
Rice Science ›› 2023, Vol. 30 ›› Issue (3): 235-246.DOI: 10.1016/j.rsci.2023.03.007
• Research Paper • Previous Articles Next Articles
Zhu Jinling1, Wei Ruping1, Wang Xin2, Zheng Chaoqun1, Wang Mengmeng1, Yang Yicheng3, Yang Liuyan1( )
)
Received:2022-07-21
Accepted:2022-11-10
Online:2023-05-28
Published:2023-03-13
Contact:
Yang Liuyan (yangly@nju.edu.cn)
Zhu Jinling, Wei Ruping, Wang Xin, Zheng Chaoqun, Wang Mengmeng, Yang Yicheng, Yang Liuyan. Polyphosphate Accelerates Transformation of Nonstructural Carbohydrates to Improve Growth of ppk-Expressing Transgenic Rice in Phosphorus Deficiency Culture[J]. Rice Science, 2023, 30(3): 235-246.
Add to citation manager EndNote|Ris|BibTeX
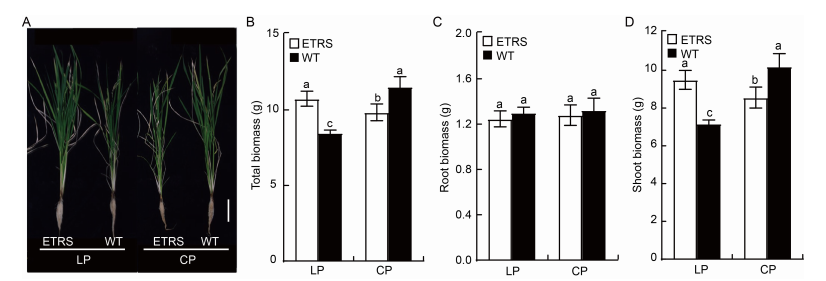
Fig. 1. Phenotypes and biomasses in ETRS and wild type (WT) in different phosphate concentration culture media. A, Morphologies of ETRS and WT in LP and CP culture media. Scale bar is 10 cm.B?D, Total (B), root (C) and shoot (D) biomasses of ETRS and WT. Data represent Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences between ETRS and WT (P < 0.05).ETRS, Polyphosphate kinase (ppk) gene-expressing transgenic rice with a single-copy line; LP, Low inorganic phosphate (Pi) culture medium (15 μmol/L); CP, Normal Pi culture medium (300 μmol/L).
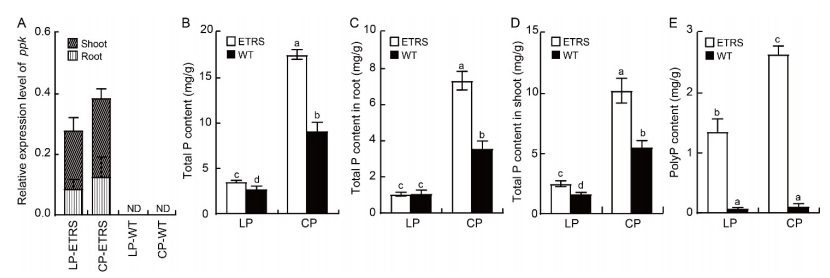
Fig. 2. Relative expression level of ppk gene, and total phosphorus (P) and polyphosphate (polyP) contents in ETRS in different phosphate concentration culture media. A, Relative expression level of polyphosphate kinase (ppk) gene in response to LP and CP. RNA was extracted from rice roots and shoots at the full heading stage. Actin was used as an internal reference. B, Total P contents of ETRS and WT. C, Total P contents in roots of ETRS and WT. D, Total P contents in shoots of ETRS and WT. E, PolyP contents of ETRS and WT. ETRS, ppk gene-expressing transgenic rice with a single-copy line; WT, Wild type; LP, Low inorganic phosphate (Pi) culture medium (15 μmol/L); CP, Normal Pi culture medium (300 μmol/L); ND, Not detected.Data represent Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences between ETRS and WT (P < 0.05).
