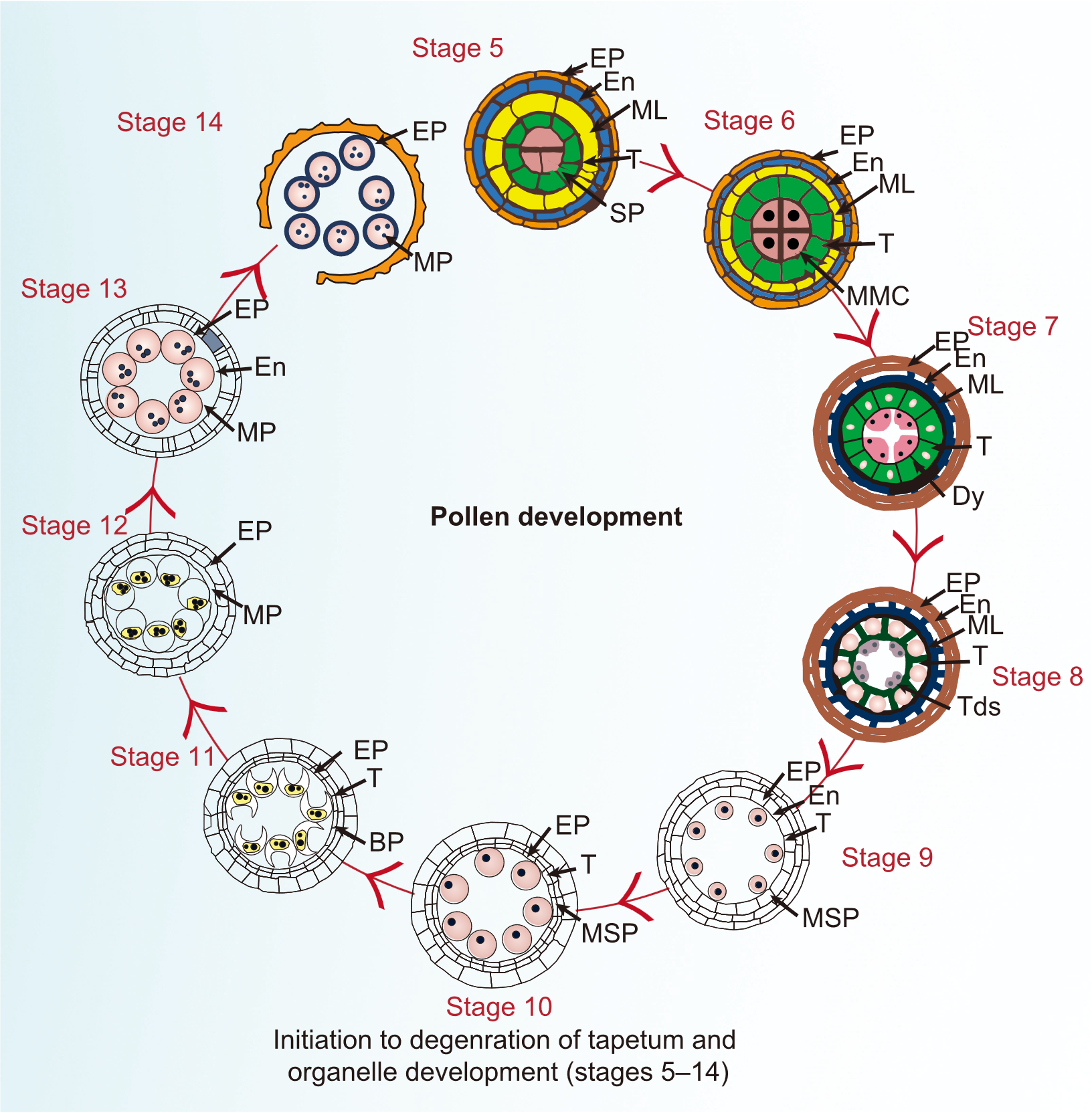
Fig. 1. Cytological morphology of anther’s cross section and development of pollen grain. Cytological development of the pollen grain during anther development stages is illustrated from stages 5-14, excluding stages 1-4 where tapetum is not present. In stage 5, cross section shows formation of T from SP. In stage 6, differentiation of the tapetal cells and formation of MMC, also known as pollen mother cell. In stage 7, meiosis I begins in MMC, and ML starts disappearing and ellipsoidal-shaped Dy are formed. In stage 8, MMC undergoes a further meiotic division (meiosis II), resulting in the formation of Tds. Among four haploid microspores, three free microspores degenerate and only one survives. In stage 9, callose wall is degraded by callases secreted from tapetal cells and Ubisch bodies are released from tapetal organelles and ML has disappeared and T starts degeneration. In stage 10, microspore continues to grow and becomes round and vacuolated. In stage 11, microspore goes into mitosis I and becomes sickle shaped to form a vegetative cell (large) and generative cell (small), T completely degenerated and its contents are released into anther locule for pollen exine formation. In stage 12, two sperm cells are formed from a generative cell as a result of mitosis II. In stage 13, pollen grain maturation is accomplished and anther dehiscence starts, whereas ML completely disappeared. In stage 14, mature pollen grains are released from anthers. Mature pollen grain lacks a complete MMC and callose walls at this stage. BP, Bicellular pollen; Dy, Dyad; EP, Epidermis, outermost layer of the anther; EN, Endothecium, inner layer of the anther; ML, Middle layer of the anther; MMC, Microspore mother cell; MP, Mature pollen; MSP, Megasporocyte; SP, Secondary parietal cell; T, Tapetum, innermost layer of the anther; Tds, Tetrads.

Fig. 2. Synchronous process of microsporogenesis and microgametogenesis. Flowers produce diploid (2n) microsporocytes, which undergo meiosis to produce four haploids. During early microspore development, free microspores have dense cytoplasm with small vacuoles. Each microspore goes into mitosis and bicellular pollen grains are formed. As they progress towards maturation, the cytoplasm moves to the periphery and vacuoles combine into a single large vacuole. Bicellular pollen grains contain multiple miniature vacuoles and numerous subcellular organelles. Vacuoles in tapetal cells are involved in nutrient storage and sporopollenin precursor degradation. Sporopollenin is crucial for microspore integrity and maturation and is produced by tapetal cells and protects pollen from harsh environments before germination.
