
Rice Science ›› 2021, Vol. 28 ›› Issue (3): 301-312.DOI: 10.1016/j.rsci.2021.04.008
• Research Paper • Previous Articles
Singh Divya1, K. Dutta Tushar1( ), N. Shivakumara Tagginahalli1, Dash Manoranjan1, Bollinedi Haritha2, Rao Uma1(
), N. Shivakumara Tagginahalli1, Dash Manoranjan1, Bollinedi Haritha2, Rao Uma1( )
)
Received:2020-05-19
Accepted:2020-08-10
Online:2021-05-28
Published:2021-05-28
Singh Divya, K. Dutta Tushar, N. Shivakumara Tagginahalli, Dash Manoranjan, Bollinedi Haritha, Rao Uma. Suberin Biopolymer in Rice Root Exodermis Reinforces Preformed Barrier Against Meloidogyne graminicola Infection[J]. Rice Science, 2021, 28(3): 301-312.
Add to citation manager EndNote|Ris|BibTeX
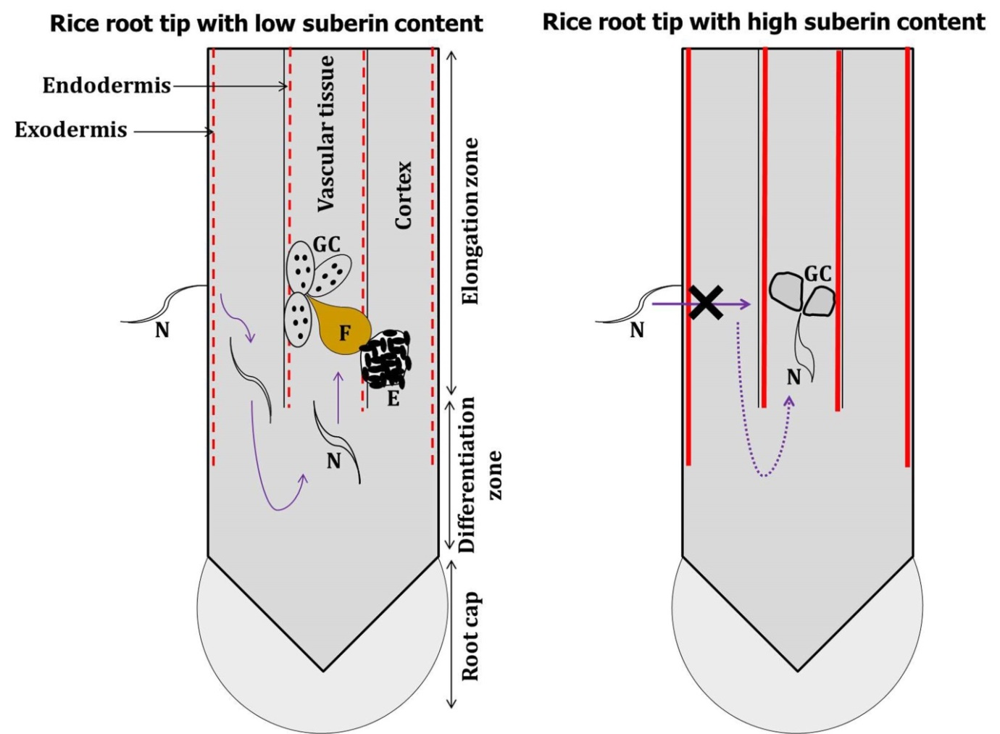
Fig. S1. Hypothesis of present study schematically represented. In low suberin containing rice root (indicated by dotted perpendicular red line), J2s of M. graminicola can penetrate the root at elongation zone, migrate to undifferentiated meristematic tissue and reach vascular cylinder by making a U-turn bypassing the differentiated endodermis. By contrast, in high suberin containing rice root (indicated by solid perpendicular red line), J2s of M. graminicola cannot cross the exodermis barrier and even if penetrated GCs induced in the vascular tissue become nonfunctional. Figures are not drawn to scale. N, Nematode; F, Adult female; E, Egg mass; GC, Giant cell.
