
Rice Science ›› 2022, Vol. 29 ›› Issue (2): 143-154.DOI: 10.1016/j.rsci.2022.01.003
• Research Paper • Previous Articles Next Articles
Wang Weixia1, Zhu Tingheng2( ), Wan Pinjun1, Wei Qi1, He Jiachun1, Lai Fengxiang1, Fu Qiang1(
), Wan Pinjun1, Wei Qi1, He Jiachun1, Lai Fengxiang1, Fu Qiang1( )
)
Received:2021-04-08
Accepted:2021-07-06
Online:2022-03-28
Published:2022-02-09
Contact:
Zhu Tingheng, Fu Qiang
Wang Weixia, Zhu Tingheng, Wan Pinjun, Wei Qi, He Jiachun, Lai Fengxiang, Fu Qiang. Cloning and Functional Analysis of Calcineurin Subunits A and B in Development and Fecundity of Nilaparvata lugens (Stål)[J]. Rice Science, 2022, 29(2): 143-154.
Add to citation manager EndNote|Ris|BibTeX
| Gene ID | Blastn NCBI (Size) | Blast N. lugens genome | Accession No. | No. of exons | Full length (bp) | ORF length (bp) | Protein sequence length (aa) | MW (kDa) | pI |
|---|---|---|---|---|---|---|---|---|---|
| NlCNB1 | XM_022349071.1 (1 396 bp) | Scaffold 5431 | MT780262 | / | 720 | 75‒587 | 171 | 19.4 | 4.31 |
| NlCNBH1 | XM_022328413.1 (1 012 bp) | Scaffold 8 | MT780263 | 4 | 994 | 31‒603 | 191 | 21.9 | 4.66 |
| NlCNA-X1 | XM_022350490.1 (2 008 bp) | Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 | MT780264 | 16 | 1 758 | 100‒1 707 | 536 | 59.8 | 6.65 |
| NlCNA-X2 | XM_022350490.1 (2 008 bp) | MT780265 | 15 | 1 743 | 100‒1 692 | 531 | 59.2 | 6.65 | |
| NlCNA-X3 | XM_022350512.1 (1 960 bp) | MT780266 | 15 | 1 710 | 100‒1 659 | 520 | 58.0 | 6.52 | |
| NlCNA-X4 | XM_022350540.1 (1 932 bp) | MT780267 | 14 | 1 686 | 100‒1 635 | 512 | 57.1 | 6.07 | |
| NlCNA-X5 | XM_022350540.1 (1 932 bp) | MT780268 | 14 | 1 683 | 100‒1 632 | 511 | 57.0 | 6.18 | |
| NlCNA-X6 | XM_022350548.1 (1 929 bp) | MT780269 | 14 | 1 680 | 100‒1 629 | 510 | 57.0 | 6.18 |
Table 1. Genes cloned from N. lugens and their sequence characteristics.
| Gene ID | Blastn NCBI (Size) | Blast N. lugens genome | Accession No. | No. of exons | Full length (bp) | ORF length (bp) | Protein sequence length (aa) | MW (kDa) | pI |
|---|---|---|---|---|---|---|---|---|---|
| NlCNB1 | XM_022349071.1 (1 396 bp) | Scaffold 5431 | MT780262 | / | 720 | 75‒587 | 171 | 19.4 | 4.31 |
| NlCNBH1 | XM_022328413.1 (1 012 bp) | Scaffold 8 | MT780263 | 4 | 994 | 31‒603 | 191 | 21.9 | 4.66 |
