
Rice Science ›› 2025, Vol. 32 ›› Issue (4): 549-560.DOI: 10.1016/j.rsci.2025.04.004
• Research Papers • Previous Articles Next Articles
Wang Weixia1, Zhu Tingheng2, Wei Qi1, Wan Pinjun1, He Jiachun1, Lai Fengxiang1, Fu Qiang1( )
)
Received:2024-10-17
Accepted:2025-01-08
Online:2025-07-28
Published:2025-08-06
Contact:
Fu Qiang
Wang Weixia, Zhu Tingheng, Wei Qi, Wan Pinjun, He Jiachun, Lai Fengxiang, Fu Qiang. A Mucin2-Like Gene, NlMuc2, is Required for Early Embryonic Development in Nilaparvata lugens[J]. Rice Science, 2025, 32(4): 549-560.
Add to citation manager EndNote|Ris|BibTeX
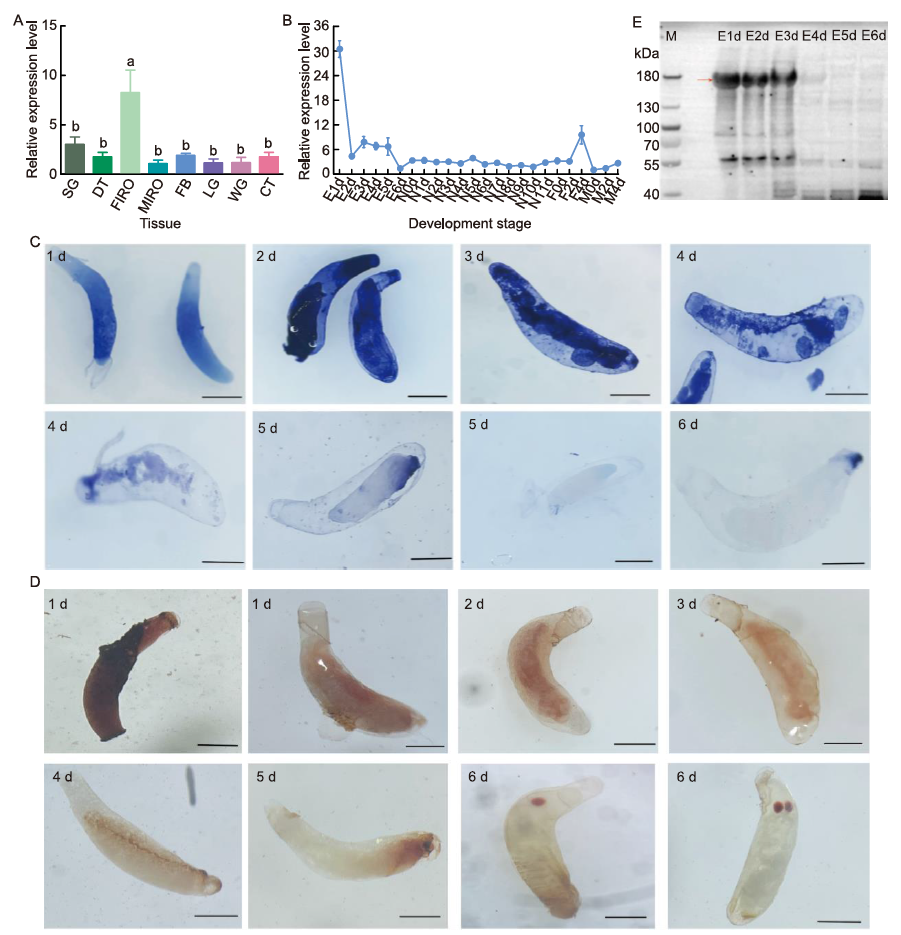
Fig. 2. Spatial and temporal expression patterns of Nilaparvata lugens mucin-2 (NlMuc2). A, Expression patterns of NlMuc2 in different tissues of N. lugens. Total RNA was extracted from salivary glands (SG), digestive tracts (DT), female internal reproductive organs (FIRO), male internal reproductive organs (MIRO), fat bodies (FB), legs (LG), wings (WG), and cuticles (CT). Tissues were dissected from adults 24‒72 h after emergence. Different lowercase letters above error bars indicate significant differences determined by one-way analysis of variance followed by Tukey’s honestly significance difference (HSD) tests (P < 0.05). B, Expression patterns of NlMuc2 across developmental stages of N. lugens. Samples were extracted from whole insects collected every 24 or 48 h from the start of each stage. E1d‒E6d, Eggs from 1 to 6 d after laying; N0d‒N11d, Nymphs from 0 to 11 d after hatching; F0d, F2d, and F4d, Newly emerged, 2-day-old, and 4-day-old females, respectively; M0d, M2d, and M4d, Newly emerged, 2-day-old, and 4-day-old males, respectively. 18S rRNA and ribosomal protein S11 were used as internal controls for qRT-PCR. Data show relative NlMuc2 mRNA levels (mean ± SE with three independent experiments). C, Dynamic expression patterns of NlMuc2 in eggs throughout embryonic development at the transcript level by in situ hybridization. Blue signals were detected using NBT/BCIP (nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl-phosphate) and