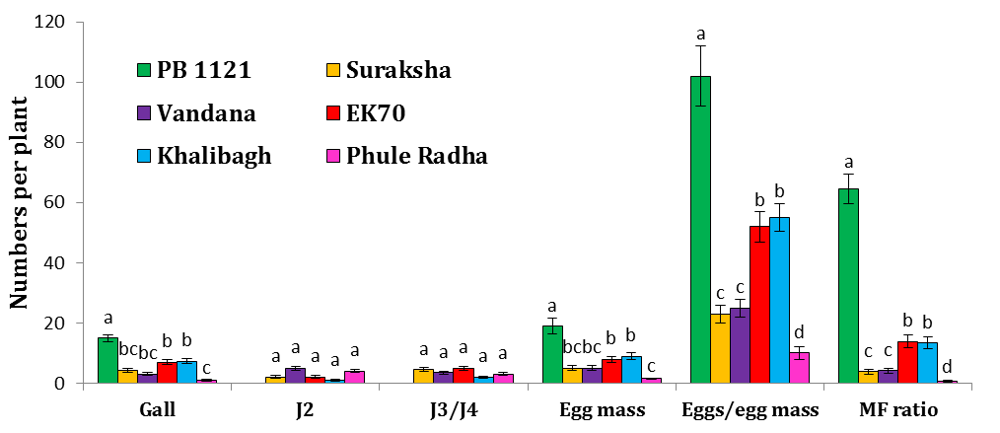
Fig. S2. Screening of rice varieties for nematode resistance.Relative numbers of gall, J2, J3/J4, egg mass and eggs/egg mass of M. graminicola in different varieties of rice (PB1121, Suraksha, Vandana, EK70, Khalibagh and Phule Radha) at 16 d after inoculation. Different lowercase letters within any parameter are significantly different at P < 0.01 by the Tukey’s HSD test. Error bars indicate standard error of mean (n = 6). Inoculum level was 30 J2s/plant.J2, J3 and J4 refer to the second, third and fourth stage juveniles, respectively.
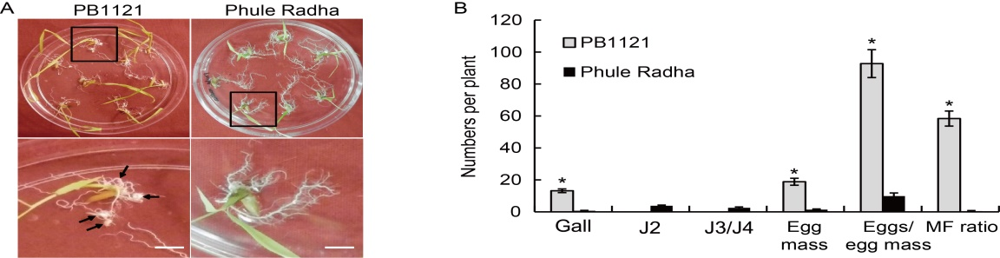
Fig. 1. Meloidogyne graminicola infected plantlets of PB1121 and Phule Radha in Petri plates containing PF-127 medium at 16 d after inoculation. A, Photographs show comparatively greater galling intensity and larger sized galls (indicated by arrows in bottom panel magnified view) in PB1121 than negligible and smaller sized galls in Phule Radha. Scale bars, 1 cm.B, Relative numbers of galls, egg masses, eggs/egg mass and multiplication factor (MF) ratio in PB1121 and Phule Radha. Inoculum level is 30 J2s/plant. J2, The second stage juveniles; J3/J4, The third/fourth stage juveniles. Data represent Mean ± SE (n = 3). Asterisks indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.
| Life cycle stage | Days after inoculation (d) | Number of nematodes/root system | |
|---|---|---|---|
| PB1121 | Phule Radha | ||
| J2 | 1 | 25.66 ± 1.20 e | 5.33 ± 0.33 b |
| 2 | 32.33 ± 2.67 f | 9.66 ± 0.67 c | |
| 3 | 34.00 ± 2.88 fg | 10.33 ± 0.58 d | |
| 4 | 35.50 ± 2.76 g | 11.25 ± 1.18 d | |
| 5 | 8.80 ± 1.53 c | 5.50 ± 0.58 b | |
| 7 | 5.00 ± 0.88 b | 2.33 ± 0.33 a | |
| J3/J4 | 5 | 27.00 ± 1.15 d | 5.00 ± 0.67 ab |
| 7 | 26.66 ± 1.45 d | 6.25 ± 1.20 b | |
| 10 | 8.00 ± 0.88 c | 4.66 ± 0.66 a | |
| 12 | 0.00 ± 0.00 a | 3.33 ± 0.33 a | |
| Female | 10 | 24.00 ± 1.67 b | 0.00 ± 0.00 a |
| 12 | 27.50 ± 2.57 c | 3.66 ± 0.33 a | |
| 16 | 33.00 ± 1.76 d | 4.66 ± 0.66 a | |
| Egg mass | 16 | 33.00 ± 1.76 b | 4.66 ± 0.66 a |
| Eggs/egg mass | 16 | 152.80 ± 8.76 b | 10.66 ± 1.58 a |
| Multiplication factor ratio | 50.42 ± 6.17 b | 0.49 ± 0.13 a | |
Table S1. Comparison of M. graminicola invasion, development and reproduction in PB1121 and Phule Radha.
