
Rice Science ›› 2024, Vol. 31 ›› Issue (3): 269-284.DOI: 10.1016/j.rsci.2023.11.012
收稿日期:2023-09-22
接受日期:2023-11-08
出版日期:2024-05-28
发布日期:2024-06-04
. [J]. Rice Science, 2024, 31(3): 269-284.

Fig. 1. Common occurrence and field morphology of rice false smut. A, Global distribution of rice false smut. The geographical locations of rice false smut occurrence in different continents were marked with different colors. B, Occurrence of rice false smut in the field. C, Diseased rice panicles with green-black false smut balls in the paddy field.
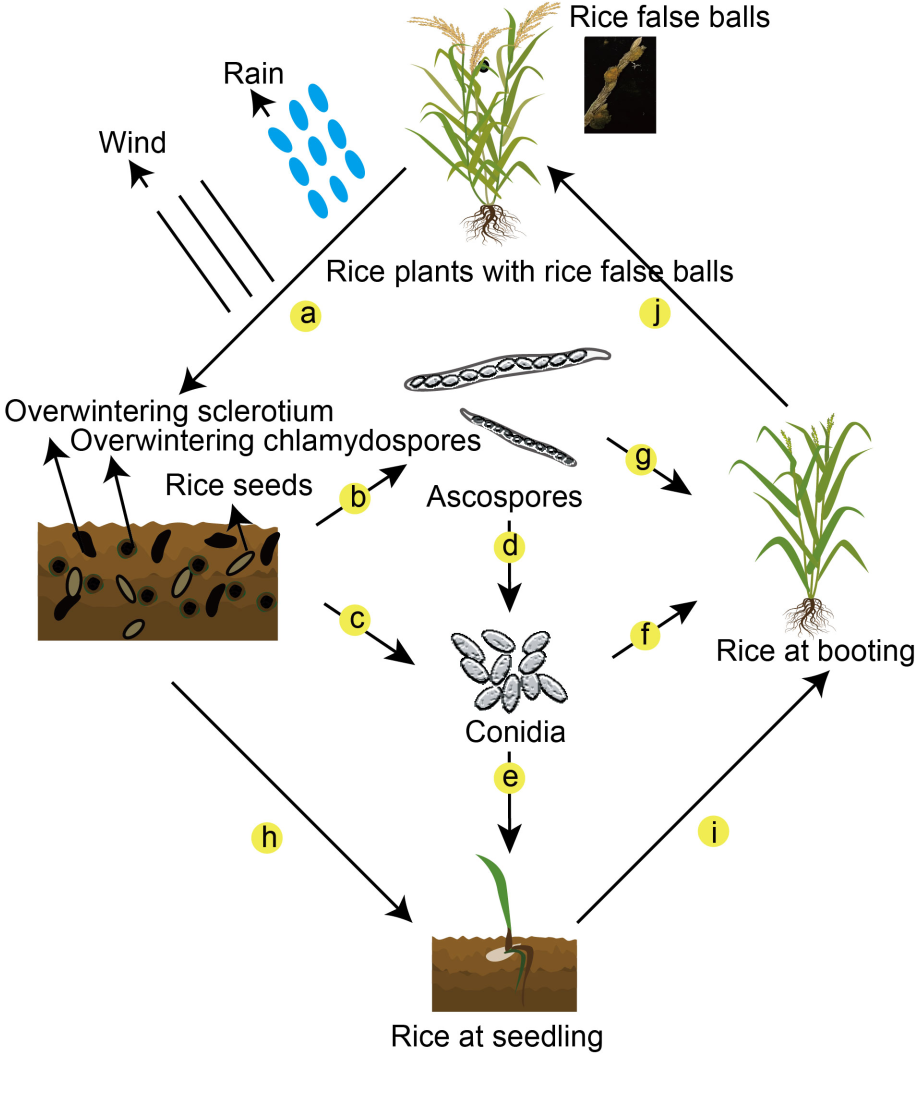
Fig. 2. Infection cycle of Ustilaginoidea virens in the field. a, Rice false smut spores on rice panicles are transmitted to the paddy field through wind and rain. b, The overwintering sclerotia germinated to form ascospores. c, Overwintering chlamydospores germinate to form conidia. d, Ascospores produce conidia. e, Conidia are epiphytic in the roots or coleoptiles of rice seedlings. f, Conidia infect panicles at the booting stage of rice. g, Ascospores infect panicles at the booting stage of rice. h‒j, Seeds with U. virens grow in the field.
| Gene | Deletion mutant | Gene function | Reference |
|---|---|---|---|
| UvSUN2 | Reduced virulence | Necessary for growth, cell wall construction, and stress response | Yu et al, |
| Uvt-1241 | Reduced virulence | Important in growth and pathogenesis | Bo et al, |
| UvPRO1 | Reduced virulence | Important in mycelial growth and conidiation as well as stress response and pathogenesis | Lv et al, |
| UvHOG1 | Reduced virulence | Important in regulating stress response, mycelial growth, and possible secondary metabolism | Zheng et al, |
| Uvt3277 | Reduced virulence | Important functions related to pathogenesis | Zheng et al, |
| Uv_1261 | Increased virulence | Crucial to virulence and inhibition defense of rice flowers | Fan et al, |
| UvAcI, UvPdeH | Reduced virulence | Important for the regulation of conidiation, stress response, virulence, and intracellular cyclic adenosine monophosphate levels | Guo et al, |
| UvBI-1 | Loss of virulence | Negative in mycelial growth and conidiation, and essential for stress tolerance, cell wall integrity, production of secondary metabolites, and pathogenicity | Xie et al, |
| UvHox2 | Reduced virulence | Regulation of chlamydial spore formation, conidiation, and pathogenicity | Yu J J et al, |
| UvGATA | Reduced virulence | Important for fungi in pathogenicity and reactive oxygen stress tolerance | Yu M N et al, |
| UvCom1 | Reduced virulence | Affects vegetative growth and division, and the ability to stably utilize host nutrients | Chen et al, |
| UvPaL1 | Reduced virulence | Affects mycelial growth, cell morphology, stress adaptation, and virulence | Chen et al, |
| UvHrip1 | Reduced virulence | Inhibits the defense response induced by pathogen-associated molecular patterns in Arabidopsis seedlings and plants and promotes disease reproduction in Arabidopsis | Li S et al, |
| UvAtg8 | Reduced virulence | Essential for fungal growth, stress response, needle formation, secondary spore formation, and pathogenicity | Meng et al, |
| UvPsr1 | Reduced virulence | Essential for mycelial growth, conidiation, stress response, and pathogenicity | Xiong et al, |
| MAT1-1-1, MAT1-1-2 | Reduced virulence | Important in division, stress response, sexual development, and pathogenicity | Yong et al, |
| MAT1-1-3 | Reduced virulence | Necessary for fruiting body and sclerotium formation, asexual development, and pathogenicity | Yong et al, |
| UvPmk1, UvCDc2 | Loss of virulence | Important in conidia, stress response, and pathogenicity | Zhang et al, |
| UvCap1 | Reduced virulence | Important in development and pathogenicity | Cao et al, |
| UvCCHC5 | Reduced virulence | Affects stress response, vegetative growth, conidiation, and virulence | Chen et al, |
| UvEC1 | Reduced virulence | Affects metabolism, protein localization, catalytic activity, binding, toxin biosynthesis, and splicing | Chen et al, |
| UvCGBP1 | Reduced virulence | Regulates fungal virulence through mitogen-activated protein kinase pathway | Chen et al, |
| UvCBP1 | Increased virulence | Manipulates plant immunity | Li et al, |
| UvKMT6 | Reduced virulence | Affects growth, division, and pathogenicity | Meng et al, |
| UvZnFTF1 | Reduced virulence | Involved in vegetative growth, conidiation, pigment biosynthesis, and pathogenicity | Song T Q et al, |
| UvMsn2 | Reduced virulence | Regulates vegetative growth, conidiation, stress response, mitochondrial morphology, and virulence | Xu et al, |
| UvSMEK1 | Reduced virulence | Regulates pathogenicity, needle formation, and spore germination | Yu J J et al, |
| UvZC1 | Reduced virulence | Involved in vegetative growth, conidium production, and rice infection, and related to the integrity of cell walls and response to oxidative stress | Yu M N et al, |
| UvSUN1 | Reduced virulence | Essential for growth, cell wall integrity, and pathogenicity | Yu M N et al, |
| Uvste50 | Reduced virulence | Affects the formation of conidia and rice false smut balls | Cao et al, |
| UvCCHC4 | Reduced virulence | Affects the expression of genes related to mitochondrial biogenesis, ribosomes, transporters, and ribosome biogenesis | Chen et al, |
| UvWhi2 | Reduced virulence | Necessary for fungal growth, stress response, and secondary spore formation | Meng et al, |
| UvKMT2 | Reduced virulence | Helpful in development, secondary spore formation, virulence, and various stress responses | Meng et al, |
| UvbZIP12 | Reduced virulence | Involved in the regulation of growth, development, and abiotic stress tolerance, but is not necessary for pathogenicity | Qu et al, |
| UvbZIP6 | Reduced virulence | Involved in growth, needle growth, stress response, and ball formation | Qu et al, |
| UvAtg14 | Reduced virulence | Contributes to mycelial growth, conidiation, and pathogenicity | Yu J J et al, |
| UvAtg7 | Reduced virulence | Contributes to mycelial growth, virulence, asexual reproduction, and cell stress response | Yu J J et al, |
| UvZnFTF2 | Reduced virulence | Involved in development and pathogenicity | Song T Q et al, |
| UvVEA | Reduced virulence | Affects the formation of chlamydia spores and the development of rice false smut balls | Yu M N et al, |
| UvSorA, UvSorB | Reduced virulence | Important in mycelial growth, sporulation, cell wall integrity, stress response, and phytotoxicity | Zhang X P et al, |
| UvATG6 | Reduced virulence | Eliminates autophagy of U. virens and reducing growth, conidium production and germination, and virulence | Gu et al, |
| UvATF21 | Reduced virulence | Crucial in vegetative growth, meristem, stress response, and full virulence | Liu Y R et al, |
| UvHst2 | Reduced virulence | As a global regulator of secondary metabolism in U. virens | Liu L et al, |
| UvSnf1 | Reduced virulence | Plays vital roles in virulence and carbon source utilization in U. virens | Wen et al, |
| UvGHF1 | Reduced virulence | An essential virulence factor and elicits plant immunity as a pathogen-associated molecular pattern | Zou et al, |
| Uv1809 | Increased virulence | Inhibits rice immunity and promotes U. virens infection | Chen et al, |
Table 1. Genes controlling virulence of Ustilaginoidea virens.