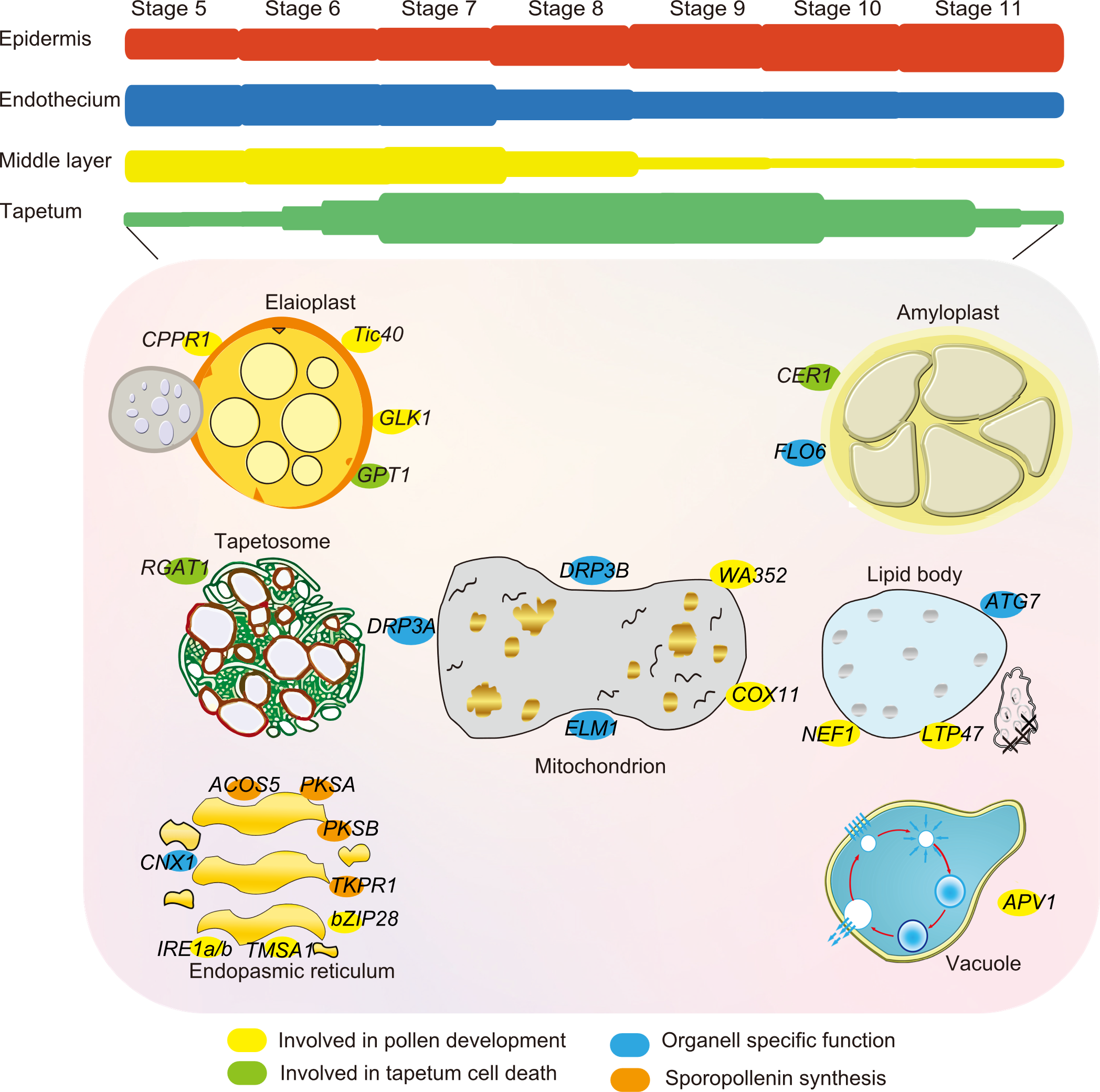
Fig. 3. Essential genes and functional roles of tapetal organelles. The development of the anther is divided into 14 distinct stages based on its cytological features. The tapetum, which is an essential tissue for pollen development, appears in stage 5 and degenerates at stage 11. During stages 5-7, tapetum cells actively synthesize and process nutrition and protection required for pollen development. In stages 9-11, these precursors are delivered. Tapetum cells also secrete enzymes, hormones, amino acids, and other nutritious materials essential for pollen development. The molecularly complex deposition of lipids is regulated by tapetal organelles such as elaioplast and tapetosomes. Essential genes with functional roles are present around the surface of these organelles, which include elaioplasts, amyloplasts, lipids bodies, mitochondria, vacuoles, tapetosomes, and endoplasmic reticulum. Specific colored genes can be observed in the key.
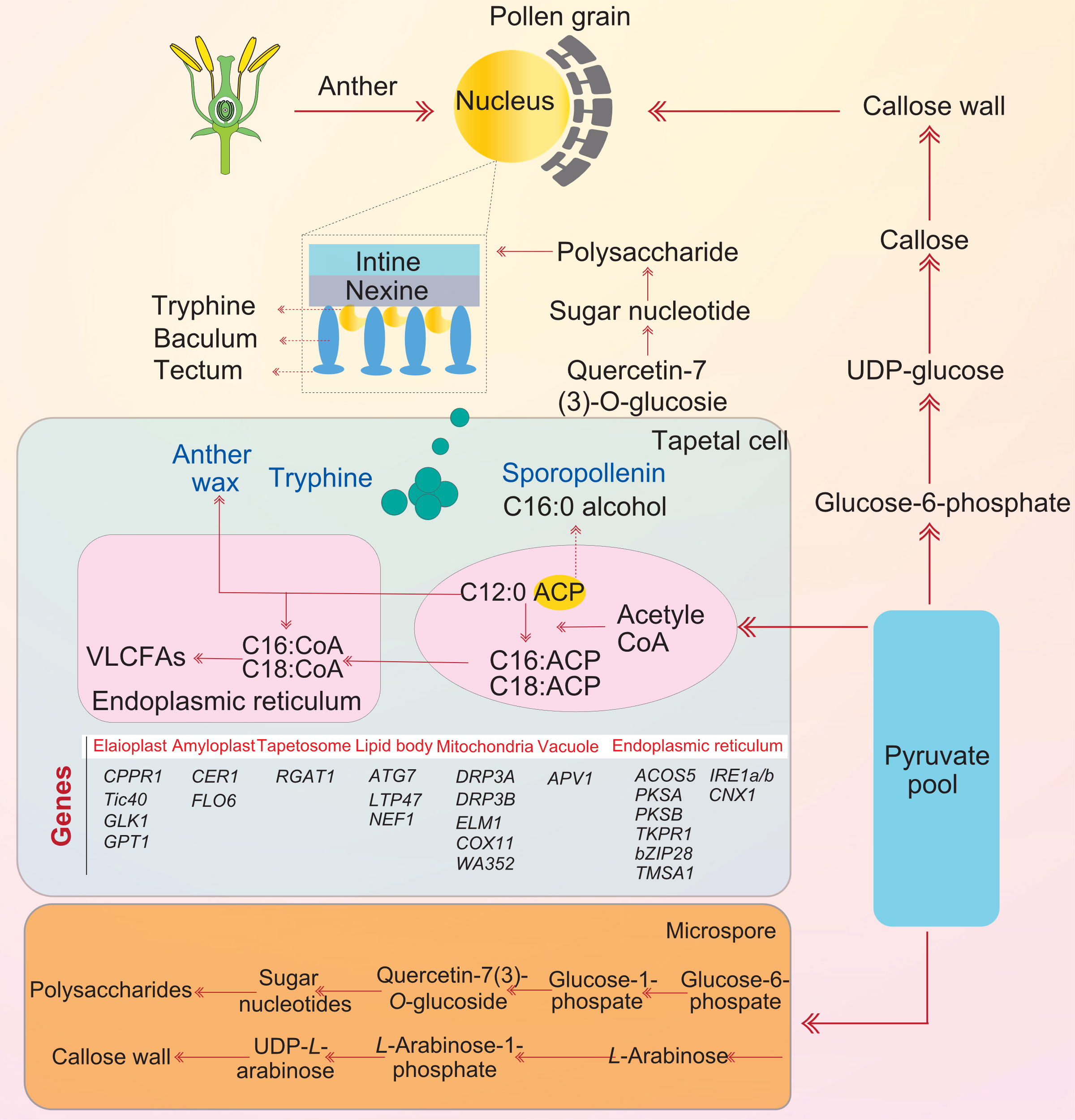
Fig. 4. Biochemical roles of tapetal organelles and their genes in pollen development-associated processes through tricarboxylic acid cycle. The development and degradation of tapetum, the deposition of callose, the production and transport of sporopollenin, and the development of anther wax are all well-known aspects of pollen development. Sporopollenin is a major component in the formation of pollen exine, and its synthesis and transport are coordinated through various molecular and biochemical pathways. Glycolysis and pyruvate pools are reported to be transformed into tapetal cells, and C12 and C16 fatty acid molecules are ultimately processed into sporopollenin synthesis. Pyruvate pool is also converted to polysaccharides and callose walls in microspores. Acetyl-CoA is moved to the tapetal endoplasmic reticulum to form very long chain fatty acids (VLCFAs) that create anther wax. These molecules are then transported from the tapetal cells to create quercetin-7-(3)-O-glucoside and sugar nucleotides, which form the pollen coat. Tapetal cells have some reported genes, which are related to the elaioplast, amyloplast, lipid body, mitochondrion, vacuole, tapetosome, and endoplasmic reticulum. Among tapetal organelles, the endoplasmic reticulum plays its role in pollen exine formation and anther development through bZIP28, IRE1a/b, TKPR1, PKSA, PKSB, and ACOS5. Similarly, CPPR1, Tic40, GLK1, and GPT1 are associated with elaioplast and plastid development and mainly involved in tapetum cell death. ACP, Acyl carrier protein.