| Life cycle stage | Days after inoculation (d) | Number of nematodes/root system | |
|---|---|---|---|
| PB1121 | Phule Radha | ||
| J2 | 1 | 25.66 ± 1.20 e | 5.33 ± 0.33 b |
| 2 | 32.33 ± 2.67 f | 9.66 ± 0.67 c | |
| 3 | 34.00 ± 2.88 fg | 10.33 ± 0.58 d | |
| 4 | 35.50 ± 2.76 g | 11.25 ± 1.18 d | |
| 5 | 8.80 ± 1.53 c | 5.50 ± 0.58 b | |
| 7 | 5.00 ± 0.88 b | 2.33 ± 0.33 a | |
| J3/J4 | 5 | 27.00 ± 1.15 d | 5.00 ± 0.67 ab |
| 7 | 26.66 ± 1.45 d | 6.25 ± 1.20 b | |
| 10 | 8.00 ± 0.88 c | 4.66 ± 0.66 a | |
| 12 | 0.00 ± 0.00 a | 3.33 ± 0.33 a | |
| Female | 10 | 24.00 ± 1.67 b | 0.00 ± 0.00 a |
| 12 | 27.50 ± 2.57 c | 3.66 ± 0.33 a | |
| 16 | 33.00 ± 1.76 d | 4.66 ± 0.66 a | |
| Egg mass | 16 | 33.00 ± 1.76 b | 4.66 ± 0.66 a |
| Eggs/egg mass | 16 | 152.80 ± 8.76 b | 10.66 ± 1.58 a |
| Multiplication factor ratio | 50.42 ± 6.17 b | 0.49 ± 0.13 a | |
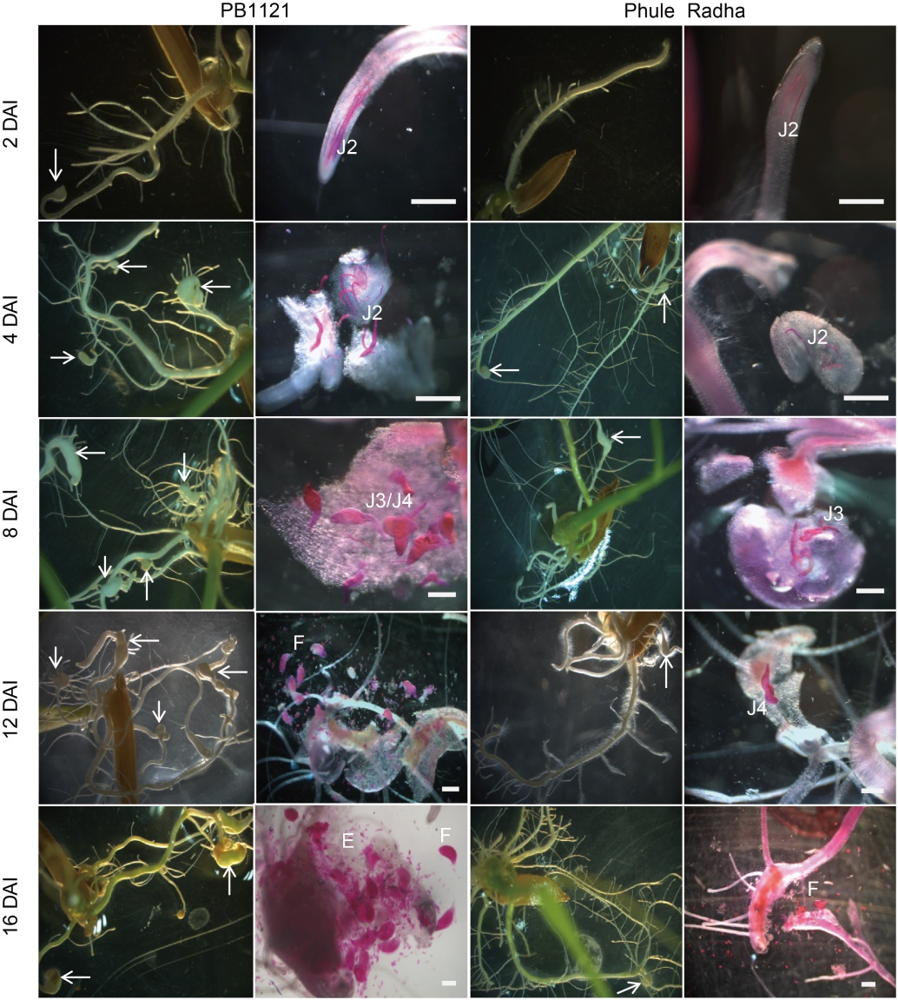
Fig. 2. Comparative life cycle progression of M. graminicola in PB1121 and Phule Radha. Typical hook-like gall formation at the root tip was evident from 2 DAI onward in PB1121. By contrast, Phule Radha supported fewer galls because of the lower J2 penetration and delayed development of invading J2s even at 12 and 16 DAI. Nematodes were stained with acid fuchsin. White arrows indicate root galls. Scale bars, 200 µm.DAI, Days after inoculation; J2, J3 and J4, The second, third and fourth stage juveniles, respectively; E, Eggs; F, Female.