| Gene | Deletion mutant | Gene function | Reference |
|---|---|---|---|
| UvSUN2 | Reduced virulence | Necessary for growth, cell wall construction, and stress response | Yu et al, |
| Uvt-1241 | Reduced virulence | Important in growth and pathogenesis | Bo et al, |
| UvPRO1 | Reduced virulence | Important in mycelial growth and conidiation as well as stress response and pathogenesis | Lv et al, |
| UvHOG1 | Reduced virulence | Important in regulating stress response, mycelial growth, and possible secondary metabolism | Zheng et al, |
| Uvt3277 | Reduced virulence | Important functions related to pathogenesis | Zheng et al, |
| Uv_1261 | Increased virulence | Crucial to virulence and inhibition defense of rice flowers | Fan et al, |
| UvAcI, UvPdeH | Reduced virulence | Important for the regulation of conidiation, stress response, virulence, and intracellular cyclic adenosine monophosphate levels | Guo et al, |
| UvBI-1 | Loss of virulence | Negative in mycelial growth and conidiation, and essential for stress tolerance, cell wall integrity, production of secondary metabolites, and pathogenicity | Xie et al, |
| UvHox2 | Reduced virulence | Regulation of chlamydial spore formation, conidiation, and pathogenicity | Yu J J et al, |
| UvGATA | Reduced virulence | Important for fungi in pathogenicity and reactive oxygen stress tolerance | Yu M N et al, |
| UvCom1 | Reduced virulence | Affects vegetative growth and division, and the ability to stably utilize host nutrients | Chen et al, |
| UvPaL1 | Reduced virulence | Affects mycelial growth, cell morphology, stress adaptation, and virulence | Chen et al, |
| UvHrip1 | Reduced virulence | Inhibits the defense response induced by pathogen-associated molecular patterns in Arabidopsis seedlings and plants and promotes disease reproduction in Arabidopsis | Li S et al, |
| UvAtg8 | Reduced virulence | Essential for fungal growth, stress response, needle formation, secondary spore formation, and pathogenicity | Meng et al, |
| UvPsr1 | Reduced virulence | Essential for mycelial growth, conidiation, stress response, and pathogenicity | Xiong et al, |
| MAT1-1-1, MAT1-1-2 | Reduced virulence | Important in division, stress response, sexual development, and pathogenicity | Yong et al, |
| MAT1-1-3 | Reduced virulence | Necessary for fruiting body and sclerotium formation, asexual development, and pathogenicity | Yong et al, |
| UvPmk1, UvCDc2 | Loss of virulence | Important in conidia, stress response, and pathogenicity | Zhang et al, |
| UvCap1 | Reduced virulence | Important in development and pathogenicity | Cao et al, |
| UvCCHC5 | Reduced virulence | Affects stress response, vegetative growth, conidiation, and virulence | Chen et al, |
| UvEC1 | Reduced virulence | Affects metabolism, protein localization, catalytic activity, binding, toxin biosynthesis, and splicing | Chen et al, |
| UvCGBP1 | Reduced virulence | Regulates fungal virulence through mitogen-activated protein kinase pathway | Chen et al, |
| UvCBP1 | Increased virulence | Manipulates plant immunity | Li et al, |
| UvKMT6 | Reduced virulence | Affects growth, division, and pathogenicity | Meng et al, |
| UvZnFTF1 | Reduced virulence | Involved in vegetative growth, conidiation, pigment biosynthesis, and pathogenicity | Song T Q et al, |
| UvMsn2 | Reduced virulence | Regulates vegetative growth, conidiation, stress response, mitochondrial morphology, and virulence | Xu et al, |
| UvSMEK1 | Reduced virulence | Regulates pathogenicity, needle formation, and spore germination | Yu J J et al, |
| UvZC1 | Reduced virulence | Involved in vegetative growth, conidium production, and rice infection, and related to the integrity of cell walls and response to oxidative stress | Yu M N et al, |
| UvSUN1 | Reduced virulence | Essential for growth, cell wall integrity, and pathogenicity | Yu M N et al, |
| Uvste50 | Reduced virulence | Affects the formation of conidia and rice false smut balls | Cao et al, |
| UvCCHC4 | Reduced virulence | Affects the expression of genes related to mitochondrial biogenesis, ribosomes, transporters, and ribosome biogenesis | Chen et al, |
| UvWhi2 | Reduced virulence | Necessary for fungal growth, stress response, and secondary spore formation | Meng et al, |
| UvKMT2 | Reduced virulence | Helpful in development, secondary spore formation, virulence, and various stress responses | Meng et al, |
| UvbZIP12 | Reduced virulence | Involved in the regulation of growth, development, and abiotic stress tolerance, but is not necessary for pathogenicity | Qu et al, |
| UvbZIP6 | Reduced virulence | Involved in growth, needle growth, stress response, and ball formation | Qu et al, |
| UvAtg14 | Reduced virulence | Contributes to mycelial growth, conidiation, and pathogenicity | Yu J J et al, |
| UvAtg7 | Reduced virulence | Contributes to mycelial growth, virulence, asexual reproduction, and cell stress response | Yu J J et al, |
| UvZnFTF2 | Reduced virulence | Involved in development and pathogenicity | Song T Q et al, |
| UvVEA | Reduced virulence | Affects the formation of chlamydia spores and the development of rice false smut balls | Yu M N et al, |
| UvSorA, UvSorB | Reduced virulence | Important in mycelial growth, sporulation, cell wall integrity, stress response, and phytotoxicity | Zhang X P et al, |
| UvATG6 | Reduced virulence | Eliminates autophagy of U. virens and reducing growth, conidium production and germination, and virulence | Gu et al, |
| UvATF21 | Reduced virulence | Crucial in vegetative growth, meristem, stress response, and full virulence | Liu Y R et al, |
| UvHst2 | Reduced virulence | As a global regulator of secondary metabolism in U. virens | Liu L et al, |
| UvSnf1 | Reduced virulence | Plays vital roles in virulence and carbon source utilization in U. virens | Wen et al, |
| UvGHF1 | Reduced virulence | An essential virulence factor and elicits plant immunity as a pathogen-associated molecular pattern | Zou et al, |
| Uv1809 | Increased virulence | Inhibits rice immunity and promotes U. virens infection | Chen et al, |
| Name | Source | Antibacterial mechanism | Reference |
|---|---|---|---|
| Microbial inoculum agent | |||
| JN005 | Bacillus subtilis | Inhibition of mycelial growth | Guan et al, |
| E337 | Antennariella placitae | Increase rice yield and reduce the severity of rice false smut in susceptible rice plants | Andargie et al, |
| RSE5814 | Paenibacillus polymyxa | Inhibition of mycelial growth | Liu et al, |
| NKG-2 | Bacillus velezensis | Inhibition of mycelial growth | Myo et al, |
| Jt84 | Bacillus amyloliquefaciens | Inhibition of mycelial growth | Zhang et al, |
| BFC-33 | Bacillus fluminensis | Enhance the defense response of plant seedlings | Al-Shwaiman et al, |
| BR-01 | Bacillus velezensis | Produce antimicrobial peptides | Zhou et al, |
| Chemical agent | |||
| Azoxystrobin and Difenconazole, Metiram and Pyraclostrobin | Mixed bactericides | Reduce the incidence of disease in the field and increase yield | Muniraju et al, |
| Ethylicin | Organosulfur compound | Inhibition of mycelial growth | Fu et al, |
| Quicklime | CaO | Reduce the number of chlamydia spores in field soil | Ashizawa, |
| Trifloxystrobin-Tebuconazole | Triazole group and strobilurin | Reduce disease intensity and increase yield | Duraisamy et al, |
| Kresoxim methyl | A new broad spectrum strobilurin group of fungicide | Interfere with respiration in plant pathogenic fungi | Duraisamy et al, |
| Propiconazole | Triazole fungicide | Demethylation inhibitor of fungal sterol biosynthesis | Duraisamy et al, |
| Chelerythrine | Alkaloid | Broken mycelium membrane and spore reactive oxygen species accumulation | Wei et al, |
| Osthole@guar gum | Composite material | Destroy the cell wall of U. viren | Hu et al, |
| Sanmate | Benzimidazole methyl carbamate | Reduce rice disease ear rate and disease index | Song J H et al, |
| Salicyl hydroxamic acid | Extraquinone inhibitor | Inhibition of mycelial growth, conidial germination, peroxidase, and esterase activities | Song J H et al, |
| Pyraclostrobin, azoxystrobin | Extraquinone inhibitor | Reduce rice disease ear rate and disease index | Song J H et al, |
Table 2. Biological and chemical agents for controlling Ustilaginoidea virens.