alkaline phosphatase-conjugated anti-DIG (digoxigenin) antibodies after hybridization with DIG-labeled antisense RNA probes. Scale bars, 300 μm. D, Dynamic expression patterns of NlMuc2 in eggs at the protein level by immunohistochemistry. Brown signals were visualized using DAB (3,3′-diaminobenzidine) and HRP (horseradish peroxidase)-conjugated goat anti-rabbit IgG. Eggs were collected every 24 h from rice leaves. Scale bars, 300 μm. E, Analysis of NlMuc2 protein levels during egg development stages with Western blotting. E1d‒E6d, Eggs from 1 to 6 d after laying. Immunoblotting used anti-NlMuc2 antibodies. Red arrow indicates NlMuc2 (166.9 kDa). For electrophoresis, 30 μg of protein was load into each well of a 3%-8% NuPAGE gel.
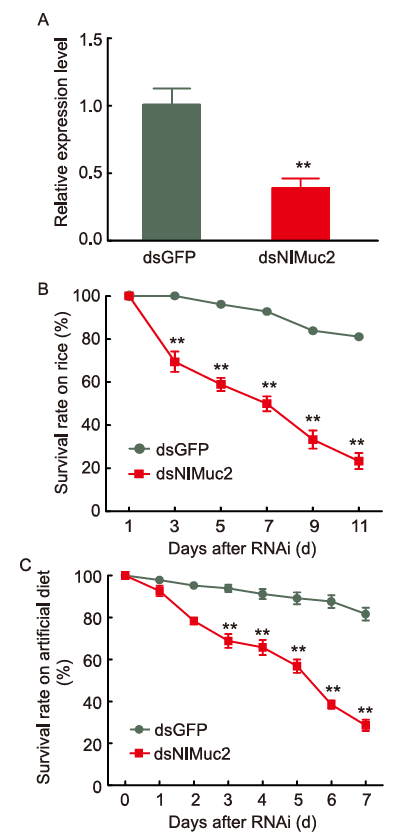
Fig. 3. Knockdown of NlMuc2 causes lethality. A, NlMuc2 expression after dsNlMuc2 injection. B, Survival rates of 3rd-instar nymphs on rice after dsNlMuc2 injection. C, Survival rates of 3rd-instar nymphs on artificial diet after dsNlMuc2 injection. RNAi, RNA interference. dsRNA (70 ng per nymph; n = 30) was injected into 3rd-instar nymphs. dsGFP was used as control. Data are represented as mean ± SE from six independent experiments and analyzed by student’s t-test (**, P < 0.05).

Fig. 4. Effects on fecundity after dsNlMuc2 injection on 5th-instar nymphs of Nilaparvata lugens. A‒C, Number of laid eggs (A), hatched nymphs (B), and hatching rate (C) of 5th-instar nymphs of N. lugens after dsNlMuc2 injection. D, Morphology of unhatched eggs dissected from rice leaf sheaths. The red eyespots were marked by the red arrow. Yolk-like mass was marked by the yellow arrow. In dsGFP, unhatched eggs contained a normally developed embryo with a white-to-yellow yolk-like mass at the posterior or the red eyespot at the anterior or near the micropylar cap. In dsNlmuc2, unhatched eggs contained white-to-yellow yolk-like mass (yellow arrow) at the anterior, the red eyespot at the posterior. Scale bars, 500 μm Data are represented as mean ± SE from 15 independent experiments and analyzed by student’s t-test (**, P < 0.05).