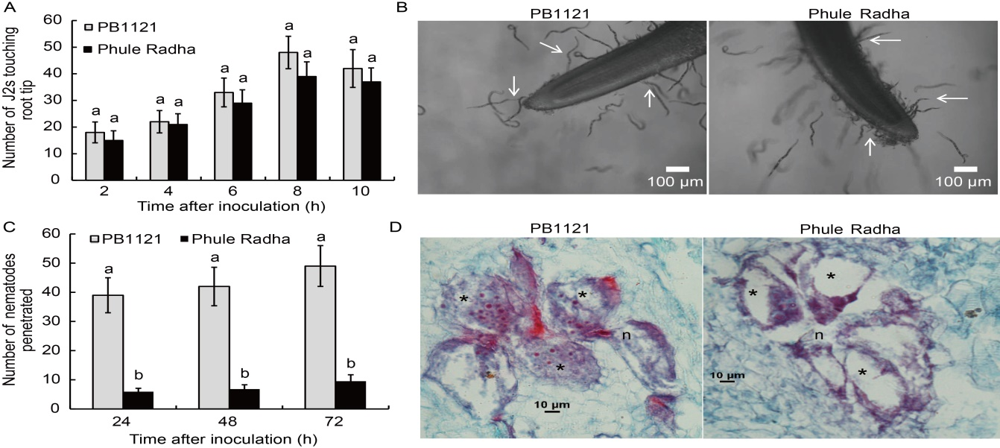
Fig. 3. Comparative attraction to and penetration of PB1121 and Phule Radha root tips by M. graminicola. A, Attraction of the second stage juveniles (J2s) towards rice root tips in the PF-127 medium at 2, 4, 6, 8 and 10 h after inoculation. Data represent Mean ± SE (n = 3) and bars with unshared letters (within identical time point) indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.B, Photomicrographs showing attraction of J2s to the root tips of PB1121 and Phule Radha at 8 h after inoculation. Head of the young female is localized among the multinucleate giant cells with densely stained cytoplasm at the vascular cylinder of PB1121. By contrast, nematode head surrounded by the giant cells are devoid of cytoplasm at the vascular cylinder of Phule Radha. C, Penetration of J2s in rice root tips at 24, 48 and 72 h after inoculation. Data represent Mean ± SE (n = 3) and bars with unshared letters (within identical time point) indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.D, Cross-sections of rice roots infected by M. graminicola at 7 d after inoculation. n, Nematode; *, Giant cell.
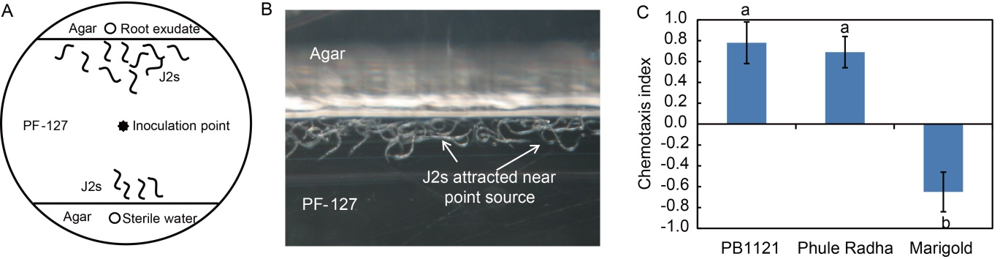
Fig. 4. Chemotaxis of M. graminicola towards root exudates of PB1121 and Phule Radha. A, Assay was conducted in a 50 mm × 10 mm Petri dish containing PF-127 in central area and agar in the peripheral area. Wells containing 10 µL of root exudate (sterile water as control) were 1.5 cm distant from the nematode inoculation point (100 J2s applied). J2s, The second stage juveniles.B, A close-up view of the assay plate shows that after 1 h of inoculation, J2s were accumulated near the point source at agar-PF-127 interface when exposed to root exudates of PB1121. C, Chemotaxis index of nematodes towards rice root exudates. Marigold was used as a negative control. Values indicate attraction (positive index) or repulsion (negative index). Data represent Mean ± SE (n = 3) and bars with unshared letters indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.
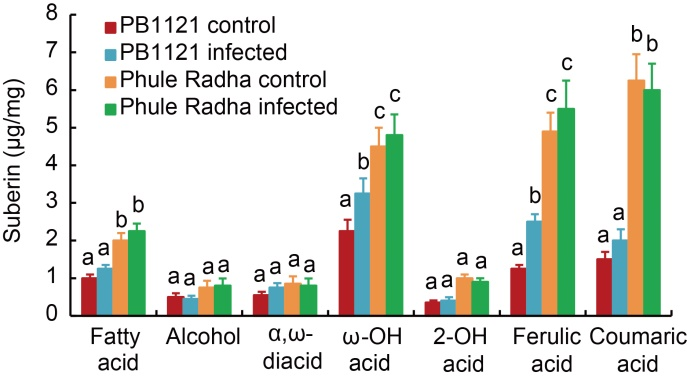
Fig. 5. Suberin content in root tips of PB1121 and Phule Radha. Aliphatic and aromatic suberin released from the exodermis of control and nematode infected [5-day-old plantlets were inoculated with 100 J2s (the second stage juveniles) of M. graminicola in the PF-127 medium and plantlets harvested at 2 d after inoculation] roots after transesterification with BF3-methanol. Total aliphatic suberin amount and monomer compositions were performed via gas chromatography- mass spectrometry analysis. Bars represent Mean ± SE (n = 3) and bars with unshared letters (within each substance class) indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.