| NlCNA-X1 | XM_022350490.1 (2 008 bp) | Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 Scaffold 262 | MT780264 | 16 | 1 758 | 100‒1 707 | 536 | 59.8 | 6.65 |
| NlCNA-X2 | XM_022350490.1 (2 008 bp) | MT780265 | 15 | 1 743 | 100‒1 692 | 531 | 59.2 | 6.65 | |
| NlCNA-X3 | XM_022350512.1 (1 960 bp) | MT780266 | 15 | 1 710 | 100‒1 659 | 520 | 58.0 | 6.52 | |
| NlCNA-X4 | XM_022350540.1 (1 932 bp) | MT780267 | 14 | 1 686 | 100‒1 635 | 512 | 57.1 | 6.07 | |
| NlCNA-X5 | XM_022350540.1 (1 932 bp) | MT780268 | 14 | 1 683 | 100‒1 632 | 511 | 57.0 | 6.18 | |
| NlCNA-X6 | XM_022350548.1 (1 929 bp) | MT780269 | 14 | 1 680 | 100‒1 629 | 510 | 57.0 | 6.18 |
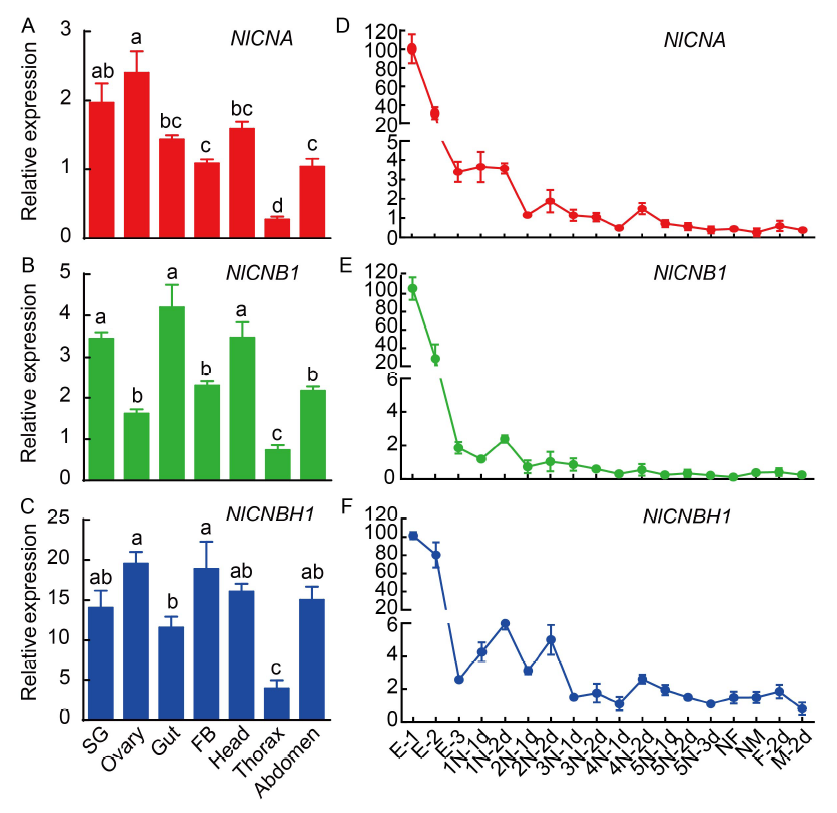
Fig. 1. Expression analyses of NlCNA, NlCNB1 and NlCNBH1 at various tissues (A?C) and developmental stages (D?F) in N. lugens. SG, Salivary gland; FB, Fat body; E-1, 0?48 h embryo; E-2, 72?96 h embryo; E-3, 120?144 h embryo; 1N-1d?5N-3d, From the first day of the 1st instar nymph to the third day of the 5th instar nymph; NF, Newly emerged female; NM, Newly emerged male; F-2d, Adult females at 2 d after eclosion; M-2d, Adult males at 2 d after eclosion. Three biological replications (Mean ± SE) were carried out for each gene based on independent samples, and relative expression levels of target genes were calculated by the equation Y = 10 (Ct internal ? Ct target) / 3 × 100%. Different lowercase letters above the bars indicate significant differences among different tissues by the Duncan's multiple range test at P < 0.05.
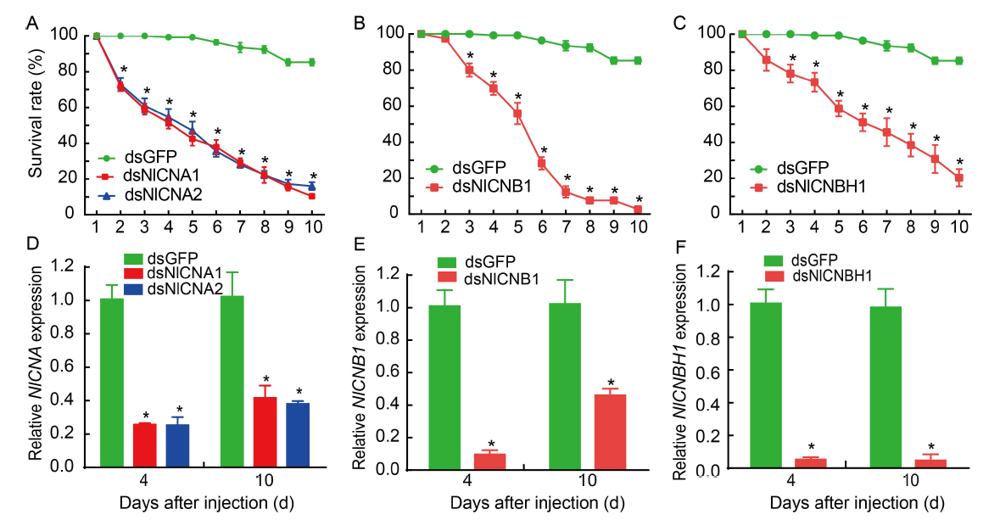
Fig. 2. Effects of dsRNA on survivorship of N. lugens (A?C) and mRNA levels of target genes (D?F). N. lugens (A?C) and mRNA levels of target genes (D?F). The 3rd instar nymphs were injected with specific dsRNA and observed for phenotypic variations at 24 h intervals. Individuals treated with dsGFP were used as a control. The survival rate was calculated from four biological replicates (Mean ± SE). For each treatment, 30 nymphs were used. mRNA levels of target genes from five nymphs at 4 and 10 d after injection in N. lugens were analyzed by qRT-PCR with the 2?ΔΔCT method from three biological replicates (Mean ± SE). Significant differences between the treatment and control are indicated with asterisks (*, P < 0.05).