| Name | ID | Protein | Function | Reference | |
|---|---|---|---|---|---|
| Pollen wall formation | |||||
| AMS | AT2G16910 | bHLH transcription factor | Early tapetum development | Sorensen et al, | |
| DYT1 | AT4G21330 | bHLH transcription factor | Early tapetum development | Zhang et al, | |
| MYB80 | AT5G56110 | MYB transcription factor | Tapetum programmed cell death, microspore release, exine formation | Zhang et al, | |
| TDF1 | AT3G28470 | MYB transcription factor | Early tapetum development | Zhu et al, | |
| CYP704B2 | Os03g07250 | Cytochrome P450 | Required for anther cutin biosynthesis and pollen exine development | Li et al, | |
| ABCG15 | AT3G21090 | ATP binding cassette transporter | Sporopollenin transportation | Qin et al, | |
| CYP703A3 | Os08g0131100 | Cytochrome P450 protein | Development of anther cuticle and pollen exine | Yang et al, | |
| DPW | Os01g70025 | A cytoplasmically localized BAHD acyltransferase | Pollen development | Xu et al, | |
| OsPKS2 | Os07g0411300 | PKS III superfamily protein | Pollen exine and Ubisch body patterning | Zhu et al, | |
| OsPKS1 | Os10g34360 | PKS III superfamily protein | Pollen exine formation and sporopollenin precursor biosynthetic pathway | Zou et al, | |
| Elaioplast/plastid | |||||
| Tic40 | AT5G16620 | A putative plastid inner envelope membrane translocon | Tapetal function and microspore development | Dun et al, | |
| OsGPT1 | Os08g08840 | Glucose 6-phosphate/Phosphate translocator 1 protein family | Tapetum function and pollen development | Zhang et al, | |
| OsCPPR1 | Os02g0110400 | P-type subfamily of pentatricopeptide repeat proteins | Tapetal programmed cell death and pollen development | Zheng et al, | |
| OsGLK1 | Os06g0348800 | A transcription factor belonging to the MYB GARP family | Plastid differentiation and maintenance | Zheng et al, | |
| Tapetosome | |||||
| RGAT1 | AT1G19530 | DELLA proteins | Gibberellic acid-mediated tapetum and pollen development | Qian et al, | |
| Mitochondrion | |||||
| ELM1 | AT5G22350 | Serine/threonine protein kinase | Mitochondrial fission | Souid et al, | |
| COX11 | LOC4330832 | Nuclear-encoded mitochondrial protein | Induce plant cytoplasmic male sterility | Luo et al, | |
| DRP3A | AT4G33650 | Dynamin-related protein family | Mitochondrial fission | Chen et al, | |
| DRP3B | AT2G14120 | Dynamin-related protein family | Mitochondrial fission | Chen et al, | |
| Endoplasmic reticulum | |||||
| SHD/ HSP90 | AT4G24190 | Heat shock protein 90-7 | Assist in the correct folding and activation of CLV proteins, which are essential for maintaining stem cell development in the shoot apical meristem and floral meristem of Arabidopsis | Ishiguro et al, | |
| ACOS5 | AT1G62940 | Fatty acyl-CoA synthetase | Sporopollenin synthesis | de Azevedo Souza et al, | |
| ERdj3A/ TMS1 | AT3G08970 | Hsp40-homologous proteins, encodes a protein with DnaJ and protein disulfide isomerase, a_ERdj5_C domain | Role in thermotolerance of pollen tubes | Yang et al, | |
| TKPR1 | AT4G35420 | Tetraketide alpha-pyrone reductase | Sporopollenin synthesis | Grienenberger et al, | |
| PKSB | AT4G34850 | Acyltransferase | Sporopollenin synthesis | Kim et al, | |
| BiP1 | AT5G28540 | Hsp70 protein family | Molecular chaperone Hsp70 in the endoplasmic reticulum, involved in the fusion of polar nuclei during female gametophyte development | Maruyama et al, | |
| PKSA | AT1G02050 | Acyltransferase | Sporopollenin synthesis | Chen et al, | |
| BiP2 | AT5G42020 | Hsp70 family of proteins | Expressed and functional in pollen and pollen tubes | Maruyama et al, | |
| IRE1a | AT2G17520 | Protein family of endoribonuclease | Plant development and stress responses, protecting pollen development from elevated temperature | Deng et al, | |
| IRE1b | AT5G24360 | Protein family of endoribonuclease | Plant development and stress responses, protecting pollen development from elevated temperature | Deng et al, | |
| CNX1 | AT5G61790 | CNX/CRT protein family | Roles in vegetative growth and male gametophyte development | Vu et al, | |
| CRT1 | AT1G56340 | CNX/CRT protein family | Roles in vegetative growth and male gametophyte development | Vu et al, et al, 2021 | |
| bZIP28 | AT3G10800 | Basic leucine zipper family of transcription factors | Regulator of reproductive development and fertility genes in plants and participates in unfolded protein response in response to heat stress | Zhang et al, | |
| Vacuole | |||||
| APV1 | GRMZM5G830329 | A member of P450 subfamily | Role in development of pollen exine and anther cuticle | Somaratne et al, | |
| Lipid body | |||||
| nef1 | AT5g13390 | A novel plant protein of 1123 amino acids | Role in pollen development and lipid accumulation within the tapetum | Ariizumi et al, | |
| ATG7 | Os01g0614900 | Autophagy-related protein | Involved in metabolic regulation and nutrient supply in anthers, critical for post-meiotic anther development and pollen maturation | Kurusu et al, | |
| OsLTP47 | Os02g51590 | Non-specific lipid transfer protein | Function in a lipid transfer relay essential for pollen wall development | Chen et al, | |
| Amyloplast | |||||
| FLO6 | Os06g46350 | A CBM48 domain-containing protein | Involved in compound granule formation and starch synthesis in rice endosperm | Peng et al, | |
| OsCER1 | AK066386 | Ceramide synthase family | Biosynthesis of very long-chain alkanes and tapetum degeneration | Ni et al, | |
| Autophagosome | |||||
| SlMYB72 | Solyc07g055000 | MYB transcription factor | Tapetum degradation and pollen development | Wu et al, | |
Table 1. Genes and proteins involved in regulating the functions of various tapetal organelles.
| Name | ID | Protein | Function | Reference | |
|---|---|---|---|---|---|
| Pollen wall formation | |||||
| AMS | AT2G16910 | bHLH transcription factor | Early tapetum development | Sorensen et al, | |