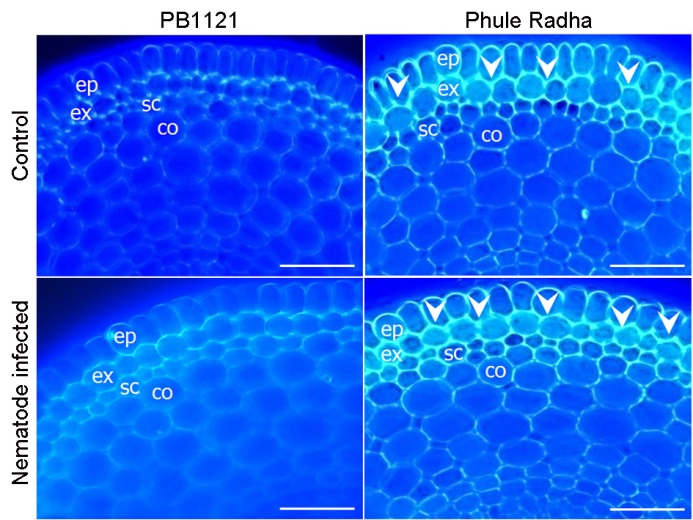
Fig. 6. Comparative suberin lamellae deposition in roots of PB1121 and Phule Radha. Cross sections of the control and nematode-infected rice roots stained with Fluorol Yellow 088. Intense yellowish green fluorescence (indicated by white arrowheads) in the suberized exodermis of Phule Radha is evident. Blue fluorescence indicates auto fluorescence. Sections of both genotypes were taken at the identical distance from root tip (about 1 cm from root apex). Scale bars, 50 µm. ep, Epidermis; ex, Exodermis/hypodermis; sc, Sclerenchyma; co, Cortex.
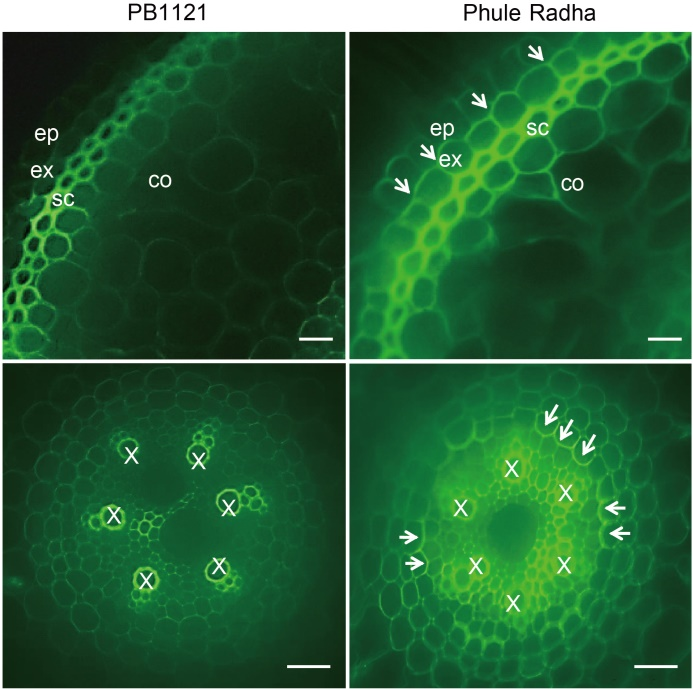
Fig. 7. Comparative Casparian strip (CS) deposition in roots of PB1121 and Phule Radha. Cross sections were obtained from 5-day-old control roots and stained with berberine hemisulfate and aniline blue. Intense green fluorescence (indicated by white arrows) in the CS lining of Phule Radha exodermis (top panel) and endodermis (bottom panel) is evident. Berberine causes intense xylem (X) fluorescence. Aniline blue counterstains berberine- induced fluorescence of lignin and reveals endodermal CS. Sections of both genotypes were taken at the identical distance from root tips (about 1 cm from root apex). Scale bars, 20 µm. ep, Epidermis; ex, Exodermis/hypodermis; sc, Sclerenchyma; co, Cortex.