| Name | Source | Antibacterial mechanism | Reference |
|---|---|---|---|
| Microbial inoculum agent | |||
| JN005 | Bacillus subtilis | Inhibition of mycelial growth | Guan et al, |
| E337 | Antennariella placitae | Increase rice yield and reduce the severity of rice false smut in susceptible rice plants | Andargie et al, |
| RSE5814 | Paenibacillus polymyxa | Inhibition of mycelial growth | Liu et al, |
| NKG-2 | Bacillus velezensis | Inhibition of mycelial growth | Myo et al, |
| Jt84 | Bacillus amyloliquefaciens | Inhibition of mycelial growth | Zhang et al, |
| BFC-33 | Bacillus fluminensis | Enhance the defense response of plant seedlings | Al-Shwaiman et al, |
| BR-01 | Bacillus velezensis | Produce antimicrobial peptides | Zhou et al, |
| Chemical agent | |||
| Azoxystrobin and Difenconazole, Metiram and Pyraclostrobin | Mixed bactericides | Reduce the incidence of disease in the field and increase yield | Muniraju et al, |
| Ethylicin | Organosulfur compound | Inhibition of mycelial growth | Fu et al, |
| Quicklime | CaO | Reduce the number of chlamydia spores in field soil | Ashizawa, |
| Trifloxystrobin-Tebuconazole | Triazole group and strobilurin | Reduce disease intensity and increase yield | Duraisamy et al, |
| Kresoxim methyl | A new broad spectrum strobilurin group of fungicide | Interfere with respiration in plant pathogenic fungi | Duraisamy et al, |
| Propiconazole | Triazole fungicide | Demethylation inhibitor of fungal sterol biosynthesis | Duraisamy et al, |
| Chelerythrine | Alkaloid | Broken mycelium membrane and spore reactive oxygen species accumulation | Wei et al, |
| Osthole@guar gum | Composite material | Destroy the cell wall of U. viren | Hu et al, |
| Sanmate | Benzimidazole methyl carbamate | Reduce rice disease ear rate and disease index | Song J H et al, |
| Salicyl hydroxamic acid | Extraquinone inhibitor | Inhibition of mycelial growth, conidial germination, peroxidase, and esterase activities | Song J H et al, |
| Pyraclostrobin, azoxystrobin | Extraquinone inhibitor | Reduce rice disease ear rate and disease index | Song J H et al, |
| [1] | Al-Shwaiman H A, Shahid M, Elgorban A M, Siddique K H M, Syed A. 2022. Beijerinckia fluminensis BFC-33, a novel multi- stress-tolerant soil bacterium: Deciphering the stress amelioration, phytopathogenic inhibition and growth promotion in Triticum aestivum (L.). Chemosphere, 295: 133843. |
| [2] | Andargie M, Li L Y, Feng A Q, Li J X. 2015. Colonization of rice roots by a green fluorescent protein-tagged isolate of Ustilaginoidea virens. Am J Plant Sci, 6(14): 2272-2279. |
| [3] | Andargie M, Li J X. 2016. Potential of mold mites (Acari: Acaridae) as a biocontrol agent of Ustilaginoidea virens. Entomol Gen, 36: 177-191. |
| [4] | Andargie M, Zhu C Y, Yun Y, Li J X. 2017. Identification and evaluation of potential bio-control fungal endophytes against Ustilagonoidea virens on rice plants. World J Microbiol Biotechnol, 33(6): 120. |
| [5] | Andargie M, Li J X. 2019a. Expression of the Arabidopsis SWEET genes during rice false smut infection in the transgenic Arabidopsis thaliana containing increased levels of ATP and sucrose. J Plant Biochem Biotechnol, 28(4): 509-520. |
| [6] | Andargie M, Li J X. 2019b. Antifungal activity against plant pathogens by compounds from Streptoverticillium morookaense. J Plant Pathol, 101(3): 547-558. |
| [7] | Ashizawa T. 2019. Application of calcined lime with simeconazole suppresses rice false smut disease. J Gen Plant Pathol, 85(5): 401-403. |
| [8] | Ashizawa T, Takahashi M, Arai M, Arie T. 2012. Rice false smut pathogen, Ustilaginoidea virens, invades through small gap at the apex of a rice spikelet before heading. J Gen Plant Pathol, 78(4): 255-259. |
| [9] | Atia M M M. 2004. Rice false smut (Ustilaginoidea virens) in Egypt. J Plant Dis Prot, 111(1): 71-82. |
| [10] | Bo H W, Yu M N, Yu J J, Yin X L, Ding H, Wang Y H, Liu Y F. 2016. Molecular cloning flanking sequences of T-DNA insertion from the Ustilaginoidea virens mutant strain B1241. Sci Agric Sin, 49(9): 1685-1695. (in Chinese with English abstract) |
| [11] | Brooks S A, Anders M M, Yeater K M. 2009. Effect of cultural management practices on the severity of false smut and kernel smut of rice. Plant Dis, 93(11): 1202-1208. |
| [12] | Brooks S A, Anders M M, Yeater K M. 2011. Influences from long-term crop rotation, soil tillage, and fertility on the severity of rice grain smuts. Plant Dis, 95(8): 990-996. |
| [13] | Cao H J, Zhang J J, Yong M L, Yu M N, Song T Q, Yu J J, Pan X Y, Liu Y F. 2021. The cyclase-associated protein UvCap1 is required for mycelial growth and pathogenicity in the rice false smut fungus. Phytopathol Res, 3(1): 5. |
| [14] | Cao H J, Gong H, Song T Q, Yu M N, Pan X Y, Yu J J, Qi Z Q, Du Y, Liu Y F. 2022. The adaptor protein UvSte50 governs fungal pathogenicity of Ustilaginoidea virens via the MAPK signaling pathway. J Fungi, 8(9): 954. |
| [15] | Cao Z Y, Sun L H, Mou R X, Lin X Y, Zhou R, Ma Y N, Chen M X. 2016. Analysis of ustiloxins in rice using polymer cation exchange cleanup followed by liquid chromatography-tandem mass spectrometry. J Chromatogr A, 1476: 46-52. |
| [16] | Chao J Q, Jin J, Wang D, Han R, Zhu R S, Zhu Y G, Li S Q. 2014. Cytological and transcriptional dynamics analysis of host plant revealed stage-specific biological processes related to compatible rice-Ustilaginoidea virens interaction. PLoS One, 9(3): e91391. |
| [17] | Chen X Y, Hai D, Tang J T, Liu H, Huang J B, Luo C X, Hsiang T, Zheng L. 2020a. UvCom1 is an important regulator required for development and infection in the rice false smut fungus Ustilaginoidea virens. Phytopathology, 110(2): 483-493. |
| [18] | Chen X Y, Tang J T, Pei Z X, Liu H, Huang J B, Luo C X, Tom H, Zheng L. 2020b. The ‘pears and lemons’ protein UvPal1 regulates development and virulence of Ustilaginoidea virens. Environ Microbiol, 22(12): 5414-5432. |
| [19] | Chen X Y, Pei Z X, Peng L, Qin Q, Duan Y H, Liu H, Chen X L, Zheng L, Luo C X, Huang J B. 2021a. Genome-wide identification and functional characterization of CCHC-type zinc finger genes in Ustilaginoidea virens. J Fungi, 7(11): 947. |
| [20] | Chen X Y, Pei Z X, Li P P, Li X B, Duan Y H, Liu H, Chen X L, Zheng L, Luo C X, Huang J B. 2021b. Quantitative proteomics analysis reveals the function of the putative ester cyclase UvEC1 in the pathogenicity of the rice false smut fungus Ustilaginoidea virens. Int J Mol Sci, 22(8): 4069. |
| [21] | Chen X Y, Li P P, Liu H, Chen X L, Huang J B, Luo C X, Li G T, Hsiang T, Collinge D B, Zheng L. 2021c. A novel transcription factor UvCGBP1 regulates development and virulence of rice false smut fungus Ustilaginoidea virens. Virulence, 12(1): 1563-1579. |
| [22] | Chen X Y, Li X B, Duan Y H, Pei Z X, Liu H, Yin W X, Huang J B, Luo C X, Chen X L, Li G T, Xie K B, Hsiang T, Zheng L. 2022a. A secreted fungal subtilase interferes with rice immunity via degradation of SUPPRESSOR OF G2 ALLELE OF skp1. Plant Physiol, 190(2): 1474-1489. |
| [23] | Chen X Y, Duan Y H, Qiao F G, Liu H, Huang J B, Luo C X, Chen X L, Li G T, Xie K B, Hsiang T, Zheng L. 2022b. A secreted fungal effector suppresses rice immunity through host histone hypoacetylation. New Phytol, 235(5): 1977-1994. |