| DYT1 | AT4G21330 | bHLH transcription factor | Early tapetum development | Zhang et al, | |
| MYB80 | AT5G56110 | MYB transcription factor | Tapetum programmed cell death, microspore release, exine formation | Zhang et al, | |
| TDF1 | AT3G28470 | MYB transcription factor | Early tapetum development | Zhu et al, | |
| CYP704B2 | Os03g07250 | Cytochrome P450 | Required for anther cutin biosynthesis and pollen exine development | Li et al, | |
| ABCG15 | AT3G21090 | ATP binding cassette transporter | Sporopollenin transportation | Qin et al, | |
| CYP703A3 | Os08g0131100 | Cytochrome P450 protein | Development of anther cuticle and pollen exine | Yang et al, | |
| DPW | Os01g70025 | A cytoplasmically localized BAHD acyltransferase | Pollen development | Xu et al, | |
| OsPKS2 | Os07g0411300 | PKS III superfamily protein | Pollen exine and Ubisch body patterning | Zhu et al, | |
| OsPKS1 | Os10g34360 | PKS III superfamily protein | Pollen exine formation and sporopollenin precursor biosynthetic pathway | Zou et al, | |
| Elaioplast/plastid | |||||
| Tic40 | AT5G16620 | A putative plastid inner envelope membrane translocon | Tapetal function and microspore development | Dun et al, | |
| OsGPT1 | Os08g08840 | Glucose 6-phosphate/Phosphate translocator 1 protein family | Tapetum function and pollen development | Zhang et al, | |
| OsCPPR1 | Os02g0110400 | P-type subfamily of pentatricopeptide repeat proteins | Tapetal programmed cell death and pollen development | Zheng et al, | |
| OsGLK1 | Os06g0348800 | A transcription factor belonging to the MYB GARP family | Plastid differentiation and maintenance | Zheng et al, | |
| Tapetosome | |||||
| RGAT1 | AT1G19530 | DELLA proteins | Gibberellic acid-mediated tapetum and pollen development | Qian et al, | |
| Mitochondrion | |||||
| ELM1 | AT5G22350 | Serine/threonine protein kinase | Mitochondrial fission | Souid et al, | |
| COX11 | LOC4330832 | Nuclear-encoded mitochondrial protein | Induce plant cytoplasmic male sterility | Luo et al, | |
| DRP3A | AT4G33650 | Dynamin-related protein family | Mitochondrial fission | Chen et al, | |
| DRP3B | AT2G14120 | Dynamin-related protein family | Mitochondrial fission | Chen et al, | |
| Endoplasmic reticulum | |||||
| SHD/ HSP90 | AT4G24190 | Heat shock protein 90-7 | Assist in the correct folding and activation of CLV proteins, which are essential for maintaining stem cell development in the shoot apical meristem and floral meristem of Arabidopsis | Ishiguro et al, | |
| ACOS5 | AT1G62940 | Fatty acyl-CoA synthetase | Sporopollenin synthesis | de Azevedo Souza et al, | |
| ERdj3A/ TMS1 | AT3G08970 | Hsp40-homologous proteins, encodes a protein with DnaJ and protein disulfide isomerase, a_ERdj5_C domain | Role in thermotolerance of pollen tubes | Yang et al, | |
| TKPR1 | AT4G35420 | Tetraketide alpha-pyrone reductase | Sporopollenin synthesis | Grienenberger et al, | |
| PKSB | AT4G34850 | Acyltransferase | Sporopollenin synthesis | Kim et al, | |
| BiP1 | AT5G28540 | Hsp70 protein family | Molecular chaperone Hsp70 in the endoplasmic reticulum, involved in the fusion of polar nuclei during female gametophyte development | Maruyama et al, | |
| PKSA | AT1G02050 | Acyltransferase | Sporopollenin synthesis | Chen et al, | |
| BiP2 | AT5G42020 | Hsp70 family of proteins | Expressed and functional in pollen and pollen tubes | Maruyama et al, | |
| IRE1a | AT2G17520 | Protein family of endoribonuclease | Plant development and stress responses, protecting pollen development from elevated temperature | Deng et al, | |
| IRE1b | AT5G24360 | Protein family of endoribonuclease | Plant development and stress responses, protecting pollen development from elevated temperature | Deng et al, | |
| CNX1 | AT5G61790 | CNX/CRT protein family | Roles in vegetative growth and male gametophyte development | Vu et al, | |
| CRT1 | AT1G56340 | CNX/CRT protein family | Roles in vegetative growth and male gametophyte development | Vu et al, et al, 2021 | |
| bZIP28 | AT3G10800 | Basic leucine zipper family of transcription factors | Regulator of reproductive development and fertility genes in plants and participates in unfolded protein response in response to heat stress | Zhang et al, | |
| Vacuole | |||||
| APV1 | GRMZM5G830329 | A member of P450 subfamily | Role in development of pollen exine and anther cuticle | Somaratne et al, | |
| Lipid body | |||||
| nef1 | AT5g13390 | A novel plant protein of 1123 amino acids | Role in pollen development and lipid accumulation within the tapetum | Ariizumi et al, | |
| ATG7 | Os01g0614900 | Autophagy-related protein | Involved in metabolic regulation and nutrient supply in anthers, critical for post-meiotic anther development and pollen maturation | Kurusu et al, | |
| OsLTP47 | Os02g51590 | Non-specific lipid transfer protein | Function in a lipid transfer relay essential for pollen wall development | Chen et al, | |
| Amyloplast | |||||
| FLO6 | Os06g46350 | A CBM48 domain-containing protein | Involved in compound granule formation and starch synthesis in rice endosperm | Peng et al, | |
| OsCER1 | AK066386 | Ceramide synthase family | Biosynthesis of very long-chain alkanes and tapetum degeneration | Ni et al, | |
| Autophagosome | |||||
| SlMYB72 | Solyc07g055000 | MYB transcription factor | Tapetum degradation and pollen development | Wu et al, | |
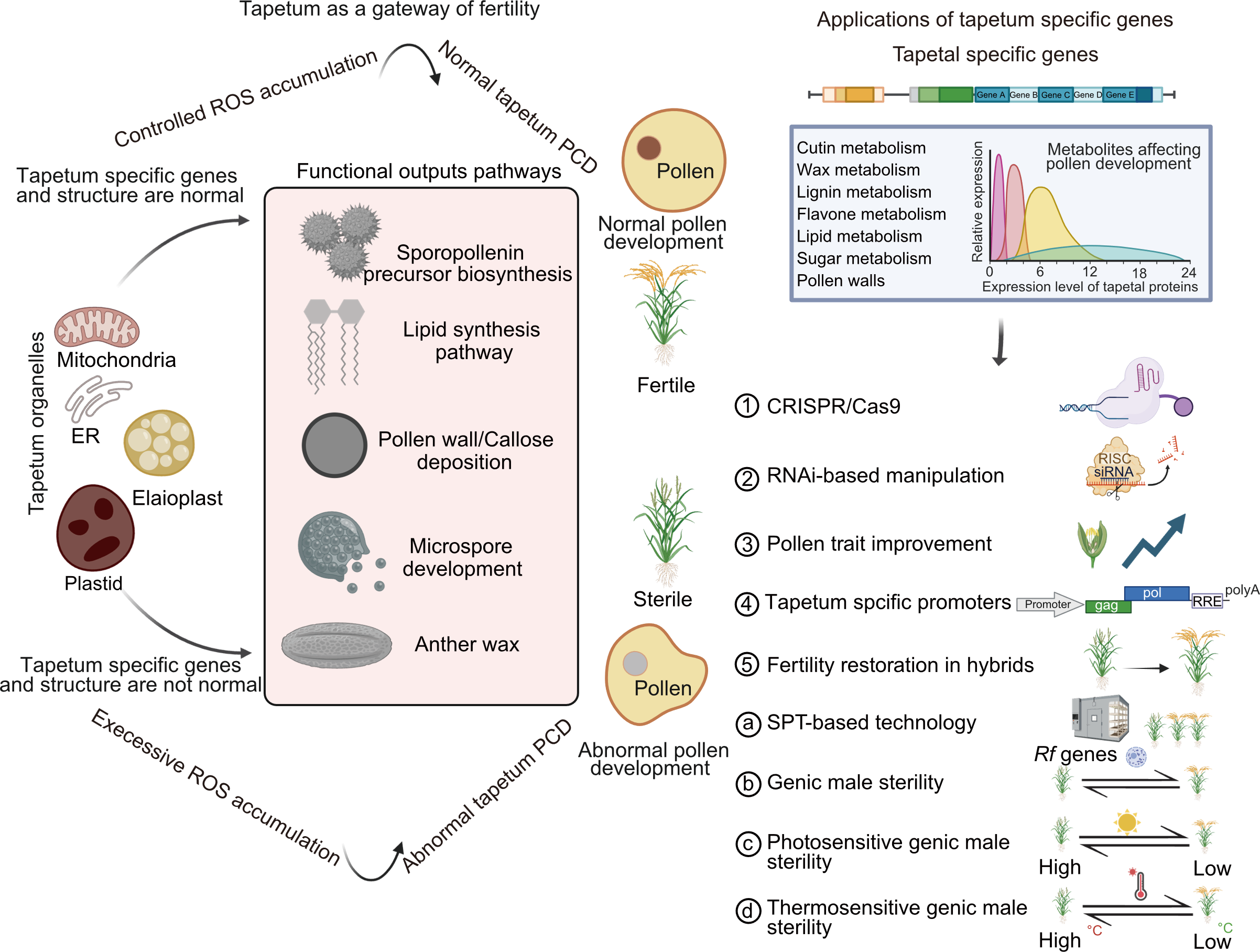
Fig. 5. Tapetum and its organelle-associated genes: A gateway to pollen fertility and applications in crop breeding. Schematic overview of tapetum organelles contributing to pollen wall formation, nutrient secretion, and sporopollenin synthesis, enabling advances in pollen fertility, male sterility, and crop breeding program. ER, Endoplasmic reticulum; PCD, Programmed cell death; ROS, Reactive oxygen species; SPT, Seed production technology.