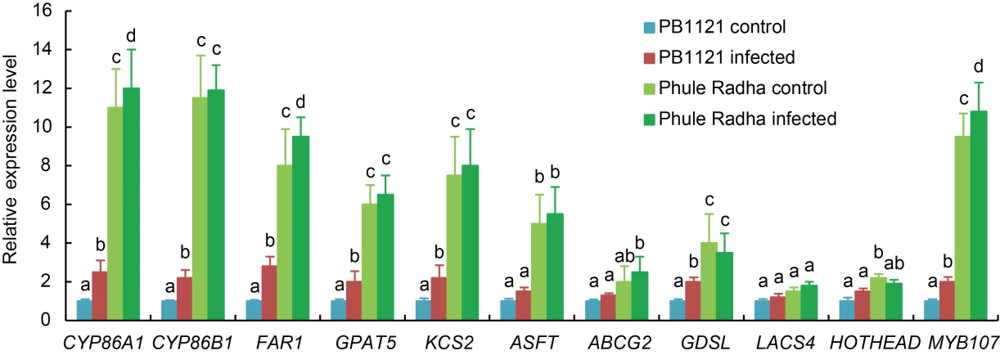
Fig. 8. Relative expression levels of suberin biosynthesis genes in root tip exodermis of PB1121 and Phule Radha. Five-day-old plantlets were inoculated with 100 J2s (the second stage juveniles) of M. graminicola in the PF-127 medium and harvested at 2 d after inoculation. Uninfected roots served as the control. qRT-PCR data are expressed as the fold change in expression from three biological and three technical replicates each containing a pool of 10 plants. Gene expression levels were normalized using the internal reference gene of O. sativa, i.e. 18S rRNA. Data represent Mean ± SE and bars with unshared letters (within each gene) indicate significant difference at P < 0.01 by the post-hoc Tukey’s test.
| Gene name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| CYP86A1 | CTCTAAACTTCGACGAGCTG | CCCACTGAGTAGATGGAGTA |
| CYP86B1 | CCGGTTTCATAGCGAAACTAC | GAAGGTGCAAATCCCTCTT |
| FAR1 | CAAGTACTCCTTCGTCATGC | GTCCCAGTCGATGTTCTTG |
| GPAT5 | CCAACTACGTGCAGAGGATAC | GAAGCCGAGAACCTCCTTG |
| KCS2 | CAACTGCAGCCTCTTCAAC | CAGTTGAGCGTGATGTTCTC |
| ASFT | CTCTACTACCTCTCCAACCT | AGGGTAGTAGTGGACAAGGA |
| ABCG2 | AGGTGTTTCAGAGAGAGAGG | AGGACGAAGAAGAGGTAGTG |
| GDSL | CTTCTACTTCGTGGCAATCG | CGCTGAAGAGGAGGAAGTA |
| LACS4 | CATGCGGTGGTTCTATTCTG | CAACATATGGGCACACACTC |
| HOTHEAD | GTGGAATTGGACCCAGAAAG | CGTCGGTTATTCCAACAGTC |
| MYB107 | CTCATCGCCTACATCCAGAA | CTTGATGATGGTGTCCTCCT |
| 18S rRNA | CGCGCAAATTACCCAATCCTGACA | TCCCGAAGGCCAACGTAAATAGGA |
Table S2. List of oligonucleotides employed for qRT-PCR analysis of suberin biosynthesis genes in rice.
| Gene name | Forward primer (5′-3′) | Reverse primer (5′-3′) |
|---|---|---|
| CYP86A1 | CTCTAAACTTCGACGAGCTG | CCCACTGAGTAGATGGAGTA |
| CYP86B1 | CCGGTTTCATAGCGAAACTAC | GAAGGTGCAAATCCCTCTT |
| FAR1 | CAAGTACTCCTTCGTCATGC | GTCCCAGTCGATGTTCTTG |
| GPAT5 | CCAACTACGTGCAGAGGATAC | GAAGCCGAGAACCTCCTTG |
| KCS2 | CAACTGCAGCCTCTTCAAC | CAGTTGAGCGTGATGTTCTC |
| ASFT | CTCTACTACCTCTCCAACCT | AGGGTAGTAGTGGACAAGGA |
| ABCG2 | AGGTGTTTCAGAGAGAGAGG | AGGACGAAGAAGAGGTAGTG |
| GDSL | CTTCTACTTCGTGGCAATCG | CGCTGAAGAGGAGGAAGTA |
| LACS4 | CATGCGGTGGTTCTATTCTG | CAACATATGGGCACACACTC |
| HOTHEAD | GTGGAATTGGACCCAGAAAG | CGTCGGTTATTCCAACAGTC |
| MYB107 | CTCATCGCCTACATCCAGAA | CTTGATGATGGTGTCCTCCT |
| 18S rRNA | CGCGCAAATTACCCAATCCTGACA | TCCCGAAGGCCAACGTAAATAGGA |
| [1] | Bais H P, Weir T L, Perry L G, Gilroy S, Vivanco J M. 2006. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Biol, 57: 233‒266. |
| [2] | Balhadere P, Evans A. 1995. Cytochemical investigation of resistance to root-knot nematode Meloidogyne naasi in cereals and grasses using cryosections of roots. Fundam Appl Nematol, 18: 539-547. |
| [3] | Brundrett M C, Kendrick B, Peterson C A. 1991. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotechnol Histochem, 66: 111‒116. |
| [4] | Cabasan M T N, Kumar A, Bellafiore S, De Waele D. 2014. Histopathology of the rice root-knot nematode, Meloidogyne graminicola, on Oryza sativa and O. glaberrima. Nematology, 16: 73‒81. |
| [5] | Cai X, Chen T, Zhou Q Y, Xu L, Qu L Q, Hua X J, Lin J X. 2011. Development of Casparian strip in rice cultivars. Plant Signal Behav, 6: 59‒65. |
| [6] | Curtis R H C. 2008. Plant-nematode interactions: Environmental signals detected by the nematode’s chemosensory organs control changes in the surface cuticle and behaviour. Parasite, 15: 310‒316. |
| [7] | Dash M, Dutta T K, Phani V, Papolu P K, Shivakumara T N, Rao U. 2017. RNAi-mediated disruption of neuropeptide genes, nlp-3 and nlp-12, cause multiple behavioral defects in Meloidogyne incognita. Biochem Biophys Res Commun, 490: 933‒940. |