| [24] | Chen X Y, Pei Z X, Liu H, Huang J B, Chen X L, Luo C X, Hsiang T, Zheng L. 2022c. Host-induced gene silencing of fungal-specific genes of Ustilaginoidea virens confers effective resistance to rice false smut. Plant Biotechnol J, 20(2): 253-255. |
| [25] | Chen X Y, Liu C, Wang H L, Liu Q, Yue Y P, Duan Y H, Wang Z Y, Zheng L, Chen X L, Wang Y H, Huang J B, Xu Q T, Pan Y M. 2024. Ustilaginoidea virens-secreted effector Uv1809 suppresses rice immunity by enhancing OsSRT2-mediated histone deacetylation. Plant Biotechnol J, 22(1): 148-164. |
| [26] | Chen Y, Zhang Y, Yao J, Li Y F, Yang X, Wang W X, Zhang A F, Gao T C. 2013. Frequency distribution of sensitivity of Ustilaginoidea virens to four EBI fungicides, prochloraz, difenoconazole, propiconazole and tebuconazole, and their efficacy in controlling rice false smut in Anhui Province of China. Phytoparasitica, 41(3): 277-284. |
| [27] | Cheng S Y, Liu H, Sun Q, Kong R, Letcher R J, Liu C S. 2019. Occurrence of the fungus mycotoxin, ustiloxin A, in surface waters of paddy fields in Enshi, Hubei, China, and toxicity in Tetrahymena thermophila. Environ Pollut, 251: 901-909. |
| [28] | Cooke M C. 1878. Some extra: European fungi. Grevillea, 7: 13-15. |
| [29] | Dou T X, Shao X H, Hu C H, Liu S W, Sheng O, Bi F C, Deng G M, Ding L J, Li C Y, Dong T, Gao H J, He W D, Peng X X, Zhang S, Huo H Q, Yang Q S, Yi G J. 2020. Host-induced gene silencing of Foc TR4 ERG6/11 genes exhibits superior resistance to Fusarium wilt of banana. Plant Biotechnol J, 18(1): 11-13. |
| [30] | Duraisamy L, Madamsetty S P, Vellaichamy P, Donempudi K, Banda S, Singh R, Prasad V, Lore J S, Jain J, Mariappan S, Laha G S. 2019. Geographic distribution of false smut disease of rice in India and efficacy of selected fungicides for its management. Int J Pest Manag, 65: 177-185. |
| [31] | Elshafie H S, Camele I, Mohamed A A. 2023. A comprehensive review on the biological, agricultural and pharmaceutical properties of secondary metabolites based-plant origin. Int J Mol Sci, 24(4): 3266. |
| [32] | Fan J, Du N, Li L, Li G B, Wang Y Q, Zhou Y F, Hu X H, Liu J, Zhao J Q, Li Y, Huang F, Wang W M. 2019. A core effector UV_1261 promotes Ustilaginoidea virens infection via spatiotemporally suppressing plant defense. Phytopathol Res, 1(1): 11. |
| [33] | Fang A F, Han Y Q, Zhang N, Zhang M, Liu L J, Li S, Lu F, Sun W X. 2016. Identification and characterization of plant cell death- inducing secreted proteins from Ustilaginoidea virens. Mol Plant Microbe Interact, 29(5): 405-416. |
| [34] | Fu R T, Ding L, Zhu J, Li P, Zheng A P. 2012. Morphological structure of propagules and electrophoretic karyotype analysis of false smut Villosiclava virens in rice. J Microbiol, 50(2): 263-269. |
| [35] | Fu R T, Ke Y X, Chen C, Wang J, Gong X S, Lu D H, Liao Y. 2018. Toxicity test and field control effect of ethylicin against several plant pathogens. Agrochemicals, 57(8): 611-613. (in Chinese with English abstract) |
| [36] | Fu R T, Wang J, Chen C, Zhao L Y, Chen X J, Lu D H. 2021a. Effects of mycotoxins of Ustilaginoidea virens on physiological- biochemical characteristics of different resistant rice varieties. Chin J Ecol, 40(9): 2793-2801. (in Chinese with English abstract) |
| [37] | Fu R T, Chen C, Wang J, Chen X J, Lu D H. 2021b. Control conditions and effects of plant protection unmanned aerial vehicle (UAV) on diseases and insect pests of rice. J Agric Sci Technol, 23(4): 103-109. (in Chinese with English abstract) |
| [38] | Fu R T, Wang J, Chen C, Zhao L Y, Chen X J, Lu D H. 2022a. Transcriptome analysis of young rice panicles in early response to exposure to mycotoxin of Ustilaginoidea virens. Chin J Rice Sci, 36(5): 447-458. (in Chinese with English abstract) |
| [39] | Fu R T, Chen C, Wang J, Zhao L Y, Chen X J, Lu D H. 2022b. Evaluation and screening of rice germplasm resources resistant to rice false smut. J Southern Agric, 53(1): 78-87. (in Chinese with English abstract) |
| [40] | Fu X X, Xie R S, Wang J, Chen X J, Wang X H, Sun W B, Meng J J, Lai D W, Zhou L G, Wang B M. 2017. Development of colloidal gold-based lateral flow immunoassay for rapid qualitative and semi-quantitative analysis of ustiloxins A and B in rice samples. Toxins, 9(3): 79. |
| [41] | Gu L F, Wang Y F, Xie S L, Liu Y R, Yan J L, Yin W X, Luo C X. 2023. UvATG6 interacts with BAX inhibitor 1 proteins and plays critical roles in growth, conidiation, and virulence in Ustilaginoidea virens. Microbiol Spectr, 11(3): e0489822. |
| [42] | Guan L L, Ren Z H, Li J J, Liu E M. 2016. Isolation and identification of an antagonistic bacterial strain against rice blast fungus. Biotechnol Bull, 32(6): 168-173. (in Chinese with English abstract) |
| [43] | Guo W W, Gao Y X, Yu Z M, Xiao Y H, Zhang Z G, Zhang H F. 2019. The adenylate cyclase UvAc1 and phosphodiesterase UvPdeH control the intracellular cAMP level, development, and pathogenicity of the rice false smut fungus Ustilaginoidea virens. Fungal Genet Biol, 129: 65-73. |
| [44] | Heslop-Harrison Y, Heslop-Harrison J S. 1996. Lodicule function and filament extension in the grasses: Potassium ion movement and tissue specialization. Ann Bot, 77(6): 573-582. |
| [45] | Hu M L, Luo L X, Wang S, Liu Y F, Li J Q. 2014. Infection processes of Ustilaginoidea virens during artificial inoculation of rice panicles. Eur J Plant Pathol, 139(1): 67-77. |
| [46] | Hu X F, Wang J, Li R Y, Wu X M, Gao X B, Li M. 2022a. Establishment of an artificial inoculation method of Ustilaginoidea virens without damaging the rice panicle sheaths. Plant Dis, 106(1): 289-296. |
| [47] | Hu X F, Wang J, Wu X M, Gao X B, Mo F X, Ding Y, Li R Y, Li M. 2022b. Preparation and characterization of sustainable osthole@guar gum composite film and antifungal mechanism against Ustilaginoidea virens. Ind Crops Prod, 185: 115144. |
| [48] | Hu X H, Shen S, Wu J L, Liu J, Wang H, He J X, Yao Z L, Bai Y F, Zhang X, Zhu Y, Li G B, Zhao J H, You X M, Xu J, Ji Y P, Li D Q, Pu M, Zhao Z X, Zhou S X, Zhang J W, Huang Y Y, Li Y, Ning Y S, Lu Y L, Huang F, Wang W M, Fan J. 2023. A natural allele of proteasome maturation factor improves rice resistance to multiple pathogens. Nat Plants, 9(2): 228-237. |
| [49] | Hu Z, Dang Y, Liu C S, Zhou L G, Liu H. 2019. Acute exposure to ustiloxin A affects growth and development of early life zebrafish, Danio rerio. Chemosphere, 226: 851-857. |
| [50] | Hu Z, Zheng L, Huang J B, Zhou L G, Liu C S, Liu H. 2020. Ustiloxin A is produced early in experimental Ustilaginoidea virens infection and affects transcription in rice. Curr Microbiol, 77(10): 2766-2774. |
| [51] | Huang Y, Tang X Q, Zheng L, Huang J B, Zhang Q, Liu H. 2021. Development of generic immuno-magnetic bead-based enzyme- linked immunoassay for ustiloxins in rice coupled with enrichment. Toxins, 13(12): 907. |
| [52] | Jones J D G, Dangl J L. 2006. The plant immune system. Nature, 444: 323-329. |
| [53] | Koeck M, Hardham A R, Dodds P N. 2011. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell Microbiol, 13(12): 1849-1857. |
| [54] | Koiso Y, Natori M, Iwasaki S, Sato S, Sonoda R, Fujita Y, Yaegashi H, Sato Z. 1992. Ustiloxin: A phytotoxin and a mycotoxin from false smut balls on rice panicles. Tetrahedron Lett, 33(29): 4157-4160. |
| [55] | Koiso Y, Li Y, Iwasaki S, Hanaoka K, Kobayashi T, Sonoda R, Fujita Y, Yaegashi H, Sato Z. 1994. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J Antibiot, 47(7): 765-773. |