| [1] | Ali A, Wu T K, Zhang H Y, et al. 2022. A putative SUBTILISIN-LIKE SERINE PROTEASE 1 (SUBSrP1) regulates anther cuticle biosynthesis and panicle development in rice. J Adv Res, 42: 273-287. |
| [2] | An X L, Zhang S W, Jiang Y L, et al. 2024. CRISPR/Cas9-based genome editing of 14 lipid metabolic genes reveals a sporopollenin metabolon ZmPKSB-ZmTKPR1-1/-2 required for pollen exine formation in maize. Plant Biotechnol J, 22(1): 216-232. |
| [3] | Ariizumi T, Toriyama K. 2011. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu Rev Plant Biol, 62: 437-460. |
| [4] | Ariizumi T, Hatakeyama K, Hinata K, et al. 2004. Disruption of the novel plant protein NEF 1 affects lipid accumulation in the plastids of the tapetum and exine formation of pollen, resulting in male sterility in Arabidopsis thaliana. Plant J, 39(2): 170-181. |
| [5] | Åstrand J, Knight C, Robson J, et al. 2021. Evolution and diversity of the angiosperm anther: Trends in function and development. Plant Reprod, 34(4): 307-319. |
| [6] | Barros J A S, Siqueira J A B, Cavalcanti J H F, et al. 2020. Multifaceted roles of plant autophagy in lipid and energy metabolism. Trends Plant Sci, 25(11): 1141-1153. |
| [7] | Benning C. 2009. Mechanisms of lipid transport involved in organelle biogenesis in plant cells. Annu Rev Cell Dev Biol, 25: 71-91. |
| [8] | Biswas R, Chaudhuri S. 2024. The tale of tapetum: From anther walls to pollen wall. Nucleus, 67(3): 611-630. |
| [9] | Blancaflor E B. 2013. Regulation of plant gravity sensing and signaling by the actin cytoskeleton. Am J Bot, 100(1): 143-152. |
| [10] | Buono R A, Hudecek R, Nowack M K. 2019. Plant proteases during developmental programmed cell death. J Exp Bot, 70(7): 2097-2112. |
| [11] | Cai C F, Zhu J, Lou Y, et al. 2015. The functional analysis of OsTDF1 reveals a conserved genetic pathway for tapetal development between rice and Arabidopsis. Sci Bull, 60(12): 1073-1082. |
| [12] | Chen L B, Ji C H, Zhou D G, et al. 2022. OsLTP47 may function in a lipid transfer relay essential for pollen wall development in rice. J Genet Genom, 49(5): 481-491. |
| [13] | Chen P Y, Wu C C, Lin C C, et al. 2019. 3D imaging of tapetal mitochondria suggests the importance of mitochondrial fission in pollen growth. Plant Physiol, 180(2): 813-826. |
| [14] | Chen W W, Yu X H, Zhang K S, et al. 2011. Male Sterile2 encodes a plastid-localized fatty acyl carrier protein reductase required for pollen exine development in Arabidopsis. Plant Physiol, 157(2): 842-853. |
| [15] | Chen X Y, Li Y F, Sun H Y, et al. 2024. Molecular mechanisms of male sterility in maize. Plant Mol Biol Rep, 42(3): 483-491. |
| [16] | Choi H, Jin J Y, Choi S, et al. 2011. An ABCG/WBC-type ABC transporter is essential for transport of sporopollenin precursors for exine formation in developing pollen. Plant J, 65(2): 181-193. |
| [17] | Christensen A, Svensson K, Thelin L, et al. 2010. Higher plant calreticulins have acquired specialized functions in Arabidopsis. PLoS One, 5(6): e11342. |
| [18] | de Azevedo Souza C, Kim S S, Koch S, et al. 2009. A novel fatty Acyl-CoA Synthetase is required for pollen development and sporopollenin biosynthesis in Arabidopsis. Plant Cell, 21(2): 507-525. |
| [19] | de Vita E, Lucy D, Tate E W. 2021. Beyond targeted protein degradation: LD·ATTECs clear cellular lipid droplets. Cell Res, 31(9): 945-946. |
| [20] | Deng Y, Srivastava R, Quilichini T D, et al. 2016. IRE 1, a component of the unfolded protein response signaling pathway, protects pollen development in Arabidopsis from heat stress. Plant J, 88(2): 193-204. |
| [21] | Dobritsa A A, Shrestha J, Morant M, et al. 2009. CYP704B1 is a long-chain fatty acid ω-hydroxylase essential for sporopollenin synthesis in pollen of Arabidopsis. Plant Physiol, 151(2): 574-589. |
| [22] | Dun X L, Zhou Z F, Xia S Q, et al. 2011. BnaC.Tic40, a plastid inner membrane translocon originating from Brassica oleracea, is essential for tapetal function and microspore development in Brassica napus. Plant J, 68(3): 532-545. |
| [23] | Dündar G, Shao Z H, Higashitani N, et al. 2019. Autophagy mitigates high-temperature injury in pollen development of Arabidopsis thaliana. Dev Biol, 456(2): 190-200. |
| [24] | Fan Y Z, Asao S, Furbank R T, et al. 2022. The crucial roles of mitochondria in supporting C4 photosynthesis. New Phytol, 233(3): 1083-1096. |
| [25] | Farooq M, Rehman A, Wahid A, et al. 2021. Physiology of grain development in cereals. In: Handbook of Plant and Crop Physiology. 4th edition. FL, USA: CRC Press: 246-260. |
| [26] | Gabarayeva N I. 2025. Tapetum uncommon behavior, orbicule development, and pollenkitt: Mini-review, with new data on orbicule simulations. Protoplasma, doi: 10.1007/s00709-025-02053-1. |
| [27] | Gabarayeva N I, Grigorjeva V V, Shavarda A L. 2019. Mimicking pollen and spore walls: Self-assembly in action. Ann Bot, 123(7): 1205-1218. |
| [28] | Gómez-Virgilio L, Silva-Lucero M D C, Flores-Morelos D S, et al. 2022. Autophagy: A key regulator of homeostasis and disease: An overview of molecular mechanisms and modulators. Cells, 11(15): 2262. |
| [29] | Gotelli M, Lattar E, Zini L M, et al. 2023. Review on tapetal ultrastructure in angiosperms. Planta, 257(6): 100. |
| [30] | Grienenberger E, Kim S S, Lallemand B, et al. 2010. Analysis of TETRAKETIDE α-PYRONE REDUCTASE function in Arabidopsis thaliana reveals a previously unknown, but conserved, biochemical pathway in sporopollenin monomer biosynthesis. Plant Cell, 22(12): 4067-4083. |
| [31] | Gu J N, Zhu J, Yu Y, et al. 2014. DYT1 directly regulates the expression of TDF1 for tapetum development and pollen wall formation in Arabidopsis. Plant J, 80(6): 1005-1013. |
| [32] | Guo Y, Zhang S H, Li Y, et al. 2023. A transcriptomic evaluation of the mechanism of programmed cell death of the replaceable bud in Chinese chestnut. Open Life Sci, 18(1): 20220635. |
| [33] | Hafidh S, Fíla J, Honys D. 2016. Male gametophyte development and function in angiosperms: A general concept. Plant Reprod, 29(1/2): 31-51. |
| [34] | Han S J, Yu B J, Wang Y, et al. 2011. Role of plant autophagy in stress response. Protein Cell, 2(10): 784-791. |
| [35] | Han Y, Zhou S D, Fan J J, et al. 2021. OsMS188 is a key regulator of tapetum development and sporopollenin synthesis in rice. Rice, 14(1): 4. |
| [36] | Hanamata S, Sawada J, Ono S, et al. 2020. Impact of autophagy on gene expression and tapetal programmed cell death during pollen development in rice. Front Plant Sci, 11: 172. |
| [37] | Hanson M R, Conklin P L. 2020. Stromules, functional extensions of plastids within the plant cell. Curr Opin Plant Biol, 58: 25-32. |
| [38] | Harbauer A B, Schneider A, Wohlleber D. 2022. Analysis of mitochondria by single-organelle resolution. Annu Rev Anal Chem, 15(1): 1-16. |
| [39] | Hou X L, Han X Y, Meng Y, et al. 2024. Acyl carrier protein OsMTACP2 confers rice cold tolerance at the booting stage. Plant Physiol, 195(2): 1277-1292. |
| [40] | Hsieh K, Huang A H C. 2004. Endoplasmic reticulum, oleosins, and oils in seeds and tapetum cells. Plant Physiol, 136(3): 3427-3434. |
| [41] | Hsieh K, Huang A H C. 2005. Lipid-rich tapetosomes in Brassica tapetum are composed of oleosin-coated oil droplets and vesicles, both assembled in and then detached from the endoplasmic reticulum. Plant J, 43(6): 889-899. |
| [42] | Hu K K, Nguyen T D K, Rabasco S, et al. 2021. Chemical analysis of single cells and organelles. Anal Chem, 93(1): 41-71. |