Fig. 3. Effects of dsRNA injection on nymphal molting. A, Normal phenotype nymphs injected with dsGFP. B?F, Abnormal phenotype nymphs injected with dsNlCNB1. About 90% nymphs with silenced NlCNB1 failed to shed the exuviae completely and did not molt into next stage.
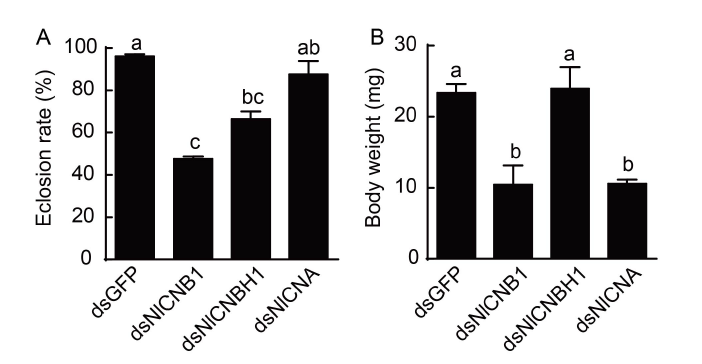
Fig. 4. Effects of dsRNAs on eclosion rate (A) of N. lugens and body weight of newly emerged females (B). The 4th instar nymphs were injected with specific dsRNA. Individuals treated with dsGFP were used as a control. The eclosion rate was calculated from three biological replicates (Mean ± SE). For each treatment, 30 nymphs were used. The newly emerged females were weighted with an electronic balance from three biological replicates (Mean ± SE). For each treatment, five nymphs were used. Different lowercase letters above the bars indicate significant differences among different treatments by the Duncan's multiple range tests at P < 0.05.
| dsRNA | No. of eggs per female | No. of offspring | Hatchability (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | No. of samples | Mean ± SE | No. of samples | Mean ± SE | No. of samples | |||
| dsGFP | 504.5 ± 56.9 a | 12 | 465.5 ± 50.3 a | 12 | 94.0 ± 2.3 a | 12 | ||
| dsNlCNA | 326.9 ± 54.6 b | 13 | 311.0 ± 55.5 b | 13 | 86.2 ± 7.7 a | 13 | ||
| dsNlCNBH1 | 258.0 ± 62.3 b | 14 | 244.4 ± 57.6 b | 14 | 96.9 ± 1.4 a | 12 | ||
| dsNlCNB1 | 75.0 ± 38.6 c | 14 | 62.3 ± 39.3 c | 14 | 59.9 ± 18.1 b | 7 | ||
Table 2. Effects of NlCNA, NlCNB1 and NlCNBH1 on fecundity of N. lugens.