Fig. 5. Effects of NlMuc2 knockdown on ovarian (A) and embryonic (B) development. Newly emerged females were injected with 70 ng dsNlMuc2, the control group was treated with dsGFP. Ovaries from at least 10 females for each group were dissected at 4, 6, and 8 d post-injection and photographed using a stereo microscopy. At 4 d post-injection, mature banana-shaped eggs appeared in the ovarioles of the dsGFP females, whereas in the dsNMuc2 females, the ovarioles were transparent and adhered together. At 6 d post-injection, mature eggs filled all ovarioles in the dsGFP group, whereas in the dsNlMuc2 group, less than 50% of the ovarioles filled mature eggs. At 8 d post-injection, the number of mature oocytes in the dsNlMuc2-injected group was lower than in the dsGFP group. The eggs laid within the rice leaf sheath were dissected and photographed at 5 d after oviposition. The red eyespot was marked by the red arrow. The yellow arrow indicated the micropylar cap of the egg. In the eggs laid by the dsGFP group, the red eyespot on the anterior side of the egg was observed at 4 or 5 d after laying. In the dsNlMuc2 eggs, 51.0% had the eyespot at the posterior. Scale bars, 500 μm.
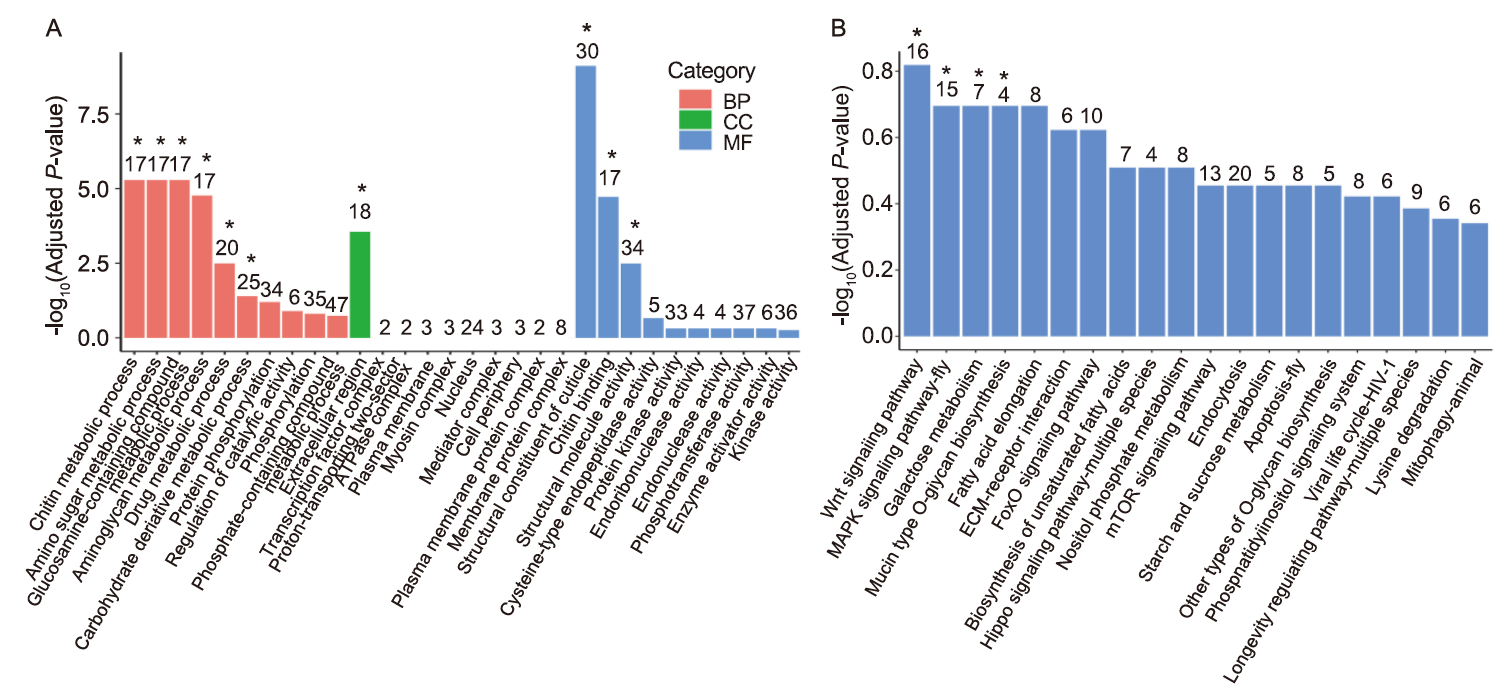
Fig. 6. Transcriptome analysis of eggs from dsNlMuc2- and dsGFP-injected females. A, Top 10 enriched Gene Ontology (GO) terms for differentially expressed genes (DEGs). BP, Biological process; CC, Cellular component; MF, Molecular function. B, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment of downregulated DEGs. The x-axis indicates the subcategories and the y-axis indicates -log10(Adjusted P-value). Asterisks (*) above the bars represent significant differences (adjusted P-value < 0.05).