| [56] | Koiso Y, Morisaki N, Yamashita Y, Mitsui Y, Shirai R, Hashimoto Y, Iwasaki S. 1998. Isolation and structure of an antimitotic cyclic peptide, Ustiloxin F: Chemical interrelation with a homologous peptide, Ustiloxin B. J Antibiot, 51(4): 418-422. |
| [57] | Koyama K, Natori S. 1988. Further characterization of seven bis(naphtho-γ-pyrone) congeners of ustilaginoidins, coloring matters of Claviceps virens (Ustilaginoidea virens). Chem Pharm Bull, 36(1): 146-152. |
| [58] | Kumagai T, Ishii T, Terai G, Umemura M, Machida M, Asai K. 2016. Genome sequence of Ustilaginoidea virens IPU010, a rice pathogenic fungus causing false smut. Genome Announc, 4(3): e00306-e00316. |
| [59] | Kumar A, Sahu T K, Bhalla A, Solani S. 2014. Influence of Trichoderma spp. against Ustilaginoidea virens inciting false smut of rice. Environ Ecol, 32: 163-168. |
| [60] | Lai D, Meng J, Xu D, Zhang X P, Liang Y F, Han Y, Jiang C, Liu H Q, Wang C F, Zhou L G, Xu J R. 2019. Determination of the absolute configurations of the stereogenic centers of ustilaginoidins by studying the biosynthetic monomers from a gene knockout mutant of Villosiclava virens. Sci Rep, 9(1): 1855. |
| [61] | Li H, Ni D H, Duan Y B, Chen Y, Li J, Song F S, Li L, Wei P C, Yang J B. 2013. Quantitative detection of the rice false smut pathogen Ustilaginoidea virens by real-time PCR. Genet Mol Res, 12(4): 6433-6441. |
| [62] | Li J, Wei S H, Xu Q Y, Kong L C, Yang M R, Wang Y L, Gu S H, Wang H N. 2020. Screening and identification of antagonistic bacteria against rice blast smut. Agrochemicals, 59(9): 676-679. |
| [63] | Li L, Zhu X M, Zhang Y R, Cai Y Y, Wang J Y, Liu M Y, Wang J Y, Bao J D, Lin F C. 2022. Research on the molecular interaction mechanism between plants and pathogenic fungi. Int J Mol Sci, 23(9): 4658. |
| [64] | Li S, Xiang S B, Wang Y L, Zhou J M, Hai Y F, Peng X W, Wang Y, Wei S H. 2020. UvHrip1, an effector secreted by Ustilaginoidea virens, suppresses basal defense and promotes disease development in Arabidopsis thaliana. Gene, 751: 144776. |
| [65] | Li W L, Li L Y, Feng A Q, Zhu X Y, Li J X. 2013. Rice false smut fungus, Ustilaginoidea virens, inhibits pollen germination and degrades the integuments of rice ovule. Am J Plant Sci, 4(12): 2295-2304. |
| [66] | Li X D, Huang R Y, Liu J Y, Xu G Y, Yuan M. 2021. Engineering false smut resistance rice via host-induced gene silencing of two chitin synthase genes of Ustilaginoidea virens. Plant Biotechnol J, 19: 2386-2388. |
| [67] | Li X J, Li J, Zhou H, Ren Z H, Liu E M. 2019. DNA methylation sensitive amplification polymorphism analysis of dormant and non-dormant chlamydospores in Ustilaginoidea virens. Plant Prot, 45(1): 129-134. (in Chinese with English) |
| [68] | Li X J, Xu L X, Lv Z C, Li F M, Xue J H, Peng Y H, Wei X Y, Li L. 2022. Antifungal mechanism of MTE-1, a novel oligosaccharide ester, against Ustilaginoidea virens. J Agric Food Chem, 70(24): 7441-7446. |
| [69] | Li Y J, Wang M, Liu Z H, Zhang K, Cui F H, Sun W X. 2019. Towards understanding the biosynthetic pathway for ustilaginoidin mycotoxins in Ustilaginoidea virens. Environ Microbiol, 21(8): 2629-2643. |
| [70] | Liang Y F, Han Y, Wang C F, Jiang C, Xu J R. 2018. Targeted deletion of the USTA and UvSLT2 genes efficiently in Ustilaginoidea virens with the CRISPR-Cas9 system. Front Plant Sci, 9: 699. |
| [71] | Lin X Y, Bian Y F, Mou R X, Cao Z Y, Cao Z Z, Zhu Z W, Chen M X. 2018. Isolation, identification, and characterization of Ustilaginoidea virens from rice false smut balls with high ustilotoxin production potential. J Basic Microbiol, 58(8): 670-678. |
| [72] | Liu J P, Tang T, Zhang S B, Zheng H B. 2009. Preliminary studies on initial infection sources and pathogen-infecting favorable stage for rice false smut. Hybrid Rice, 24(1): 74-77. (in Chinese with English abstract) |
| [73] | Liu L, Wang B, Duan G H, Wang J, Pan Z Q, Ou M M, Bai X L, Wang P Y, Zhao D, Nan N, Li D Y, Sun W X. 2023. Histone deacetylase UvHST2 is a global regulator of secondary metabolism in Ustilaginoidea virens. J Agric Food Chem, 71(35): 13124-13136. |
| [74] | Liu L M, Zhao K H, Cai L B, Zhang Y L, Fu Q, Huang S W. 2023. Combination effects of tebuconazole with Bacillus subtilis to control rice false smut and the related synergistic mechanism. Pest Manag Sci, 79(1): 234-243. |
| [75] | Liu X Y, Matsumoto H, Lv T X, Zhan C F, Fang H D, Pan Q Q, Xu H R, Fan X Y, Chu T Y, Chen S L, Qiao K, Ma Y N, Sun L, Wang Q W, Wang M C. 2023. Phyllosphere microbiome induces host metabolic defence against rice false-smut disease. Nat Microbiol, 8(8): 1419-1433. |
| [76] | Liu Y, Bai F R, Li N, Wang W P, Cheng C. 2017. Identification of endophytic bacterial strain RSE1 from seeds of super hybrid rice Shenliangyou 5814 (Oryza sativa L.) and evaluation of its antagonistic activity. Plant Growth Regul, 82(3): 403-408. |
| [77] | Liu Y J, Chen T Q, Lü C Y, Liang W S, Hu D W. 2018. Primary study on induction of the para-sclerotia of Villosiclava virens in artificial medium. Acta Phytopathol Sin, 48(5): 594-600. (in Chinese with English abstract) |
| [78] | Liu Y R, Qu J S, Wang Y F, Yin W X, Luo C X. 2023. bZIP transcription factor UvATF21 mediates vegetative growth, conidiation, stress tolerance and is required for full virulence of rice false smut fungus Ustilaginoidea virens. Rice Sci, 30(1): 50-57. |
| [79] | Lore J S, Jain J, Kumar S, Kamboj I, Khanna R, Dhillon B S, Zaidi N W, Singh U S. 2021. Prevention of false smut (Ustilaginoidea virens) on rice hybrids and pure-line cultivars by manipulating planting date. J Phytopathol, 169(10): 597-606. |
| [80] | Lu D H, Mao J H, Wang P, He Z Q, Chen F. 2008. Resistant differences of indica rice hybrid varieties to rice false smut. Acta Phytophyl Sin, 35: 289-294. (in Chinese with English abstract) |
| [81] | Lv B, Zheng L, Liu H, Tang J T, Hsiang T, Huang J B. 2016. Use of random T-DNA mutagenesis in identification of gene UvPRO1, A regulator of conidiation, stress response, and virulence in Ustilaginoidea virens. Front Microbiol, 7: 2086. |
| [82] | Maliang H D, Wang P W, Chen A L, Liu H B, Lin H P, Ma J Y. 2021. Bamboo tar as a novel fungicide: Its chemical components, laboratory evaluation, and field efficacy against false smut and sheath blight of rice and powdery mildew and fusarium wilt of cucumber. Plant Dis, 105(2): 331-338. |
| [83] | Mendes R, Kruijt M, de Bruijn I, Dekkers E, van der Voort M, Schneider J H M, Piceno Y M, DeSantis T Z, Andersen G L, Bakker P A H M, Raaijmakers J M. 2011. Deciphering the rhizosphere microbiome for disease-suppressive bacteria. Science, 332: 1097-1100. |
| [84] | Meng J J, Sun W B, Mao Z L, Xu D, Wang X H, Lu S Q, Lai D W, Liu Y, Zhou L G, Zhang G Z. 2015. Main ustilaginoidins and their distribution in rice false smut balls. Toxins, 7(10): 4023-4034. |
| [85] | Meng J J, Gu G, Dang P Q, Zhang X P, Wang W X, Dai J G, Liu Y, Lai D W, Zhou L G. 2019. Sorbicillinoids from the fungus Ustilaginoidea virens and their phytotoxic, cytotoxic, and antimicrobial activities. Front Chem, 7: 435. |
| [86] | Meng S, Xiong M, Jagernath J S, Wang C C, Qiu J H, Shi H B, Kou Y J. 2020. UvAtg8-mediated autophagy regulates fungal growth, stress responses, conidiation, and pathogenesis in Ustilaginoidea virens. Rice, 13(1): 56. |