| dsRNA | No. of eggs per female | No. of offspring | Hatchability (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean ± SE | No. of samples | Mean ± SE | No. of samples | Mean ± SE | No. of samples | |||
| dsGFP | 504.5 ± 56.9 a | 12 | 465.5 ± 50.3 a | 12 | 94.0 ± 2.3 a | 12 | ||
| dsNlCNA | 326.9 ± 54.6 b | 13 | 311.0 ± 55.5 b | 13 | 86.2 ± 7.7 a | 13 | ||
| dsNlCNBH1 | 258.0 ± 62.3 b | 14 | 244.4 ± 57.6 b | 14 | 96.9 ± 1.4 a | 12 | ||
| dsNlCNB1 | 75.0 ± 38.6 c | 14 | 62.3 ± 39.3 c | 14 | 59.9 ± 18.1 b | 7 | ||
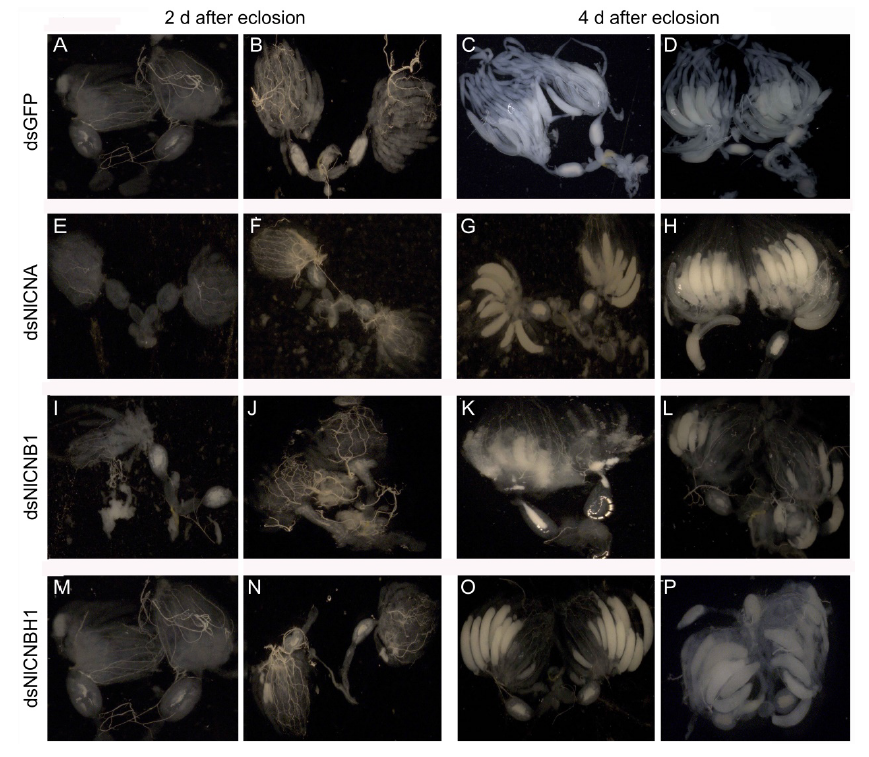
Fig. 5. Developmental status of N. lugens ovaries. A?D, Normal ovaries were dissected and photo- graphed under a dissection microscope from females injected with dsGFP at 2 and 4 d after eclosion. E?H, Ovaries from females injected with dsNlCNA at 2 and 4 d after eclosion. I?L, Abnormal ovaries from females injected with dsNlCNB1 at 2 and 4 d after eclosion. M?P, Ovaries from females injected with dsNlCNBH1 at 2 and 4 d after eclosion.
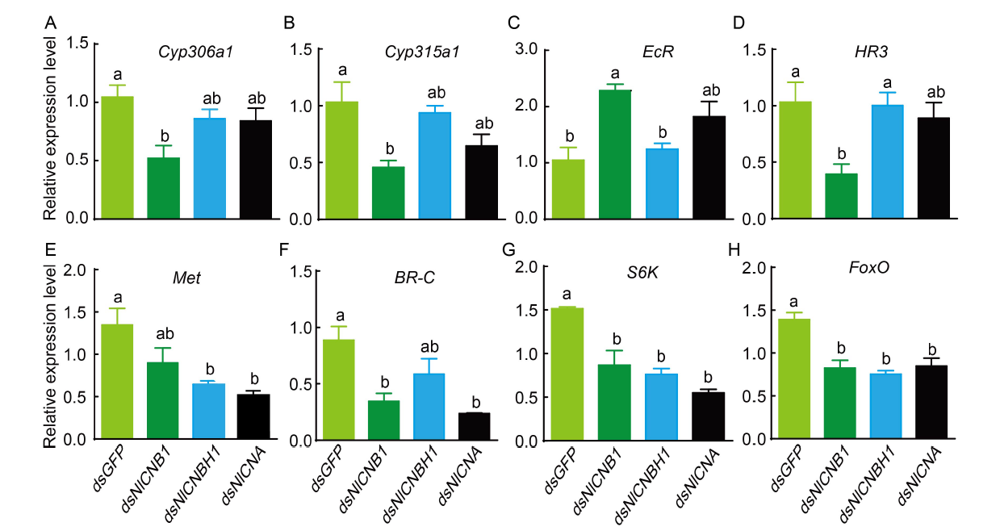
Fig. 6. Expression analysis of 8 among 10 selected genes in response to RNAi. The 4th instar nymphs were injected with specific dsRNA. Nymphs treated with dsGFP were used as a control. Five newly emerged females were collected for RNA extraction and mRNA levels of target genes were analyzed by qRT-PCR with the 2?ΔΔCT method from three biological replicates (Mean ± SE). Different lowercase letters above the bars indicate significant differences among different treatments by the Duncan's multiple range tests at P < 0.05.