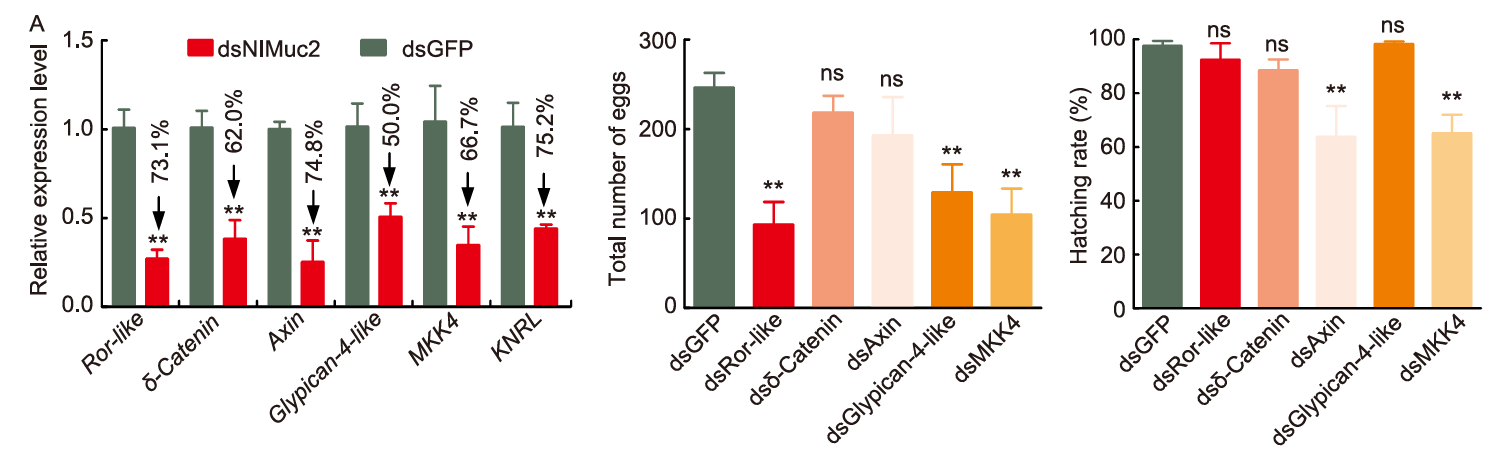
Fig. 7. Effects of differentially expressed gene knockdown on female fecundity. A, RNA-seq validation by qRT-PCR analysis. Nilaparvata lugens 18S rRNA and ribosomal protein S11 were used as internal controls. B and C, Effects of differentially expressed gene knockdown on the total number of laid eggs (B) and hatching rate (C) after dsDEGs injection in 5th-instar nymphs of N. lugen. The results were calculated using dsGFP as control. Data were expressed as mean ± SE from three independent experiments and analyzed using a two-tailed unpaired t-test at the significant levels at P < 0.05 (**). ns, Not significant. Fecundity analysis is based on three biological replicates with each includes five pairs of females and males as a pool.