| [87] | Meng S, Liu Z Q, Shi H B, Wu Z L, Qiu J H, Wen H, Lin F C, Tao Z, Luo C X, Kou Y J. 2021. UvKmt6-mediated H3K27 trimethylation is required for development, pathogenicity, and stress response in Ustilaginoidea virens. Virulence, 12(1): 2972-2988. |
| [88] | Meng S, Qiu J H, Xiong M, Liu Z Q, Jagernath J S, Lin F C, Shi H B, Kou Y J. 2022a. UvWhi2 is required for stress response and pathogenicity in Ustilaginoidea virens. Rice Sci, 29(1): 47-54. |
| [89] | Meng S, Shi H B, Lin C Y, Wu Z L, Lin F C, Tao Z, Kou Y J. 2022b. UvKmt2-mediated H3K4 trimethylation is required for pathogenicity and stress response in Ustilaginoidea virens. J Fungi, 8(6): 553. |
| [90] | Miyazaki S, Matsumoto Y, Uchihara T, Morimoto K. 2009. High- performance liquid chromatographic determination of ustiloxin A in forage rice silage. J Vet Med Sci, 71(2): 239-241. |
| [91] | Muniraju K, Pranesh D, Mallesh S B, Guruprasad G S. 2017. Novel fungicides for the management of false smut disease of rice caused by Ustilaginoidea virens. Int J Curr Microbiol App Sci, 6(11): 2664-2669. |
| [92] | Myo E M, Liu B H, Ma J J, Shi L M, Jiang M G, Zhang K C, Ge B B. 2019. Evaluation of Bacillus velezensis NKG-2 for bio-control activities against fungal diseases and potential plant growth promotion. Biol Contr, 134: 23-31. |
| [93] | Pan Z Y, Jiang X Y, Miao J K, Ma L, Jiang H B, Dong L Q, Liu B, Han Y, Bai Y J. 2022. Effects of the number of rice false smut balls and the position for the diseased ear on yield and quality of northern japonica rice. China Rice, 28(6): 118-121. (in Chinese with English abstract) |
| [94] | Pramesh D, Prasannakumar M K, Muniraju K M, Mahesh H B, Pushpa H D, Manjunatha C, Saddamhusen A, Chidanandappa E, Yadav M K, Kumara M K, Sharanabasav H, Rohith B S, Banerjee G, Das A J. 2020. Comparative genomics of rice false smut fungi Ustilaginoidea virens Uv-Gvt strain from India reveals genetic diversity and phylogenetic divergence. 3 Biotech, 10(8): 342. |
| [95] | Qiu J H, Meng S, Deng Y Z, Huang S W, Kou Y J. 2019. Ustilaginoidea virens: A fungus infects rice flower and threats world rice production. Rice Sci, 26(4): 199-206. |
| [96] | Qiu J H, Lu F F, Wang H, Xie J H, Wang C C, Liu Z Q, Meng S, Shi H B, Shen X H, Kou Y J. 2020. A candidate gene for the determination of rice resistant to rice false smut. Mol Breed, 40(12): 105. |
| [97] | Qiu S S, Fang A F, Zheng X H, Wang S Z, Wang J Y, Fan J, Sun Z T, Gao H, Yang J Y, Zeng Q T, Cui F H, Wang W M, Chen J P, Sun W X. 2022. Ustilaginoidea virens nuclear effector SCRE4 suppresses rice immunity via inhibiting expression of a positive immune regulator OsARF17. Int J Mol Sci, 23(18): 10527. |
| [98] | Qu J S, Wang Y F, Wang R, Liu Y R, Gu L F, Zhou P, Yin W X, Luo C X. 2022a. Functional study on bZIP transcription factor UvbZIP12 in Ustilaginoidea virens. Acta Phytopathol Sin, 52: 555-564. (in Chinese with English abstract) |
| [99] | Qu J S, Wang Y F, Cai M Z, Liu Y R, Gu L F, Zhou P, Du Y L, Xu C H, Wang R, Yin W X, Luo C X. 2022b. The bZIP transcription factor UvbZIP6 mediates fungal growth, stress response, and false smut formation in Ustilaginoidea virens. Phytopathol Res, 4(1): 32. |
| [100] | Rangel L I, Bolton M D. 2022. The unsung roles of microbial secondary metabolite effectors in the plant disease cacophony. Curr Opin Plant Biol, 68: 102233. |
| [101] | Rao H Z, Luo H C, Shi T W, Li Y, Huang S W. 2019. Investigation of heading dynamic states of Yongyou 12 and optimal time to spray chemicals for prevention rice false smut. China Rice, 25(1): 74-79. (in Chinese with English abstract) |
| [102] | Ren Z H, Mao Y, Yu X J, Li Y, Liu E M. 2012. Research on the composition of the fatty acids in chalmydospores of Ustilagioidea virens. Microbiol China, 39(6): 789-796. (in Chinese with English abstract) |
| [103] | Rong N H, Yong M L, Xu Y, Hu D W. 2017. Relationship between ultrastructure of cell walls of rice spikelets and infection specificity of Villosiclava virens. Acta Bot Boreal Occident Sin, 37(1): 1-7. (in Chinese with English abstract) |
| [104] | Saleh M M, Zidan N E H A. 2021. Impact of Ustilaginoidea virens mycotoxins on rice seed germination and their feeding risk. Egypt J Agric Res, 99(1): 1-9. |
| [105] | Sidhu A, Ghatelwal S R, Gumber K, Bala A. 2017. Augmented antifungal potential of benzothiazol-2-ylcarbamodithioates as hybrid-silver aqua nanoformulations. Appl Nanosci, 7(8): 617-623. |
| [106] | Song J H, Wei W, Lv B, Lin Y, Yin W X, Peng Y L, Schnabel G, Huang J B, Jiang D H, Luo C X. 2016. Rice false smut fungus hijacks the rice nutrients supply by blocking and mimicking the fertilization of rice ovary. Environ Microbiol, 18(11): 3840-3849. |
| [107] | Song J H, Wang Y F, Yin W X, Huang J B, Luo C X. 2021. Effect of chemical seed treatment on rice false smut control in field. Plant Dis, 105(10): 3218-3223. |
| [108] | Song J H, Wang Z Y, Wang Y, Zhang S J, Lei T Y, Liang Y, Dai Q G, Huo Z Y, Xu K, Chen S N. 2022a. Prevalence of carbendazin resistance in field populations of the rice false smut pathogen Ustilaginoidea virens from Jiangsu, China: Molecular mechanisms, and fitness stability. J Fungi, 8(12): 1311. |
| [109] | Song J H, Wang Z Y, Zhang S J, Wang Y, Liang Y, Dai Q G, Huo Z Y, Xu K. 2022b. The toxicity of salicylhydroxamic acid and its effect on the sensitivity of Ustilaginoidea virens to azoxystrobin and pyraclostrobin. J Fungi, 8(11): 1231. |
| [110] | Song J H, Zhang S J, Wang Y, Chen Y T, Luo J F, Liang Y, Zhang H C, Dai Q G, Xu K, Huo Z Y. 2022c. Baseline sensitivity and control efficacy of two quinone outside inhibitor fungicides, azoxystrobin and pyraclostrobin, against Ustilaginoidea virens. Plant Dis, 106(11): 2967-2973. |
| [111] | Song T Q, Zhang X, Zhang Y, Liang D, Yan J L, Yu J J, Yu M N, Cao H J, Yong M L, Pan X Y, Qi Z Q, Du Y, Zhang R S, Liu Y F. 2021. Genome-wide identification of Zn2Cys6 class fungal-specific transcription factors (ZnFTFs) and functional analysis of UvZnFTF1 in Ustilaginoidea virens. Rice Sci, 28(6): 567-578. |
| [112] | Song T Q, Yu J J, Yu M N, Cao H J, Pan X Y, Qi Z Q, Du Y, Liang D, Zhang R S, Liu Y F. 2022. A fungal-specific transcription factor UvZnFTF2 is required for vegetative growth, conidiation, and pathogenicity of Ustilaginoidea virens. Acta Phytopathol Sin, 52(6): 891-898. (in Chinese with English abstract) |
| [113] | Sun Q, Liu H, Zhang Y K, Kong R, Yi X E, Liu C S. 2021. Detection of Ustiloxin A in urine by ultra-high-performance liquid chromatography-tandem mass spectrometry coupled with two-step solid-phase extraction. J Chromatogr B, 1181: 122916. |
| [114] | Sun Q, Liu H, Zhang Y K, Yi X E, Kong R, Cheng S Y, Man J G, Zheng L, Huang J B, Su G Y, Letcher R J, Giesy J P, Liu C S. 2022. Global distribution of ustiloxins in rice and their male- biased hepatotoxicity. Environ Pollut, 301: 118992. |
| [115] | Sun W B, Dong X J, Xu D, Meng J J, Fu X X, Wang X H, Lai D W, Zhou L G, Liu Y. 2016. Preparative separation of main ustilaginoidins from rice false smut balls by high-speed counter-current chromatography. Toxins, 8(1): 20. |
| [116] | Sun W B, Wang A L, Xu D, Wang W X, Meng J J, Dai J G, Liu Y, Lai D W, Zhou L G. 2017. New ustilaginoidins from rice false smut balls caused by Villosiclava virens and their phytotoxic and cytotoxic activities. J Agric Food Chem, 65(25): 5151-5160. |