| [8] | De Waele D, Elsen A. 2007. Challenges in tropical plant nematology. Annu Rev Phytopathol, 45: 457‒485. |
| [9] | De Waele D, Das K, Zhao D, Tiwari R K S, Shrivastava D K, Vera-Cruz C, Kumar A. 2013. Host response of rice genotypes to the rice root-knot nematode (Meloidogyne graminicola) under aerobic soil conditions. Arch Phytopathol Plant Prot, 46: 1‒12. |
| [10] | Dutta T K, Powers S J, Kerry B R, Gaur H S, Curtis R H C. 2011. Comparison of host recognition, invasion, development and reproduction of Meloidogyne graminicola and M. incognita on rice and tomato. Nematology, 13: 509‒520. |
| [11] | Dutta T K, Ganguly A K, Gaur H S. 2012a. Global status of rice root-knot nematode, Meloidogyne graminicola. Afr J Microbiol Res, 6: 6016‒6021. |
| [12] | Dutta T K, Powers S J, Gaur H S, Birkett M, Curtis R H. 2012b. Effect of small lipophilic molecules in tomato and rice root exudates on the behaviour of Meloidogyne incognita and M. graminicola. Nematology, 14: 309‒320. |
| [13] | Dutta T K, Lovegrove A, Gaur H S, Curtis R H. 2014. Differential immunoreactivity of the root-knot nematodes, Meloidogyne graminicola and Meloidogyne incognita to polyclonal and monoclonal antibodies and identification of antigens through proteomics approach. Afr J Microbiol Res, 8: 1245‒1254. |
| [14] | FAOSTAT. 2019. . |
| [15] | Hatzade B, Singh D, Phani V, Kumbhar S, Rao U. 2020. Profiling of defense responsive pathway regulatory genes in Asian rice ( Oryza sativa) against infection of Meloidogyne graminicola(Nematoda: Meloidogynidae). 3 Biotechnol, 10: 60. |
| [16] | Holbein J, Grundler F M W, Siddique S. 2016. Plant basal resistance to nematodes: An update. J Exp Bot, 67: 2049-2061. |
| [17] | Holbein J, Franke R B, Marhavý P, Fujita S, Górecka M, Sobczak M, Geldner N, Schreiber L, Grundler F M W, Siddique S. 2019. Root endodermal barrier system contributes to defence against plant-parasitic cyst and root-knot nematodes. Plant J, 100: 221‒236. |
| [18] | Huang W K, Ji H L, Gheysen G, Debode J, Kyndt T. 2015. Biochar-amended potting medium reduces the susceptibility of rice to root-knot nematode infections. BMC Plant Biol, 15: 267. |
| [19] | Johansen D A. 1940. Plant Microtechnique. London, UK: McGraw- Hill Book Company: 523. |
| [20] | Kreszies T, Schreiber L, Ranathunge K. 2018. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J Plant Physiol, 227: 75‒83. |
| [21] | Krishnamurthy P, Ranathunge K, Franke R, Prakash H S, Schreiber L, Mathew M K. 2009. The role of root apoplastic transport barriers in salt tolerance of rice ( Oryza sativa L.). Planta, 230: 119-134. |
| [22] | Kumari C, Dutta T K, Banakar P, Rao U. 2016. Comparing the defence related gene expression changes upon root-knot nematode attack in susceptible versus resistant cultivars of rice. Sci Rep, 6: 22846. |
| [23] | Kumari C, Dutta T K, Gahoi S, Rao U. 2017. An insight into the expression profile of defence-related genes in compatible and incompatible Oryza sativa-Meloidogyne graminicola interaction. Ind J Genet Plant Breed, 77: 42‒50. |
| [24] | Kumbhar S D, Kulwal P L, Patil J V, Sarawate C D, Gaikwad A P, Jadhav A S. 2015. Genetic diversity and population structure in landraces and improved rice varieties from India. Rice Sci, 22: 99‒107. |
| [25] | Kyndt T, Fernandez D, Gheysen G. 2014. Plant-parasitic nematode infection in rice: Molecular and cellular insights. Annu Rev Phytopathol, 52: 135‒153. |
| [26] | Lashbrooke J, Cohen H, Levy-Samocha D, Tzfadia O, Panizel I, Zeisler V, Massalha H, Stern A, Trainotti L, Schreiber L, Costa F, Aharoni A. 2016. MYB107 and MYB9 homologs regulate suberin deposition in angiosperms. Plant Cell, 28: 2097‒2116. |