| [1] | Anders S, Huber W. 2010. Differential expression analysis for sequence count data. Genome Biol, 11(10): R106. |
| [2] | Bao Y Y, Zhang C X. 2019. Recent advances in molecular biology research of a rice pest, the brown planthopper. J Integr Agric, 18(4): 716-728. |
| [3] | Cheatle Jarvela A M, Pick L. 2017. The function and evolution of nuclear receptors in insect embryonic development. Curr Top Dev Biol, 125: 39-70. |
| [4] | Chen J C, Cheng S N, Yan L M, et al. 1979. The ovarial development of the brown planthopper (Nilaparvata lugens Stål) and its relation to migration. Acta Entomol Sin, 22: 280-288. |
| [5] | Chen W W, Kang K, Yang P, et al. 2019. Identification of a sugar gustatory receptor and its effect on fecundity of the brown planthopper Nilaparvata lugens. Insect Sci, 26(3): 441-452. |
| [6] | Cheng Y B, Li Y M, Li W R, et al. 2020. Effect of hepatocyte nuclear factor 4 on the fecundity of Nilaparvata lugens: Insights from RNA interference combined with transcriptomic analysis. Genomics, 112(6): 4585-4594. |
| [7] | Dong X L, Zhai Y F, Zhang J Q, et al. 2011. Fork head transcription factor is required for ovarian mature in the brown planthopper, Nilaparvata lugens (Stål). BMC Mol Biol, 12: 53. |
| [8] | Dong Y, Chen W W, Kang K, et al. 2021. FoxO directly regulates the expression of TOR/S6K and vitellogenin to modulate the fecundity of the brown planthopper. Sci China Life Sci, 64(1): 133-143. |
| [9] | Fan X B, Pang R, Li W X, et al. 2020. An overview of embryogenesis: External morphology and transcriptome profiling in the hemipteran insect Nilaparvata lugens. Front Physiol, 11: 106. |
| [10] | Fan X B, Zhang W Q. 2022. Genome-wide identification of FAR gene family and functional analysis of NlFAR10 during embryogenesis in the brown planthopper Nilaparvata lugens. Int J Biol Macromol, 223(Pt A): 798-811. |
| [11] | Fu J P, Posnien N, Bolognesi R, et al. 2012. Asymmetrically expressed axin required for anterior development in Tribolium. Proc Natl Acad Sci USA, 109(20): 7782-7786. |
| [12] | Fu Q, Zhang Z T, Hu C, et al. 2001. A chemically defined diet enables continuous rearing of the brown planthopper, Nilaparvata lugens (Stål) (Homoptera: Delphacidae). Appl Entomol Zool, 36(1): 111-116. |
| [13] | Gao H L, Zhang H H, Yuan X W, et al. 2023. Knockdown of the salivary protein gene NlG14 caused displacement of the lateral oviduct secreted components and inhibited ovulation in Nilaparvata lugens. PLoS Genet, 19(4): e1010704. |
| [14] | Geuking P, Narasimamurthy R, Lemaitre B, et al. 2009. A non-redundant role for Drosophila Mkk4 and hemipterous/Mkk7 in TAK1-mediated activation of JNK. PLoS One, 4(11): e7709. |
| [15] | Hattori M, Nakamura M, Komatsu S, et al. 2012. Molecular cloning of a novel calcium-binding protein in the secreted saliva of the green rice leafhopper Nephotettix cincticeps. Insect Biochem Mol Biol, 42(1): 1-9. |
| [16] | Hibino H. 1996. Biology and epidemiology of rice viruses. Annu Rev Phytopathol, 34: 249-274. |
| [17] | Huang H J, Liu C W, Huang X H, et al. 2016. Screening and functional analyses of Nilaparvata lugens salivary proteome. J Proteome Res, 15(6): 1883-1896. |
| [18] | Huang H J, Liu C W, Xu H J, et al. 2017. Mucin-like protein, a saliva component involved in brown planthopper virulence and host adaptation. J Insect Physiol, 98: 223-230. |
| [19] | Huang Z J, Tian Z, Zhao Y L, et al. 2022. MAPK signaling pathway is essential for female reproductive regulation in the cabbage beetle, Colaphellus bowringi. Cells, 11(10): 1602. |
| [20] | Hursh D A, Stultz B G. 2018. Odd-paired: The Drosophila zic gene. In: Aruga J. Zic Family: Evolution, Development and Disease. Singapore: Springer Singapore: 41-58. |
| [21] | Kramerov A A, Arbatsky N P, Rozovsky Y M, et al. 1996. Mucin-type glycoprotein from Drosophila melanogaster embryonic cells: Characterization of carbohydrate component. FEBS Lett, 378(3): 213-218. |
| [22] | Li J Y, Liu J, Chi B J, et al. 2022. 20E and MAPK signal pathway involved in the effect of reproduction caused by cyantraniliprole in Bactrocera dorsalis Hendel (Diptera: Tephritidae). Pest Manag Sci, 78(1): 63-72. |