| [117] | Sun W X, Fan J, Fang A F, Li Y J, Tariqjaveed M, Li D Y, Hu D W, Wang W M. 2020. Ustilaginoidea virens: Insights into an emerging rice pathogen. Annu Rev Phytopathol, 58: 363-385. |
| [118] | Tanaka E. 2015. Life cycle and infection route of rice false smut fungus in paddy field. Mycotoxins, 65: 39-43. |
| [119] | Tanaka E, Ashizawa T, Sonoda R, Tanaka C. 2008. Villosiclava virens gen. nov., comb. nov., teleomorph of Ustilaginoidea virens, the causal agent of rice false smut. Mycotaxon, 106: 491-501. |
| [120] | Tang J T, Chen X Y, Yan Y Q, Huang J B, Luo C X, Tom H, Zheng L. 2021. Comprehensive transcriptome profiling reveals abundant long non-coding RNAs associated with development of the rice false smut fungus, Ustilaginoidea virens. Environ Microbiol, 23(9): 4998-5013. |
| [121] | Tang Y X, Jin J, Hu D W, Yong M L, Xu Y, He L P. 2013. Elucidation of the infection process of Ustilaginoidea virens (teleomorph: Villosiclava virens) in rice spikelets. Plant Pathol, 62(1): 1-8. |
| [122] | Tokpah D D, Kwoseh C, Tokpah E S, Kolleh D. 2017. Rice false smut and its management in major rice growing areas in Ashanti region of Ghana. Afr J Agric Res, 12: 3129-3136. |
| [123] | van der Linde K, Göhre V. 2021. How do smut fungi use plant signals to spatiotemporally orientate on and in planta? J Fungi, 7(2): 107. |
| [124] | Wang A L, Li P, Han P P, Gu G, Shan T J, Lai D W, Zhou L G. 2021. New nitrogen-containing metabolites from cultures of rice false smut pathogen Villosiclava virens. Nat Prod Res, 35(2): 272-281. |
| [125] | Wang B, Liu L, Li Y J, Zou J Y, Li D Y, Zhao D, Li W, Sun W X. 2021. Ustilaginoidin D induces hepatotoxicity and behaviour aberrations in zebrafish larvae. Toxicology, 456: 152786. |
| [126] | Wang L, Zeng R, Farooq M U, Zhao D M, Bao L F, He Z W, Huang F. 2019. Villosiclava virens invasion of rice floret induce an early immune response to artificial inoculation. Int J Agric Biol, 22: 531-536. |
| [127] | Wang N, Ren Z H, Deng L W, Chen J F, Liu E M. 2012. Structure and composition of polysaccharide from chlamydospore wall in Ustiloginoidea virens. Chin J Rice Sci, 26(3): 356-360. (in Chinese with English abstract) |
| [128] | Wang S, Bai Y J, Zhou Y L, Yao J M, Bai J K. 1998. The pathogen of false smut of rice. Acta Phytopathol Sin, 28(1): 20-25. (in Chinese with English abstract) |
| [129] | Wang W, Yin H, Huang N, Zhu C J, Wang Y F, Qi X T, Ma L, Fan Y X, Yu Y, Zhang H S, Bao Y M. 2021. A simple and visible detection method for the rapid diagnosis of Ustilaginoidea virens in rice seeds by a loop-mediated isothermal amplification assay. J Phytopathol, 169(6): 369-375. |
| [130] | Wang W X, Gu G, Yin R Y, Fu J J, Jing M P, Shen Z, Lai D W, Wang B M, Zhou L G. 2022. A nanobody-based immunoassay for detection of ustilaginoidins in rice samples. Toxins, 14(10): 659. |
| [131] | Wang X H, Fu X X, Lin F K, Sun W B, Meng J J, Wang A L, Lai D W, Zhou L G, Liu Y. 2016. The contents of Ustiloxins A and B along with their distribution in rice false smut balls. Toxins, 8(9): 262. |
| [132] | Wang X H, Wang J, Lai D W, Wang W X, Dai J G, Zhou L G, Liu Y. 2017. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins, 9(2): 54. |
| [133] | Wang Y, Yang L, Yang Q, Dong J, Wang Y F, Duan Y H, Yin W X, Zheng L, Sun W X, Fan J, Luo C X, Li G T. 2022. Gap-free nuclear and mitochondrial genomes of Ustilaginoidea virens JS60-2, a fungal pathogen causing rice false smut. Mol Plant Microbe Interact, 35(12): 1120-1123. |
| [134] | Wang Y Q, Fan R H, Yang B H, Liu B, Zhang J Z, Hu D W. 2010. Comparison of isolation methods for Ustilaginoidea virens, the pathogen of rice false smut. Mycosystema, 29(1): 59-63. (in Chinese with English abstract) |
| [135] | Wang Z, Yu Z X, Solanki M K, Yang L T, Xing Y X, Dong D F, Li Y R. 2020. Diversity of sugarcane root-associated endophytic Bacillus and their activities in enhancing plant growth. J Appl Microbiol, 128(3): 814-827. |
| [136] | Wang Z, Hu X H, Solanki M K, Pang F. 2023. A synthetic microbial community of plant core microbiome can be a potential biocontrol tool. J Agric Food Chem, 71(13): 5030-5041. |
| [137] | Wei Q H, Cui D Z, Liu X F, Chai Y Y, Zhao N, Wang J Y, Zhao M. 2020. In vitro antifungal activity and possible mechanisms of action of chelerythrine. Pestic Biochem Physiol, 164: 140-148. |
| [138] | Wen H, Meng S, Xie S W, Shi H B, Qiu J H, Jiang N, Kou Y J. 2023a. Sucrose non-fermenting protein kinase gene UvSnf1 is required for virulence in Ustilaginoidea virens. Virulence, 14(1): 2235460. |
| [139] | Wen H, Shi H B, Jiang N, Qiu J H, Lin F C, Kou Y J. 2023b. Antifungal mechanisms of silver nanoparticles on mycotoxin producing rice false smut fungus. iScience, 26(1): 105763. |
| [140] | Weng H Y, Tian Y, Wu N, Li X L, Yang B Y, Huang Y P, Ye D P, Wu R Y. 2020. Development of a low-cost narrow band multispectral imaging system coupled with chemometric analysis for rapid detection of rice false smut in rice seed. Sensors, 20(4): 1209. |
| [141] | Wu B, Wen X W, Li S S, Hu D W, Liang W S. 2018. Influences of mycotoxins of Villosiclava virens on the transcriptome of rice (Oryza sativa) seedling roots. J Agric Biol, 26(7): 1093-1106. |
| [142] | Wu N, Jiang H B, Bao Y D, Zhang C, Zhang J Z, Song W J, Zhao Y Y, Mi C X, He Y, Liu F. 2020. Practicability investigation of using near-infrared hyperspectral imaging to detect rice kernels infected with rice false smut in different conditions. Sens Actuator B: Chem, 308: 127696. |
| [143] | Xie S L, Wang Y F, Wei W, Li C Y, Liu Y, Qu J S, Meng Q H, Lin Y, Yin W X, Yang Y N, Luo C X. 2019. The Bax inhibitor UvBI-1, a negative regulator of mycelial growth and conidiation, mediates stress response and is critical for pathogenicity of the rice false smut fungus Ustilaginoidea virens. Curr Genet, 65(5): 1185-1197. |
| [144] | Xiong M, Meng S, Qiu J H, Shi H B, Shen X L, Kou Y J. 2020. Putative phosphatase UvPsr1 is required for mycelial growth, conidiation, stress response and pathogenicity in Ustilaginonidea virens. Rice Sci, 27(6): 529-536. |
| [145] | Xu Y D, Wu S, Yu Z M, Moeketsi E K, Yang Z X, Zhang Z G, Zhang H F. 2021. Transcription factor UvMsn2 is important for vegetative growth, conidiogenesis, stress response, mitochondrial morphology and pathogenicity in the rice false smut fungus Ustilaginoidea virens. Phytopathol Res, 3(1): 16. |
| [146] | Yang D W, He N Q, Huang F H, Jin Y D, Li S P. 2023. The genetic mechanism of the immune response to the rice false smut (RFS) fungus Ustilaginoidea virens. Plants, 12(4): 741. |
| [147] | Yang J Y, Zhang N, Wang J Y, Fang A F, Fan J, Li D Y, Li Y J, Wang S Z, Cui F H, Yu J J, Liu Y F, Wang W M, Peng Y L, He S Y, Sun W X. 2022. SnRK1A-mediated phosphorylation of a cytosolic ATPase positively regulates rice innate immunity and is inhibited by Ustilaginoidea virens effector SCRE1. New Phytol, 236(4): 1422-1440. |
| [148] | Yang N, Ji Y Y, Wang A Y, Tang J, Liu S H, Zhang X D, Xu L J, He Y. 2022. An integrated nucleic acid detection method based on a microfluidic chip for collection and culture of rice false smut spores. Lab Chip, 22(24): 4894-4904. |