| Treatment | Rice | Sucrose content | Total soluble sugar content | Starch content | NSC content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |||||
| LP | ETRS | 19.4 ± 2.2 a | 31.6 ± 4.3 a | 24.5 ± 1.1 b | 47.6 ± 4.0 a | 191 ± 18 a | 121 ± 18 a | 215.5 ± 19.1 a | 168.6 ± 22.0 a | |||
| WT | 22.0 ± 1.1 a | 28.6 ± 3.3 a | 29.3 ± 2.0 a | 45.3 ± 3.6 a | 179 ± 13 a | 99 ± 8 b | 208.3 ± 15.0 a | 144.3 ± 11.6 b | ||||
| CP | ETRS | 21.6 ± 2.6 a | 38.2 ± 5.3 a | 25.3 ± 2.9 a | 69.8 ± 3.0 a | 208 ± 38 a | 153 ± 8 a | 233.3 ± 40.9 a | 222.8 ± 11.0 a | |||
| WT | 19.3 ± 3.3 a | 35.8 ± 1.9 a | 28.9 ± 1.6 a | 61.9 ± 6.5 a | 213 ± 23 a | 149 ± 13 a | 241.9 ± 24.6 a | 210.9 ± 19.5 a | ||||
Table 1. Contents of carbohydrate componants in ETRS and WT in different phosphate concentration culture media. mg/g
| Treatment | Rice | Sucrose content | Total soluble sugar content | Starch content | NSC content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Root | Shoot | Root | Shoot | Root | Shoot | Root | Shoot | |||||
| LP | ETRS | 19.4 ± 2.2 a | 31.6 ± 4.3 a | 24.5 ± 1.1 b | 47.6 ± 4.0 a | 191 ± 18 a | 121 ± 18 a | 215.5 ± 19.1 a | 168.6 ± 22.0 a | |||
| WT | 22.0 ± 1.1 a | 28.6 ± 3.3 a | 29.3 ± 2.0 a | 45.3 ± 3.6 a | 179 ± 13 a | 99 ± 8 b | 208.3 ± 15.0 a | 144.3 ± 11.6 b | ||||
| CP | ETRS | 21.6 ± 2.6 a | 38.2 ± 5.3 a | 25.3 ± 2.9 a | 69.8 ± 3.0 a | 208 ± 38 a | 153 ± 8 a | 233.3 ± 40.9 a | 222.8 ± 11.0 a | |||
| WT | 19.3 ± 3.3 a | 35.8 ± 1.9 a | 28.9 ± 1.6 a | 61.9 ± 6.5 a | 213 ± 23 a | 149 ± 13 a | 241.9 ± 24.6 a | 210.9 ± 19.5 a | ||||
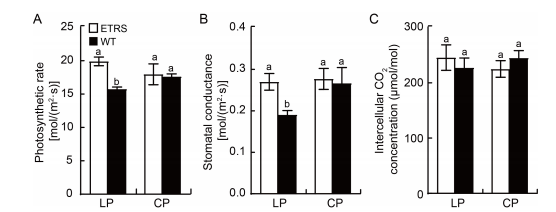
Fig. 3. Photosynthesis related attributes in shoot of ETRS and WT in different phosphate concentration culture media. A, Photosynthetic rate of ETRS and WT shoots in different phosphate concentration culture media. B, Stomatal conductance of ETRS and WT shoots in different phosphate concentration culture media.. C, Intercellular CO2 concentration of ETRS and WT shoots in different phosphate concentration culture media.. ETRS, Polyphosphate kinase (ppk) gene-expressing transgenic rice with a single-copy line; WT, Wild type; LP, Low inorganic phosphate (Pi) culture medium (15 μmol/L); CP, Normal Pi culture medium (300 μmol/L). Data represent Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences between ETRS and WT (P < 0.05).
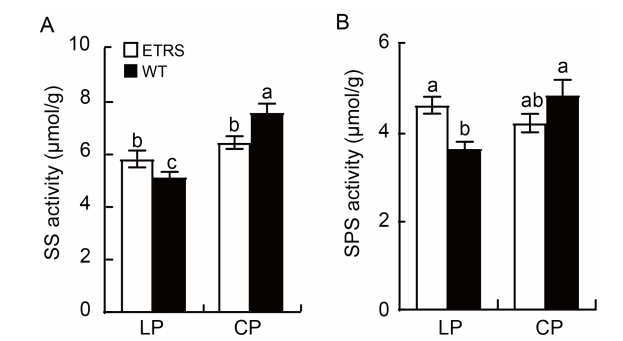
Fig. 4. Sucrose synthase (SS, A) and sucrose phosphate synthase (SPS, B) activities in shoots of ETRS and WT in different phosphate concentration culture media. ETRS, Polyphosphate kinase (ppk) gene-expressing transgenic rice with a single-copy line; WT, Wild type; LP, Low inorganic phosphate (Pi) culture medium (15 μmol/L); CP, Normal Pi culture medium (300 μmol/L). Data represent Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences between ETRS and WT (P < 0.05).
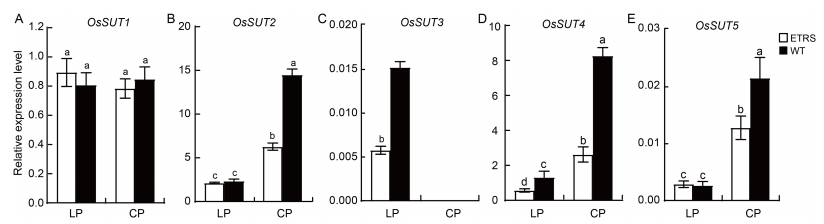
Fig. 5. Relative expression levels of OsSUT genes in shoots of ETRS and WT in different phosphate concentration culture media. A-E, Relative expression levels of OsSUT1 (A), OsSUT2 (B), OsSUT3 (C), OsSUT4 (D) and OsSUT5 (E). RNA was extracted from rice shoots at the full heading stage. Actin was used as an internal reference. ETRS, Polyphosphate kinase gene (ppk)-expressing transgenic rice with a single-copy line; WT, Wild type; LP, Low inorganic phosphate (Pi) culture medium (15 μmol/L); CP, Normal Pi culture medium (300 μmol/L). Data represent Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences between treatments (P < 0.05).