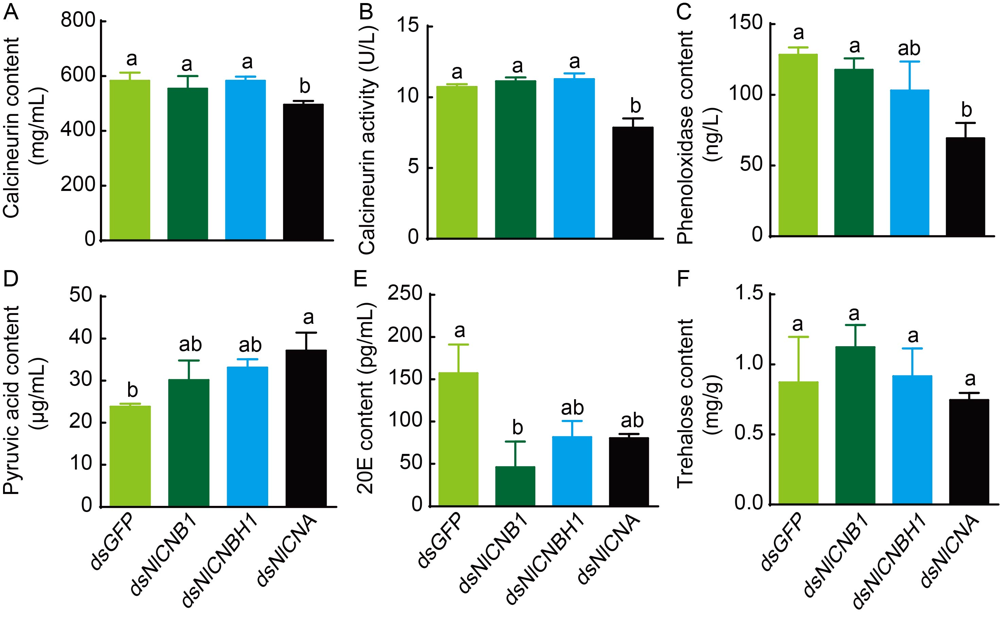
Fig. 7. Relative physiological and biochemical changes affected by dsRNAs. Calcineurin content (A), calcineurin enzyme activity (B), phenoloxidase content (C), pyruvic acid content (D), 20-hydroxyacdysone (20E) content (E) and trehalose content (F) were analyzed between different treatments of newly emerged females and males. Three replicates were conducted (Mean ± SE). Different lowercase letters above the bars indicate significant differences among different treatments by the Duncan's multiple range test at P < 0.05.
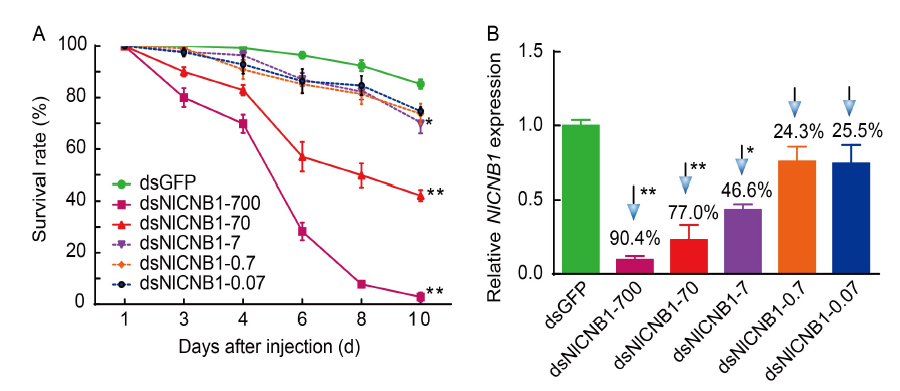
Fig. 8. Survival rate (A) and NlCNB1 mRNA abundance (B) of N. lugens that treated with different concentrations of dsNlCNB1. dsNlCNB1-700 to dsNlCNB1-0.07 refer to the concentrations of dsNlCNB1 used for injection from 700 to 0.07 ng/μL. The survival rate was calculated from three biological replicates with 30 nymphs each (Mean ± SE). mRNA level of NlCNB1 at 4 d after injection were analyzed by qRT-PCR with the 2-ΔΔCT method from three biological replicates (Mean ± SE). *, P < 0.05; **, P < 0.01.
| [1] | Allman E, Wang Q, Walker R L, Austen M, Peters M A, Nehrke K. 2016. Calcineurin homologous proteins regulate the membrane localization and activity of sodium/proton exchangers in C. elegans. Am J Physiol Cell Physiol, 310: C233-C242. |
| [2] |
Aramburu J, Rao A, Klee C B. 2000. Calcineurin: From structure to function. Curr Top Cell Regul, 36: 237-295.
PMID |
| [3] | Bottrell D G, Schoenly K G. 2012. Resurrecting the ghost of green revolutions past: The brown planthopper as a recurring threat to high-yielding rice production in tropical Asia. J Asia-Pac Entomol, 15: 122-140. |
| [4] |
Brown L, Chen M X, Cohen P T. 1994. Identification of a cDNA encoding a Drosophila calcium/calmodulin regulated protein phosphatase, which has its most abundant expression in the early embryo. FEBS Lett, 339: 124-128.