| [43] | Huang C Y, Chen P Y, Huang M D, et al. 2013. Tandem oleosin genes in a cluster acquired in Brassicaceae created tapetosomes and conferred additive benefit of pollen vigor. Proc Natl Acad Sci USA, 110(35): 14480-14485. |
| [44] | Huang M D, Wei F J, Wu C C, et al. 2009. Analyses of advanced rice anther transcriptomes reveal global tapetum secretory functions and potential proteins for lipid exine formation. Plant Physiol, 149(2): 694-707. |
| [45] | Huang M D, Chen T L, Huang A H C. 2013. Abundant type III lipid transfer proteins in Arabidopsis tapetum are secreted to the locule and become a constituent of the pollen exine. Plant Physiol, 163(3): 1218-1229. |
| [46] | Huang T H, Suen D F. 2021. Iron insufficiency in floral buds impairs pollen development by disrupting tapetum function. Plant J, 108(1): 244-267. |
| [47] | Ighodaro O M, Akinloye O A. 2018. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alex J Med, 54(4): 287-293. |
| [48] | Ischebeck T. 2016. Lipids in pollen: They are different. Biochim Biophys Acta Mol Cell Biol Lipds, 1861(9): 1315-1328. |
| [49] | Ishiguro S, Watanabe Y, Ito N, et al. 2002. SHEPHERD is the Arabidopsis GRP94 responsible for the formation of functional CLAVATA proteins. EMBO J, 21(5): 898-908. |
| [50] | Ishiguro S, Nishimori Y, Yamada M, et al. 2010. The Arabidopsis FLAKY POLLEN1 gene encodes a 3-hydroxy-3-methylglutaryl-coenzyme A synthase required for development of tapetum-specific organelles and fertility of pollen grains. Plant Cell Physiol, 51(6): 896-911. |
| [51] | Ji C H, Li H Y, Chen L B, et al. 2013. A novel rice bHLH transcription factor, DTD, acts coordinately with TDR in controlling tapetum function and pollen development. Mol Plant, 6(5): 1715-1718. |
| [52] | Jiang J, Zhang Z, Cao J. 2013. Pollen wall development: The associated enzymes and metabolic pathways. Plant Biol, 15(2): 249-263. |
| [53] | Kamogashira T, Fujimoto C, Yamasoba T. 2015. Reactive oxygen species, apoptosis, and mitochondrial dysfunction in hearing loss. BioMed Res Int, 2015(1): 617207. |
| [54] | Kim S S. 2011. Analysis of the Arabidopsis fatty acyl-CoA synthetase5 gene and co-expressed genes reveals an ancient biochemical pathway required for pollen development and sporopollenin biosynthesis. Vancouver, Canada: University of British Columbia. |
| [55] | Kim S S, Grienenberger E, Lallemand B, et al. 2010. LAP6/ POLYKETIDE SYNTHASE A and LAP5/POLYKETIDE SYNTHASE B encode hydroxyalkyl α-pyrone synthases required for pollen development and sporopollenin biosynthesis in Arabidopsis thaliana. Plant Cell, 22(12): 4045-4066. |
| [56] | Kloska A, Węsierska M, Malinowska M, et al. 2020. Lipophagy and lipolysis status in lipid storage and lipid metabolism diseases. Int J Mol Sci, 21(17): 6113. |
| [57] | Kurusu T, Koyano T, Hanamata S, et al. 2014. OsATG7 is required for autophagy-dependent lipid metabolism in rice postmeiotic anther development. Autophagy, 10(5): 878-888. |
| [58] | Kuznetsov A V, Margreiter R, Ausserlechner M J, et al. 2022. The complex interplay between mitochondria, ROS and entire cellular metabolism. Antioxidants, 11(10): 1995. |
| [59] | Lallemand B, Erhardt M, Heitz T, et al. 2013. Sporopollenin biosynthetic enzymes interact and constitute a metabolon localized to the endoplasmic reticulum of tapetum cells. Plant Physiol, 162(2): 616-625. |
| [60] | Lee K H, Piao H L, Kim H Y, et al. 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell, 126(6): 1109-1120. |
| [61] | Lee M, Mullen RTrelease R. 2007. Chloroplasts and other plastids. In: Cooper G M. The Cell: A Molecular Approach. 2nd edition. https://www.ncbi.nlm.nih.gov/books/NBK9905/. |
| [62] | Lei T, Zhang L S, Feng P, et al. 2022. OsMYB103 is essential for tapetum degradation in rice. Theor Appl Genet, 135(3): 929-945. |
| [63] | Lévesque-Lemay M, Chabot D, Hubbard K, et al. 2016. Tapetal oleosins play an essential role in tapetosome formation and protein relocation to the pollen coat. New Phytol, 209(2): 691-704. |
| [64] | Li D D, Xue J S, Zhu J, et al. 2017. Gene regulatory network for tapetum development in Arabidopsis thaliana. Front Plant Sci, 8: 1559. |
| [65] | Li F S, Phyo P, Jacobowitz J, et al. 2019. The molecular structure of plant sporopollenin. Nat Plants, 5(1): 41-46. |
| [66] | Li H, Pinot F, Sauveplane V, et al. 2010. Cytochrome P450 family member CYP704B2 catalyzes the ω-hydroxylation of fatty acids and is required for anther cutin biosynthesis and pollen exine formation in rice. Plant Cell, 22(1): 173-190. |
| [67] | Li H J, Kim Y J, Yang L, et al. 2020. Grass-specific EPAD1 is essential for pollen exine patterning in rice. Plant Cell, 32(12): 3961-3977. |
| [68] | Li L, Yuan H, Zeng Y L, et al. 2016. Plastids and carotenoid accumulation. SubCell Biochem, 79: 273-293. |
| [69] | Li M, Kim C. 2022. Chloroplast ROS and stress signaling. Plant Commun, 3(1): 100264. |
| [70] | Li N, Zhang D S, Liu H S, et al. 2006. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell, 18(11): 2999-3014. |
| [71] | Li Y B, Suen D F, Huang C Y, et al. 2012. The maize tapetum employs diverse mechanisms to synthesize and store proteins and flavonoids and transfer them to the pollen surface. Plant Physiol, 158(4): 1548-1561. |
| [72] | Li Y L, Li D D, Guo Z L, et al. 2016. OsACOS12, an orthologue of Arabidopsis acyl-CoA synthetase5, plays an important role in pollen exine formation and anther development in rice. BMC Plant Biol, 16(1): 256. |
| [73] | Liu K, Czaja M J. 2013. Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ, 20(1): 3-11. |
| [74] | Luo D P, Xu H, Liu Z L, et al. 2013. A detrimental mitochondrial-nuclear interaction causes cytoplasmic male sterility in rice. Nat Genet, 45(5): 573-577. |
| [75] | Ma X, Feng B M, Ma H. 2012. AMS-dependent and independent regulation of anther transcriptome and comparison with those affected by other Arabidopsis anther genes. BMC Plant Biol, 12: 23. |
| [76] | Manan S, Chen B B, She G B, et al. 2017. Transport and transcriptional regulation of oil production in plants. Crit Rev Biotechnol, 37(5): 641-655. |
| [77] | Marchant D B, Walbot V. 2022. Anther development: The long road to making pollen. Plant Cell, 34(12): 4677-4695. |
| [78] | Marty F. 1999. Plant vacuoles. Plant Cell, 11(4): 587-599. |
| [79] | Maruyama D, Yamamoto M, Endo T, et al. 2014. Different sets of ER-resident J-proteins regulate distinct polar nuclear-membrane fusion events in Arabidopsis thaliana. Plant Cell Physiol, 55(11): 1937-1944. |
| [80] | Mayta M L, Hajirezaei M R, Carrillo N, et al. 2019. Leaf senescence: The chloroplast connection comes of age. Plants, 8(11): 495. |
| [81] | Morita M T, Kato T, Nagafusa K, et al. 2002. Involvement of the vacuoles of the endodermis in the early process of shoot gravitropism in Arabidopsis. Plant Cell, 14(1): 47-56. |
| [82] | Murphy D J. 2001. The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res, 40(5): 325-438. |
| [83] | Ni E D, Zhou L Y, Li J, et al. 2018. OsCER1 plays a pivotal role in very-long-chain alkane biosynthesis and affects plastid development and programmed cell death of tapetum in rice (Oryza sativa L.). Front Plant Sci, 9: 1217. |
| [84] | Nikiforidis C V. 2019. Structure and functions of oleosomes (oil bodies). Adv Colloid Interface Sci, 274: 102039. |
| [85] | Nobusawa T, Umeda M. 2012. Very-long-chain fatty acids have an essential role in plastid division by controlling Z-ring formation in Arabidopsis thaliana. Genes Cells, 17(8): 709-719. |
| [86] | Pacini E, Jacquard C, Clément C. 2011. Pollen vacuoles and their significance. Planta, 234(2): 217-227. |