| [27] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402‒408. |
| [28] | Man Y, Zhao Y Y, Ye R, Lin J X, Jing Y P. 2018. In vivo cytological and chemical analysis of Casparian strips using stimulated Raman scattering microscopy. J Plant Physiol, 220: 136‒144. |
| [29] | Mantelin S, Bellafiore S, Kyndt T. 2017. Meloidogyne graminicola: A major threat to rice agriculture. Mol Plant Pathol, 18: 3‒15. |
| [30] | Molina I, Li-Beisson Y, Beisson F, Ohlrogge J B, Pollard M. 2009. Identification of an Arabidopsis feruloyl-coenzyme A transferase required for suberin synthesis. Plant Physiol, 151: 1317-1328. |
| [31] | Petitot A S, Kyndt T, Haidar R, Dereeper A, Collin M, de Almeida Engler J, Gheysen G, Fernandez D. 2017. Transcriptomic and histological responses of African rice (Oryza glaberrima) to Meloidogyne graminicola provide new insights into root-knot nematode resistance in monocots. Ann Bot, 119: 885‒899. |
| [32] | Pollard M, Beisson F, Li Y, Ohlrogge J B. 2008. Building lipid barriers: Biosynthesis of cutin and suberin. Trends Plant Sci, 13: 236‒246. |
| [33] | Ranathunge K, Lin J, Steudle E, Schreiber L. 2011. Stagnant deoxygenated growth enhances root suberization and lignifications, but differentially affects water and NaCl permeabilities in rice ( Oryza sativa L.) roots. Plant Cell Environ, 34: 1223-1240. |
| [34] | Ranathunge K, Schreiber L, Bi Y M, Rothstein S J. 2016. Ammonium- induced architectural and anatomical changes with altered suberin and lignin levels significantly change water and solute permeabilities of rice ( Oryza sativa L.) roots. Planta, 243: 231-249. |
| [35] | Ride J P. 1978. The role of cell wall alternations in resistance to fungi. Ann Appl Biol, 89: 302‒306. |
| [36] | Robbins N E, Trontin C, Duan L, Dinneny J R. 2014. Beyond the barrier: Communication in the root through the endodermis. Plant Physiol, 166: 551‒559. |
| [37] | Ruzin S E. 1999. Plant Microtechnique and Microscopy. New York, USA: Oxford University Press: 322. |
| [38] | Schreiber L, Franke R, Hartmann K D, Ranathunge K, Steudle E. 2005. The chemical composition of suberin in apoplastic barriers affects radial hydraulic conductivity differently in the roots of rice (Oryza sativa L. cv. IR64) and corn (Zea mays L. cv. Helix). J Exp Bot, 56: 1427-1436. |
| [39] | Shivakumara T N, Dutta T K, Rao U. 2018. A novel in vitro chemotaxis bioassay to assess the response of Meloidogyne incognita towards various test compounds. J Nematol, 50: 487‒494. |
| [40] | Shivakumara T N, Dutta T K, Chaudhary S, von Reuss S H, Williamson V M, Rao U. 2019. Homologs of Caenorhabditis elegans chemosensory genes have roles in behavior and chemotaxis in the root-knot nematode Meloidogyne incognita. Mol Plant-Microbe Interact, 32: 876‒887. |
| [41] | Sijmons P, Grundler F, von Mende N, Burrows P, Wyss U. 1991. Arabidopsis thaliana as a new model host for plant-parasitic nematodes. Plant J, 1: 245‒254. |
| [42] | Southey J F. 1986. Laboratory Methods for Work with Plant and Soil Nematodes. London, UK: Ministry of Agriculture, Fisheries, and Food Reference Book: 402. |
| [43] | Valette C, Andary C, Geiger J P, Sarah J L, Nicole M. 1998. Histochemical and cytochemical investigations of phenols in roots of banana infected by the burrowing nematode Radopholus similis. Phytopathology, 88: 1141-1148. |
| [44] | Wyss U, Grundler F M W, Munch A. 1992. The parasitic behaviour of second-stage juveniles of Meloidogyne incognita in roots of Arabidopsis thaliana. Nematologica, 38: 98-111. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||