PMID |
| [5] | Bueno O F, Brandt E B, Rothenberg M E, Molkentin J D. 2002. Defective T cell development and function in calcineurin A beta- deficient mice. Proc Natl Acad Sci USA, 99: 9398-9403. |
| [6] | Cai M J, Zhao W L, Jing Y P, Song Q, Zhang X Q, Wang J X, Zhao X F. 2016. 20-Hydroxyecdysone activates Forkhead box O to promote proteolysis during Helicoverpa armigera molting. Development, 143(6): 1005-1015. |
| [7] |
Chen M, Shelton A, Ye G Y. 2011. Insect-resistant genetically modified rice in China: From research to commercialization. Annu Rev Entomol, 56: 81-101.
PMID |
| [8] | Chen W W, Kang K, Yang P, Zhang W Q. 2019. Identification of a sugar gustatory receptor and its effect on fecundity of the brown planthopper Nilaparvata lugens. Insect Sci, 26(3): 441-452. |
| [9] |
Crabtree G R. 2001. Calcium, calcineurin, and the control of transcription. J Biol Chem, 276(4): 2313-2316.
PMID |
| [10] | Dijkers P F, O'Farrell P H. 2007. Drosophila calcineurin promotes induction of innate immune responses. Curr Biol, 17: 2087-2093. |
| [11] | Dong Y, Chen W W, Kang K, Pang R, Dong Y P, Liu K, Zhang W Q. 2021. FoxO directly regulates the expression of TOR/S6K and vitellogenin to modulate the fecundity of the brown planthopper. Sci China Life Sci, 64(1): 133-143. |
| [12] | Fónagy A, Yokoyama N, Ozawa R, Okano K, Tatsuki S, Maeda S, Matsumoto S. 1999. Involvement of calcineurin in the signal transduction of PBAN in the silkworm, Bombyx mori (Lepidoptera). Comp Biochem Physiol B Biochem Mol Biol, 124: 51-60. |
| [13] | Furman J L, Norris C M. 2014. Calcineurin and glial signaling: Neuroinflammation and beyond. J Neuroinflamm, 11: 158. |
| [14] | Gajewski K, Wang J B, Molkentin J D, Chen E H, Olson E N, Schulz R A. 2003. Requirement of the calcineurin subunit gene canB2 for indirect flight muscle formation in Drosophila. Proc Natl Acad Sci USA, 100: 1040-1045. |
| [15] |
Hou L, Cai M J, Liu W, Song Q, Zhao X F. 2012. Small GTPase Rab4b participates in the gene transcription of 20-hydroxyecdysone and insulin pathways to regulate glycogen level and metamorphosis. Dev Biol, 371(1): 13-22.
PMID |
| [16] |
Hu D, Luo W, Fan L F, Liu F L, Gu J, Deng H M, Zhang C, Huang L H, Feng Q L. 2016. Dynamics and regulation of glycolysis- tricarboxylic acid metabolism in the midgut of Spodoptera litura during metamorphosis. Insect Mol Biol, 25(2): 153-162.
PMID |
| [17] |
Jiménez-Vidal M, Srivastava J, Putney L K, Barber D L. 2010. Nuclear-localized calcineurin homologous protein CHP1 interacts with upstream binding factor and inhibits ribosomal RNA synthesis. J Biol Chem, 285: 36260-36266.
PMID |
| [18] | Kayukawa T, Jouraku A, Ito Y, Shinoda T. 2017. Molecular mechanism underlying juvenile hormone-mediated repression of precocious larval-adult metamorphosis. Proc Natl Acad Sci USA, 114: 1057-1062. |
| [19] |
Klee C B, Ren H, Wang X T. 1998. Regulation of the calmodulin- stimulated protein phosphatase, calcineurin. J Biol Chem, 273: 13367-13370.
PMID |
| [20] |
Kuromi H, Yoshihara M, Kidokoro Y. 1997. An inhibitory role of calcineurin in endocytosis of synaptic vesicles at nerve terminals of Drosophila larvae. Neurosci Res, 27(2): 101-113.
PMID |
| [21] |
Kuwahara H, Kamei J I, Nakamura N, Matsumoto M, Inoue H, Kanazawa H. 2003. The apoptosis-inducing protein kinase DRAK2 is inhibited in a calcium-dependent manner by the calcium-binding protein CHP. J Biochem, 134: 245-250.