| [87] | Pan C T, Yang D D, Zhao X L, et al. 2021. PIF4 negatively modulates cold tolerance in tomato anthers via temperature-dependent regulation of tapetal cell death. Plant Cell, 33(7): 2320-2339. |
| [88] | Pan X Y, Yan W, Chang Z Y, et al. 2020. OsMYB80 regulates anther development and pollen fertility by targeting multiple biological pathways. Plant Cell Physiol, 61(5): 988-1004. |
| [89] | Parish R W, Li S F. 2010. Death of a tapetum: A programme of developmental altruism. Plant Sci, 178(2): 73-89. |
| [90] | Parzych K R, Klionsky D J. 2014. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid Redox Signal, 20(3): 460-473. |
| [91] | Pei Y R, Deng Y N, Zhang H R, et al. 2022. EAR APICAL DEGENERATION1 regulates maize ear development by maintaining malate supply for apical inflorescence. Plant Cell, 34(6): 2222-2241. |
| [92] | Peng C, Wang Y H, Liu F, et al. 2014. FLOURY ENDOSPERM6 encodes a CBM48 domain-containing protein involved in compound granule formation and starch synthesis in rice endosperm. Plant J, 77(6): 917-930. |
| [93] | Phan H A, Iacuone S, Li S F, et al. 2011. The MYB80 transcription factor is required for pollen development and the regulation of tapetal programmed cell death in Arabidopsis thaliana. Plant Cell, 23(6): 2209-2224. |
| [94] | Piffanelli P, Murphy D J. 1998. Novel organelles and targeting mechanisms in the anther tapetum. Trends Plant Sci, 3(7): 250-252. |
| [95] | Prat S, Frommer W B, Höfgen R, et al. 1990. Gene expression during tuber development in potato plants. FEBS Lett, 268(2): 334-338. |
| [96] | Qian Q, Yang Y H, Zhang W B, et al. 2021. A novel Arabidopsis gene RGAT1 is required for GA-mediated tapetum and pollen development. New Phytol, 231(1): 137-151. |
| [97] | Qiao Y Y, Hou B Z, Qi X Q. 2023. Biosynthesis and transport of pollen coat precursors in angiosperms. Nat Plants, 9(6): 864-876. |
| [98] | Qin P, Tu B, Wang Y, et al. 2013. ABCG15 encodes an ABC transporter protein, and is essential for post-meiotic anther and pollen exine development in rice. Plant Cell Physiol, 54(1):138-154. |
| [99] | Quilichini T D, Douglas C J, Samuels A L. 2014a. New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann Bot, 114(6): 1189-1201. |
| [100] | Quilichini T D, Samuels A L, Douglas C J. 2014b. ABCG26-mediated polyketide trafficking and hydroxycinnamoyl spermidines contribute to pollen wall exine formation in Arabidopsis. Plant Cell, 26(11): 4483-4498. |
| [101] | Rabinowitz J D, White E. 2010. Autophagy and metabolism. Science, 330: 1344-1348. |
| [102] | Sadali N M, Sowden R G, Ling Q H, et al. 2019. Differentiation of chromoplasts and other plastids in plants. Plant Cell Rep, 38(7): 803-818. |
| [103] | Scott R J, Spielman M, Dickinson H G. 2004. Stamen structure and function. Plant Cell, 16: S46-S60. |
| [104] | Shi J X, Cui M H, Yang L, et al. 2015. Genetic and biochemical mechanisms of pollen wall development. Trends Plant Sci, 20(11): 741-753. |
| [105] | Singh M B, Lohani N, Bhalla P L. 2021. The role of endoplasmic reticulum stress response in pollen development and heat stress tolerance. Front Plant Sci, 12: 661062. |
| [106] | Singh S P, Verma R K, Goel R, et al. 2024. Arabidopsis BECLIN1-induced autophagy mediates reprogramming in tapetal programmed cell death by altering the gross cellular homeostasis. Plant Physiol Biochem, 208: 108471. |
| [107] | Somaratne Y, Tian Y H, Zhang H, et al. 2017. ABNORMAL POLLEN VACUOLATION1 (APV1) is required for male fertility by contributing to anther cuticle and pollen exine formation in maize. Plant J, 90(1): 96-110. |
| [108] | Song S F, Wang T K, Li Y X, et al. 2021. A novel strategy for creating a new system of third-generation hybrid rice technology using a cytoplasmic sterility gene and a genic male-sterile gene. Plant Biotechnol J, 19(2): 251-260. |
| [109] | Sorensen A M, Kröber S, Unte U S, et al. 2003. The Arabidopsis ABORTED MICROSPORES (AMS) gene encodes a MYC class transcription factor. Plant J, 33(2): 413-423. |
| [110] | Soto-Avellaneda A, Morrison B E. 2020. Signaling and other functions of lipids in autophagy: A review. Lipids Health Dis, 19(1): 214. |
| [111] | Souid A K, Gao C, Wang L, et al. 2006. ELM1 is required for multidrug resistance in Saccharomyces cerevisiae. Genetics, 173(4): 1919-1937. |
| [112] | Suzuki T, Tsunekawa S, Koizuka C, et al. 2013. Development and disintegration of tapetum-specific lipid-accumulating organelles, elaioplasts and tapetosomes, in Arabidopsis thaliana and Brassica napus. Plant Sci, 207: 25-36. |
| [113] | Tang H L, Song Y L, Guo J L, et al. 2018. Physiological and metabolome changes during anther development in wheat (Triticum aestivum L.). Plant Physiol Biochem, 132: 18-32. |
| [114] | Tariq N, Yaseen M, Xu D, et al. 2023. Rice anther tapetum: A vital reproductive cell layer for sporopollenin biosynthesis and pollen exine patterning. Plant Biol, 25(2): 233-245. |
| [115] | Tirichen H, Yaigoub H, Xu W W, et al. 2021. Mitochondrial reactive oxygen species and their contribution in chronic kidney disease progression through oxidative stress. Front Physiol, 12: 627837. |
| [116] | Uzair M, Xu D W, Schreiber L, et al. 2020. PERSISTENT TAPETAL CELL2 is required for normal tapetal programmed cell death and pollen wall patterning. Plant Physiol, 182(2): 962-976. |
| [117] | Vu K V, Nguyen N T, Jeong C Y, et al. 2017. Systematic deletion of the ER lectin chaperone genes reveals their roles in vegetative growth and male gametophyte development in Arabidopsis. Plant J, 89(5): 972-983. |
| [118] | Wang H, Mao Y F, Yang J, et al. 2015. TCP24 modulates secondary cell wall thickening and anther endothecium development. Front Plant Sci, 6: 436. |
| [119] | Wang N, Li X, Zhu J, et al. 2025. Molecular and cellular mechanisms of photoperiod- and thermo-sensitive genic male sterility in plants. Mol Plant, 18(1): 26-41. |
| [120] | Wang Q M, Tian Y L, Chen K Y, et al. 2025. OsPAD1, encoding a non-specific lipid transfer protein, is required for rice pollen aperture formation. Plant Mol Biol, 115(1): 11. |
| [121] | Wang S P, Zhang Y X, Song Q L, et al. 2018. Mitochondrial dysfunction causes oxidative stress and tapetal apoptosis in chemical hybridization reagent-induced male sterility in wheat. Front Plant Sci, 8: 2217. |
| [122] | Wang S S, Lu J N, Song X F, et al. 2017. Cytological and transcriptomic analyses reveal important roles of CLE19 in pollen exine formation. Plant Physiol, 175(3): 1186-1202. |
| [123] | Wang X, Guan Z Y, Gong Z, et al. 2018. Crystal structure of WA352 provides insight into cytoplasmic male sterility in rice. Biochem Biophys Res Commun, 501(4): 898-904. |
| [124] | Wang Y X, Copenhaver G P. 2018. Meiotic recombination: Mixing it up in plants. Annu Rev Plant Biol, 69: 577-609. |
| [125] | Wei S J, Ma L G. 2023. Comprehensive insight into tapetum-mediated pollen development in Arabidopsis thaliana. Cells, 12(2): 247. |
| [126] | Welte M A, Gould A P. 2017. Lipid droplet functions beyond energy storage. Biochim Biophys Acta Mol Cell Biol Lipds, 1862(10): 1260-1272. |
| [127] | Wilson Z A, Zhang D B. 2009. From Arabidopsis to rice: Pathways in pollen development. J Exp Bot, 60(5): 1479-1492. |
| [128] | Wu L Y, Jing X H, Zhang B L, et al. 2022. A natural allele of OsMS1 responds to temperature changes and confers thermosensitive genic male sterility. Nat Commun, 13(1): 2055. |
| [129] | Wu M B, Zhang Q D, Wu G L, et al. 2023. SlMYB72 affects pollen development by regulating autophagy in tomato. Hortic Res, 10(3): uhac286. |
| [130] | Wu Y Z, Fox T W, Trimnell M R, et al. 2016. Development of a novel recessive genetic male sterility system for hybrid seed production in maize and other cross-pollinating crops. Plant Biotechnol J, 14(3): 1046-1054. |