| [23] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408. |
| [24] | Lou Y H, Shen Y, Li D T, et al. 2019. A mucin-like protein is essential for oviposition in Nilaparvata lugens. Front Physiol, 10: 551. |
| [25] | Lu J B, Wang S N, Ren P P, et al. 2023. RNAi-mediated silencing of an egg-specific gene Nllet1 results in hatch failure in the brown planthopper. Pest Manag Sci, 79(1): 415-427. |
| [26] | Lu K, Cheng Y B, Li Y M, et al. 2021. The KNRL nuclear receptor controls hydrolase-mediated vitellin breakdown during embryogenesis in the brown planthopper Nilaparvata lugens. Insect Sci, 28(6): 1633-1650. |
| [27] | Nagaso H, Murata T, Day N, et al. 2001. Simultaneous detection of RNA and protein by in situ hybridization and immunological staining. J Histochem Cytochem, 49(9): 1177-1182. |
| [28] | Panfilio K A. 2008. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev Biol, 313(2): 471-491. |
| [29] | Pang R, Qiu J Q, Li T C, et al. 2017. The regulation of lipid metabolism by a hypothetical P-loop NTPase and its impact on fecundity of the brown planthopper. Biochim Biophys Acta Gen Subj, 1861(7): 1750-1758. |
| [30] | Prühs R, Beermann A, Schröder R. 2017. The roles of the Wnt-antagonists axin and Lrp4 during embryogenesis of the red flour beetle Tribolium castaneum. J Dev Biol, 5(4): 10. |
| [31] | Qiu J, He Y, Zhang J, et al. 2016. Discovery and functional identification of fecundity-related genes in the brown planthopper by large-scale RNA interference. Insect Mol Biol, 25(6): 724-733. |
| [32] | Ren Z W, Zhuo J C, Zhang C X, et al. 2018. Characterization of NlHox3, an essential gene for embryonic development in Nilaparvata lugens. Arch Insect Biochem Physiol, 98(2): e21448. |
| [33] | Roy S, Saha T T, Zou Z, et al. 2018. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol, 63: 489-511. |
| [34] | Sarauer B L, Gillott C, Hegedus D. 2003. Characterization of an intestinal mucin from the peritrophic matrix of the diamondback moth, Plutella xylostella. Insect Mol Biol, 12(4): 333-343. |
| [35] | Shangguan X X, Zhang J, Liu B F, et al. 2018. A mucin-like protein of planthopper is required for feeding and induces immunity response in plants. Plant Physiol, 176(1): 552-565. |
| [36] | Shen Y, Lu J B, Chen Y Z, et al. 2021. A lateral oviduct secreted protein plays a vital role for egg movement through the female reproductive tract in the brown planthopper. Insect Biochem Mol Biol, 132: 103555. |
| [37] | Syed Z A, Härd T, Uv A, et al. 2008. A potential role for Drosophila mucins in development and physiology. PLoS One, 3(8): e3041. |
| [38] | Tang Q Y, Feng M G. 2002. Analysis of variance. In: Tang Q Y, Feng M G. DPS Data Processing System for Practical Statistics-4. Beijing, China: Scientific Press: 47-71. (in Chinese) |
| [39] | Wagner C E, Wheeler K M, Ribbeck K. 2018. Mucins and their role in shaping the functions of mucus barriers. Annu Rev Cell Dev Biol, 34: 189-215. |
| [40] | Wu S Y, Tong X L, Li C L, et al. 2019. Genome-wide identification and expression profiling of the C2H2-type zinc finger protein genes in the silkworm Bombyx mori. PeerJ, 7: e7222. |
| [41] | Xue J, Bao Y Y, Li B L, et al. 2010. Transcriptome analysis of the brown planthopper Nilaparvata lugens. PLoS One, 5(12): e14233. |
| [42] | Xue J, Zhou X, Zhang C X, et al. 2014. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol, 15(12): 521. |
| [43] | Yang Q, Smagghe G, Staes A, et al. 2023. α-1,6-Fucosyltransferase plays a critical role during embryogenesis of the hemimetabolous insect Nilaparvata lugens. Insect Biochem Mol Biol, 154: 103918. |
| [44] | Yuan M, Lu Y H, Zhu X, et al. 2014. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS One, 9(1): e86503. |
| [45] | Zhang Z J, Aslam A F M, Liu X J, et al. 2015. Functional analysis of Bombyx Wnt1 during embryogenesis using the CRISPR/Cas9 system. J Insect Physiol, 79: 73-79. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||