PMID |
| [22] | Lee J I, Mukherjee S, Yoon K H, Dwivedi M, Bandyopadhyay J. 2013. The multiple faces of calcineurin signaling in Caenorhabditis elegans: Development, behaviour and aging. J Biosci, 38: 417-431. |
| [23] | Li K L, Fu Q, Wang W X, Lai F X, Wan P J. 2017. Molecular cloning and functional characterization of Halloween genes involved in ecdysteroid biosynthesis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae). Acta Entomol Sin, 60(10): 1129-1140. (in Chinese with English abstract) |
| [24] | Li Q H, Wang L H, Lin Y N, Chang G Q, Li H W, Jin W N, Hu R H, Pang T X. 2011. Nuclear accumulation of calcineurin B homologous protein 2 (CHP2) results in enhanced proliferation of tumor cells. Genes Cells, 16: 416-426. |
| [25] | Li Y B. 2016. The regulation of calcineurin to phenoloxidase and other biological enzymes involved in immunity of Locusta migratoria L. Shenyang, China: Shenyang Agricultural University. (in Chinese with English abstract) |
| [26] | Li Y X, Dijkers P F. 2015. Specific calcineurin isoforms are involved in Drosophila toll immune signaling. J Immunol, 194(1): 168-176. |
| [27] |
Lin X, Sikkink R A, Rusnak F, Barber D L. 1999. Inhibition of calcineurin phosphatase activity by a calcineurin B homologous protein. J Biol Chem, 274: 36125-36131.
PMID |
| [28] |
Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4): 402-408.
PMID |
| [29] | Lu A R, Zhang Q L, Zhang J, Yang B, Wu K, Xie W, Luan Y X, Ling E J. 2014. Insect prophenoloxidase: The view beyond immunity. Front Physiol, 5(1): 1-15. |
| [30] | Lu F, Qi G J, Qin R R, Hu G, Wang Z, Zhang X X, Cheng X N, Zhai B P. 2011. The processes of morphological change and grading criteria for ovarian development in the brown planthopper. Chin J Appl Entomol, 48(5): 1394-1400. (in Chinese with English abstract) |
| [31] | Nakai Y, Horiuchi J, Tsuda M, Takeo S, Akahori S, Matsuo T, Kume K, Aigaki T. 2011. Calcineurin and its regulator sra/DSCR1 are essential for sleep in Drosophila. J Neurosci, 31: 12759-12766. |
| [32] | Naoe Y, Arita K, Hashimoto H, Kanazawa H, Sato M, Shimizu T. 2005. Crystallization and preliminary X-ray crystallographic analysis of rat calcineurin B homologous protein 1. Acta Crystallogr Sect-Struct Biol Cryst Commun, F61: 612-613. |
| [33] |
Pang T X, Su X H, Wakabayashi S, Shigekawa M. 2001. Calcineurin homologous protein as an essential cofactor for Na+/H+ exchangers. J Biol Chem, 276: 17367-17372.
PMID |
| [34] |
Pfluger P T, Kabra D G, Aichler M, Schriever S C, Pfuhlmann K, García V C, Lehti M, Weber J, Kutschke M, Rozman J, Elrod J W, Hevener A L, Feuchtinger A, de Angelis M H, Walch A, Rollmann S M, Aronow B J, Müller T D, Perez-Tilve D, Jastroch M, de Luca M, Molkentin J D, Tschöp M H. 2015. Calcineurin links mitochondrial elongation with energy metabolism. Cell Metab, 22: 838-850.
PMID |
| [35] |
Preetha G, Stanley J, Suresh S, Samiyappan R. 2010. Risk assessment of insecticides used in rice on miridbug, Cyrtorhinus lividipennis Reuter, the important predator of brown planthopper, Nilaparvata lugens (Stål). Chemosphere, 80: 498-503.
PMID |
| [36] | Riddiford L M, Hiruma K, Zhou X F, Nelson C A. 2003. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochem Mol Biol, 33: 1327-1338. |
| [37] |
Rider M H, Hussain N, Dilworth S M, Storey J M, Storey K B. 2011. AMP-activated protein kinase and metabolic regulation in cold-hardy insects. J Insect Physiol, 57: 1453-1462.
PMID |
| [38] |
Rusnak F, Mertz P. 2000. Calcineurin: Form and function. Physiol Rev, 80: 1483-1521.
PMID |
| [39] |
Shibasaki F, Hallin U, Uchino H. 2002. Calcineurin as a multifunctional regulator. J Biochem, 131: 1-15.
PMID |
| [40] |
Sugiura R, Sio S O, Shuntoh H, Kuno T. 2001. Molecular genetic analysis of the calcineurin signaling pathways. Cell Mol Life Sci, 58: 278-288.