| [131] | Xie H T, Wan Z Y, Li S, et al. 2014. Spatiotemporal production of reactive oxygen species by NADPH oxidase is critical for tapetal programmed cell death and pollen development in Arabidopsis. Plant Cell, 26(5): 2007-2023. |
| [132] | Xie Y C, Li J B, Kang R, et al. 2020. Interplay between lipid metabolism and autophagy. Front Cell Dev Biol, 8: 431. |
| [133] | Xing S P, Zachgo S. 2008. ROXY1 and ROXY2 two Arabidopsis glutaredoxin genes, are required for anther development. Plant J, 53(5): 790-801. |
| [134] | Xiong S X, Lu J Y, Lou Y, et al. 2016. The transcription factors MS 188 and AMS form a complex to activate the expression of CYP 703A2 for sporopollenin biosynthesis in Arabidopsis thaliana. Plant J, 88(6): 936-946. |
| [135] | Xu D, Shi J, Rautengarten C, et al. 2017. Defective Pollen Wall 2 (DPW2) encodes an acyl transferase required for rice pollen development. Plant Physiol, 173(1): 240-255. |
| [136] | Xu D W, Qu S Y, Tucker M R, et al. 2019. Ostkpr1 functions in anther cuticle development and pollen wall formation in rice. BMC Plant Biol, 19(1): 104. |
| [137] | Xu J, Yang C Y, Yuan Z, et al. 2010. The ABORTED MICROSPORES regulatory network is required for postmeiotic male reproductive development in Arabidopsis thaliana. Plant Cell, 22(1): 91-107. |
| [138] | Xu J, Ding Z W, Vizcay-Barrena G, et al. 2014. ABORTED MICROSPORES acts as a master regulator of pollen wall formation in Arabidopsis. Plant Cell, 26(4): 1544-1556. |
| [139] | Xu X F, Wang K Q, Yu Y H, et al. 2025. The transcription factors MYB80 and TEK coordinate callose wall degradation and pollen exine formation in Arabidopsis. Plant Physiol, 197(4): kiaf124. |
| [140] | Xue J S, Feng Y F, Zhang M Q, et al. 2024. The regulatory mechanism of rapid lignification for timely anther dehiscence. J Integr Plant Biol, 66(8): 1788-1800. |
| [141] | Yamamoto Y H, Noda T. 2020. Autophagosome formation in relation to the endoplasmic reticulum. J Biomed Sci, 27(1): 97. |
| [142] | Yang K Z, Xia C, Liu X L, et al. 2009. A mutation in Thermosensitive Male Sterile 1, encoding a heat shock protein with DnaJ and PDI domains, leads to thermosensitive gametophytic male sterility in Arabidopsis. Plant J, 57(5): 870-882. |
| [143] | Yang X J, Wu D, Shi J X, et al. 2014. Rice CYP703A3, a cytochrome P450 hydroxylase, is essential for development of anther cuticle and pollen exine. J Integr Plant Biol, 56(10): 979-994. |
| [144] | Yao X Z, Hu W, Yang Z N. 2022. The contributions of sporophytic tapetum to pollen formation. Seed Biol, 1(1): 1-13. |
| [145] | Yu J P, Han J J, Kim Y J, et al. 2017. Two rice receptor-like kinases maintain male fertility under changing temperatures. Proc Natl Acad Sci USA, 114(46): 12327-12332. |
| [146] | Zhang D B, Li H. 2014. Exine export in pollen. In: Geisler M. Plant ABC Transporters: Signaling and Communication in Plants. Cham, Germany: Springer International Publishing: 49-62. |
| [147] | Zhang D B, Luo X, Zhu L. 2011. Cytological analysis and genetic control of rice anther development. J Genet Genom, 38(9): 379-390. |
| [148] | Zhang D S, Liang W Q, Yin C S, et al. 2010. OsC6, encoding a lipid transfer protein, is required for postmeiotic anther development in rice. Plant Physiol, 154(1): 149-162. |
| [149] | Zhang J L, Zhang L, Liang D, et al. 2023. ROS accumulation-induced tapetal PCD timing changes leads to microspore abortion in cotton CMS lines. BMC Plant Biol, 23(1): 311. |
| [150] | Zhang S S, Yang H, Ding L, et al. 2017. Tissue-specific transcriptomics reveals an important role of the unfolded protein response in maintaining fertility upon heat stress in Arabidopsis. Plant Cell, 29(5): 1007-1023. |
| [151] | Zhang W, Sun Y J, Timofejeva L, et al. 2006. Regulation of Arabidopsis tapetum development and function by DYSFUNCTIONAL TAPETUM1 (DYT1) encoding a putative bHLH transcription factor. Development, 133(16): 3085-3095. |
| [152] | Zhang W D, Li H J, Xue F Y, et al. 2021. Rice Glucose 6-Phosphate/Phosphate Translocator 1 is required for tapetum function and pollen development. Crop J, 9(6): 1278-1290. |
| [153] | Zhang X, Zhao G C, Tan Q, et al. 2020. Rice pollen aperture formation is regulated by the interplay between OsINP1 and OsDAF1. Nat Plants, 6(4): 394-403. |
| [154] | Zhang Y F, Li Y L, Zhong X, et al. 2022. Mutation of glucose-methanol-choline oxidoreductase leads to thermosensitive genic male sterility in rice and Arabidopsis. Plant Biotechnol J, 20(10): 2023-2035. |
| [155] | Zhang Z B, Zhu J, Gao J F, et al. 2007. Transcription factor AtMYB103 is required for anther development by regulating tapetum development, callose dissolution and exine formation in Arabidopsis. Plant J, 52(3): 528-538. |
| [156] | Zhao Q, Guan X Y, Zhou L J, et al. 2023. ABA-triggered ROS burst in rice developing anthers is critical for tapetal programmed cell death induction and heat stress-induced pollen abortion. Plant Cell Environ, 46(5): 1453-1471. |
| [157] | Zheng H Q, Rowland O, Kunst L. 2005. Disruptions of the Arabidopsis enoyl-CoA reductase gene reveal an essential role for very-long-chain fatty acid synthesis in cell expansion during plant morphogenesis. Plant Cell, 17(5): 1467-1481. |
| [158] | Zheng S Y, Dong J F, Lu J Q, et al. 2022. A cytosolic pentatricopeptide repeat protein is essential for tapetal plastid development by regulating OsGLK1 transcript levels in rice. New Phytol, 234(5): 1678-1695. |
| [159] | Zhu J, Chen H, Li H, et al. 2008. Defective in Tapetal Development and Function 1 is essential for anther development and tapetal function for microspore maturation in Arabidopsis. Plant J, 55(2): 266-277. |
| [160] | Zhu J, Zhang G Q, Chang Y H, et al. 2010. AtMYB103 is a crucial regulator of several pathways affecting Arabidopsis anther development. Sci China Life Sci, 53(9): 1112-1122. |
| [161] | Zhu J, Lou Y, Xu X F, et al. 2011. A genetic pathway for tapetum development and function in Arabidopsis. J Integr Plant Biol, 53(11): 892-900. |
| [162] | Zhu L, Shi J X, Zhao G C, et al. 2013. Post-meiotic deficient anther1 (PDA1) encodes an ABC transporter required for the development of anther cuticle and pollen exine in rice. J Plant Biol, 56(1): 59-68. |
| [163] | Zhu L P, Zhang T, Teeri T H. 2021. Tetraketide α-pyrone reductases in sporopollenin synthesis pathway in Gerbera hybrida: Diversification of the minor function. Hortic Res, 8: 207. |
| [164] | Zhu X, Yu J, Shi J, et al. 2017. The polyketide synthase OsPKS2 is essential for pollen exine and Ubisch body patterning in rice. J Integr Plant Biol, 59(9): 612-628. |
| [165] | Zou T, Xiao Q, Li W, et al. 2017. OsLAP6/OsPKS1, an orthologue of Arabidopsis PKSA/LAP6, is critical for proper pollen exine formation. Rice, 10(1): 53. |
| [1] | XIONG Fei, WANG Zhong, GU Yun-jie, CHEN Gang, ZHOU Peng. Effects of Nitrogen Application Time on Caryopsis Development and Grain Quality of Rice Variety Yangdao 6 [J]. RICE SCIENCE, 2008, 15(1): 57-62 . |
| [2] | YANG Jian-chang, CHANG Er-hua, TANG Cheng, ZHANG Hao, WANG Zhi-qin. Relationships of Ethylene Evolution Rate and 1-Aminocylopropane -1-Carboxylic Acid Concentration in Grains during Filling Period with Appearance Quality of Rice [J]. RICE SCIENCE, 2007, 14(1): 33-41 . |
| [3] | XIONG Fei, WANG Zhong, CHENG Gang, WANG Jue . Caryopsis Development and Main Quality Characteristics in Different indica Rice Varieties [J]. RICE SCIENCE, 2005, 12(4): 238-242 . |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||