| [149] | Yao L, Ye H L, Hu R P, Wu J, Lu D H, Mao J H, He Z Q. 2012. Preliminary study on infection mechanism of rice false smut. Southwest China J Agric Sci, 25(4): 1273-1276. (in Chinese with English abstract) |
| [150] | Yin X L, Chen Z Y, Liu Y F, Liu Y Z, Wang X Y, Luo C P, Yu J J, Nie Y F. 2011. Screening and evaluation of antagonistic bacteria against rice false smut. Jiangsu J Agric Sci, 27(5): 983-989. (in Chinese with English abstract) |
| [151] | Yong M L, Fan L L, Li D Y, Liu Y J, Cheng F M, Xu Y, Wang Z Y, Hu D W. 2016. Villosiclava virens infects specifically rice and barley stamen filaments due to the unique host cell walls. Microsc Res Tech, 79(9): 838-844. |
| [152] | Yong M L, Yu J J, Pan X Y, Yu M N, Cao H J, Song T Q, Qi Z Q, Du Y, Zhang R S, Yin X L, Liu W D, Liu Y F. 2020a. Two mating-type genes MAT1-1-1 and MAT1-1-2 with significant functions in conidiation, stress response, sexual development, and pathogenicity of rice false smut fungus Villosiclava virens. Curr Genet, 66(5): 989-1002. |
| [153] | Yong M L, Yu J J, Pan X Y, Yu M N, Cao H J, Qi Z Q, Du Y, Zhang R S, Song T Q, Yin X L, Chen Z Y, Liu W D, Liu Y F. 2020b. MAT1-1-3, a mating type gene in the Villosiclava virens, is required for fruiting bodies and Sclerotia formation, asexual development and pathogenicity. Front Microbiol, 11: 1337. |
| [154] | Yu J J, Yu M N, Song T Q, Cao H J, Pan X Y, Yong M L, Qi Z Q, Du Y, Zhang R S, Yin X L, Liu Y F. 2019. A homeobox transcription factor UvHOX2 regulates chlamydospore formation, conidiogenesis, and pathogenicity in Ustilaginoidea virens. Front Microbiol, 10: 1071. |
| [155] | Yu J J, Yu M N, Song T Q, Cao H J, Yong M L, Pan X Y, Qi Z Q, Du Y, Zhang R S, Yin X L, Liang D, Liu Y F. 2021. UvSMEK1, a suppressor of MEK null, regulates pathogenicity, conidiation and conidial germination in rice false smut fungus Ustilaginoidea virens. Rice Sci, 28(5): 457-465. |
| [156] | Yu J J, He X, Xu C F, Yu M N, Song T Q, Cao H J, Pan X Y, Qi Z Q, Du Y, Zhang R S, Liang D, Liu Y F. 2022. Autophagy-related protein UvAtg7 contributes to mycelial growth, virulence, asexual reproduction and cell stress response in rice false smut fungus Ustilaginoidea virens. Fungal Genet Biol, 159: 103668. |
| [157] | Yu M N, Yu J J, Hu J K, Huang L, Wang Y H, Yin X L, Nie Y F, Meng X K, Wang W D, Liu Y F. 2015. Identification of pathogenicity-related genes in the rice pathogen Ustilaginoidea virens through random insertional mutagenesis. Fungal Genet Biol, 76: 10-19. |
| [158] | Yu M N, Yu J J, Cao H J, Yong M L, Liu Y F. 2019. Genome-wide identification and analysis of the GATA transcription factor gene family in Ustilaginoidea virens. Genome, 62(12): 807-816. |
| [159] | Yu M N, Yu J J, Cao H J, Pan X Y, Song T Q, Liu Y F. 2021a. Clone and functional research of Zn2(II)Cys6 transcription factor UvZC1 gene in Ustilaginoidea virens. Jiangsu J Agric Sci, 37(6): 1400-1408. (in Chinese with English abstract) |
| [160] | Yu M N, Yu J J, Cao H J, Song T Q, Pan X Y, Qi Z Q, Du Y, Zhang R S, Huang S W, Liu W D, Liu Y F. 2021b. SUN-family protein UvSUN1 regulates the development and virulence of Ustilaginoidea virens. Front Microbiol, 12: 739453. |
| [161] | Yu M N, Yu J J, Cao H J, Pan X Y, Song T Q, Qi Z Q, Du Y, Huang S W, Liu Y F. 2022. The velvet protein UvVEA regulates conidiation and chlamydospore formation in Ustilaginoidea virens. J Fungi, 8(5): 479. |
| [162] | Yu S W, Liu P W, Wang J Y, Li D Y, Zhao D, Yang C, Shi D Y, Sun W X. 2023. Molecular mechanisms of Ustilaginoidea virens pathogenicity and their utilization in disease control. Phytopathol Res, 5(1): 16. |
| [163] | Zhang J C, Chen Z Y, Zhang B X, Liu Y F, Lu F. 2003. Study on morphology of Ustilaginoidea virens. Acta Phytopathol Sin, 33(6): 517-523. (in Chinese with English abstract) |
| [164] | Zhang N, Yang J Y, Fang A F, Wang J Y, Li D Y, Li Y J, Wang S Z, Cui F H, Yu J J, Liu Y F, Peng Y L, Sun W X. 2020. The essential effector SCRE1 in Ustilaginoidea virens suppresses rice immunity via a small peptide region. Mol Plant Pathol, 21(4): 445-459. |
| [165] | Zhang R S, Yu J J, Qi Z Q, Zhang H, Du Y, Yu M N, Song T Q, Cao H J, Pan X Y, Yong M L, Chen Z Y, Liu Y F. 2021. Study on the field application technology of Bacillus amyloliquefaciens Jt84 against rice false smut. Chin J Biol Control, 37(3): 525-530. (in Chinese with English abstract) |
| [166] | Zhang X D, Guo B X, Wang Y F, Hu L, Yang N, Mao H P. 2022. A detection method for crop fungal spores based on microfluidic separation enrichment and AC impedance characteristics. J Fungi, 8(11): 1168. |
| [167] | Zhang X P, Xu D, Hou X W, Wei P L, Fu J J, Zhao Z T, Jing M P, Lai D W, Yin W B, Zhou L G. 2022. UvSorA and UvSorB involved in sorbicillinoid biosynthesis contribute to fungal development, stress response and phytotoxicity in Ustilaginoidea virens. Int J Mol Sci, 23(19): 11056. |
| [168] | Zhang Y, Liao X L, Zeng X N, Huang H, Gao W J, Xue Z J. 2010. Identification of an antagonistic bacterial strain from duck manure against Rhizoctonia solani. Acta Phytopathol Sin, 40(5): 517-521. (in Chinese with English abstract) |
| [169] | Zhang Y, Zhang K, Fang A F, Han Y Q, Yang J, Xue M F, Bao J D, Hu D W, Zhou B, Sun X Y, Li S J, Wen M, Yao N, Ma L J, Liu Y F, Zhang M, Huang F, Luo C X, Zhou L G, Li J Q, Chen Z Y, Miao J K, Wang S, Lai J S, Xu J R, Hsiang T, Peng Y L, Sun W X. 2014. Specific adaptation of Ustilaginoidea virens in occupying host florets revealed by comparative and functional genomics. Nat Commun, 5: 3849. |
| [170] | Zhang Z, Du X F, Chai R Y, Mao X Q, Qiu H P, Wang Y L, Wang J Y, Sun G C. 2006. Agrobacterium tumefaciens-mediated transformation of the pathogen of Ustilaginoidea virens. Chin J Rice Sci, 20(4): 440-442. (in Chinese with English abstract) |
| [171] | Zhang Z W, Chen L, Ding K J, Pan W, Ye Z H. 2013. Infection characteristics of Ustilaginoidea virens and optimal time for disease control. J Anhui Agric Univ, 40(4): 656-659. (in Chinese with English abstract) |
| [172] | Zheng D W, Wang Y, Han Y, Xu J R, Wang C F. 2016. UvHOG1 is important for hyphal growth and stress responses in the rice false smut fungus Ustilaginoidea virens. Sci Rep, 6: 24824. |
| [173] | Zheng M T, Ding H, Huang L, Wang Y H, Yu M N, Zheng R, Yu J J, Liu Y F. 2017. Low-affinity iron transport protein Uvt3277 is important for pathogenesis in the rice false smut fungus Ustilaginoidea virens. Curr Genet, 63(1): 131-144. |
| [174] | Zheng X H, Fang A F, Qiu S S, Zhao G S, Wang J Y, Wang S Z, Wei J J, Gao H, Yang J Y, Mou B H, Cui F H, Zhang J, Liu J, Sun W X. 2022. Ustilaginoidea virens secretes a family of phosphatases that stabilize the negative immune regulator OsMPK6 and suppress plant immunity. Plant Cell, 34(8): 3088-3109. |
| [175] | Zhou J P, Xie Y Q, Liao Y H, Li X Y, Li Y M, Li S P, Ma X G, Lei S M, Lin F, Jiang W, He Y Q. 2022. Characterization of a Bacillus velezensis strain isolated from Bolbostemmatis Rhizoma displaying strong antagonistic activities against a variety of rice pathogens. Front Microbiol, 13: 983781. |
| [176] | Zou J Y, Jiang C Q, Qiu S S, Duan G H, Wang G Q, Li D Y, Yu S W, Zhao D, Sun W X. 2023. An Ustilaginoidea virens glycoside hydrolase 42 protein is an essential virulence factor and elicits plant immunity as a PAMP. Mol Plant Pathol, 24(11): 1414-1429. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||