PMID |
| [41] | Takeo S, Hawley R S, Aigaki T. 2010. Calcineurin and its regulation by Sra/RCAN is required for completion of meiosis in Drosophila. Dev Biol, 344(2): 957-967. |
| [42] |
Tomita J, Mitsuyoshi M, Ueno T, Aso Y, Tanimoto H, Nakai Y, Aigaki T, Kume S, Kume K. 2011. Pan-neuronal knockdown of calcineurin reduces sleep in the fruit fly Drosophila melanogaster. J Neurosci, 31: 13137-13146.
PMID |
| [43] | Tonks N K. 2006. Protein tyrosine phosphatases: From genes, to function, to disease. Nat Rev Mol Cell Biol, 7: 833-846. |
| [44] | Tonks N K. 2013. Protein tyrosine phosphatases: From housekeeping enzymes to master regulators of signal transduction. FEBS J, 280: 346-378. |
| [45] | Voss M, Fechner L, Walz B, Baumann O. 2010. Calcineurin activity augments cAMP/PKA-dependent activation of V-ATPase in blowfly salivary glands. Am J Physiol Cell Physiol, 298: C1047-C1056. |
| [46] | Wagner J, Allman E, Taylor A, Ulmschneider K, Kovanda T, Ulmschneider B, Nehrke K, Peters M A. 2011. A calcineurin homologous protein is required for sodium-proton exchange events in the C. elegans intestine. Am J Physiol Cell Physiol, 301: C1389-C1403. |
| [47] | Wang W X, Li K L, Chen Y, Lai F X, Fu Q. 2015. Identification and function analysis of enolase gene NlEno1 from Nilaparvata lugens (Stål) (Hemiptera: Delphacidae). J Insect Sci, 15(1): 66. |
| [48] | Wang Y L, Xie C S, Diao Z Q, Liang B. 2017. Calcineurin antagonizes AMPK to regulate lipolysis in Caenorhabditis elegans. Molecules, 22: 1062. |
| [49] | Wei J Z, Yao X, Yang S, Liu S K, Zhou S, Cen J J, Liu X G, Du M F, Tang Q B, An S H. 2021. Suppression of calcineurin enhances the toxicity of Cry1Ac to Helicoverpa armigera. Front Microbiol, 12: 634619. |
| [50] |
Yamanaka N, Rewitz K F, O'Connor M B. 2013. Ecdysone control of developmental transitions: Lessons from Drosophila research. Annu Rev Entomol, 58: 497-516.
PMID |
| [51] |
Yin Z J, Dong X L, Kang K, Chen H, Dai X Y, Wu G G, Zheng L, Yu Y, Zhai Y F. 2018. FoxO transcription factor regulate hormone mediated signaling on nymphal diapause. Front Physiol, 9: 1654.
PMID |
| [52] | Yoshiga T, Yokoyama N, Imai N, Ohnishi A, Moto K, Matsumoto S. 2002. cDNA cloning of calcineurin heterosubunits from the pheromone gland of the silkmoth, Bombyx mori. Insect Biochem Mol Biol, 32: 477-486. |
| [53] | Zhang Q, Yang J G, Chen P, Liu T H, Xiao Q, Zhou X L, Wang L, Long Y B, Dong Z Q, Pan M H, Lu C. 2020. BmFoxO gene regulation of the cell cycle induced by 20-hydroxyecdysone in BmN-SWU1 cells. Insects, 11(10): 700. |
| [1] | Lü Jun, Liu Jinhui, Chen Lin, Sun Jiawei, Su Qin, Li Shihui, Yang Jianhua, Zhang Wenqing. Screening of Brown Planthopper Resistant miRNAs in Rice and Their Roles in Regulation of Brown Planthopper Fecundity [J]. Rice Science, 2022, 29(6): 559-568. |
| [2] | Mamunur Rashid Md, Jahan Mahbuba, Shariful Islam Khandakar. Impact of Nitrogen, Phosphorus and Potassium on Brown Planthopper and Tolerance of Its Host Rice Plants [J]. Rice Science, 2016, 23(3): 119-131. |
| [3] | Singh Sarao Preetinder, Sanmallappa Bentur Jagadaish. Antixenosis and Tolerance of Rice Genotypes Against Brown Planthopper [J]. Rice Science, 2016, 23(2): 96-103. |
| [4] | Shu-hua Liu, Jian Tang, Ju Luo, Bao-jun Yang, Ai-ying Wang, Jin-cai Wu. Cloning and Characterization of karmoisin Homologue Gene (Nlka) in Two Brown Planthopper Strains with Different Eye Colors [J]. Rice Science, 2016, 23(2): 104-110. |
| [5] | MA Bo-jun, GU Zhi-min, TANG Hai-juan, CHEN Xi-feng, LIU Feng, ZHANG Hong-sheng . Preliminary Study on Function of Calcineurin B-Like Protein Gene OsCBL8 in Rice [J]. RICE SCIENCE, 2010, 17(1): 10-18 . |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||