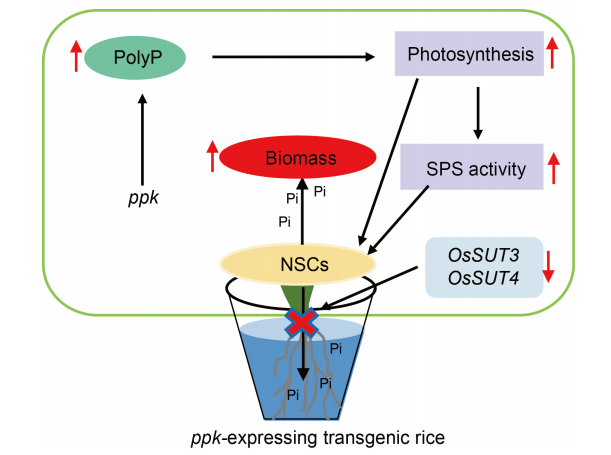
Fig. 6. Mechanism of polyP promoting NSC synthesis and reducing their transport from shoot to root in ETRS in low phosphate concentration culture medium. NSC, Nonstructural carbohydrate; Pi, Inorganic phosphate; SPS, Phosphate synthase.
| [1] |
Achbergerová L, Nahálka J. 2011. Polyphosphate: An ancient energy source and active metabolic regulator. Microb Cell Fact, 10: 63.
PMID |
| [2] | Ahn D J, Won J G, Rico C M, Lee S C. 2010. Influence of variety, location, growing year, and storage on the total phosphorus, phytate-phosphorus, and phytate-phosphorus to total phosphorus ratio in rice. J Agric Food Chem, 58(5): 3008-3011. |
| [3] | Anur R M, Mufithah N, Sawitri W D, Sakakibara H, Sugiharto B. 2020. Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants, 9(2): 200. |
| [4] |
Aoki N, Hirose T, Scofield G N, Whitfeld P R, Furbank R T. 2003. The sucrose transporter gene family in rice. Plant Cell Physiol, 44(3): 223-232.
PMID |
| [5] |
Ayre B G. 2011. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant, 4(3): 377-394.
PMID |
| [6] |
Bihmidine S, Hunter C T, Johns C E, Koch K E, Braun D M. 2013. Regulation of assimilate import into sink organs: Update on molecular drivers of sink strength. Front Plant Sci, 4: 177.
PMID |
| [7] |
Braun D M, Wang L, Ruan Y L. 2014. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J Exp Bot, 65(7): 1713-1735.
PMID |
| [8] | Cakmak I, Hengeler C, Marschner H. 1994. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J Exp Bot, 45(9): 1245-1250. |
| [9] | Chen J Y, Liu S L, Wei S A, Wang S J. 2010. Hormone and sugar effects on rice sucrose transporter OsSUT1 expression in germinating embryos. Acta Physiol Plant, 32(4): 749-756. |
| [10] | Chtouki M, Naciri R, Garré S, Nguyen F, Oukarroum A. 2022. Chickpea plant responses to polyphosphate fertiliser forms and drip fertigation frequencies: Effect on photosynthetic performance and phenotypic traits. Funct Plant Biol, 49(6): 505-516. |
| [11] | Chung P, Hsiao H H, Chen H J, Chang C W, Wang S J. 2014. Influence of temperature on the expression of the rice sucrose transporter 4 gene, OsSUT4, in germinating embryos and maturing pollen. Acta Physiol Plant, 36(1): 217-229. |
| [12] | Ciereszko I, Barbachowska A. 2000. Sucrose metabolism in leaves and roots of bean (Phaseolus vulgaris L.) during phosphate deficiency. J Plant Physiol, 156(5/6): 640-644. |
| [13] |
Elling L. 1995. Effect of metal ions on sucrose synthase from rice grains: A study on enzyme inhibition and enzyme topography. Glycobiology, 5(2): 201-206.
PMID |
| [14] | Eom J S, Cho J I, Reinders A, Lee S W, Yoo Y, Tuan P Q, Choi S B, Bang G, Park Y I, Cho M H, Bhoo S H, An G, Hahn T R, Ward J M, Jeon J S. 2011. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol, 157(1): 109-119. |
| [15] |
Farrar J, Pollock C, Gallagher J. 2000. Sucrose and the integration of metabolism in vascular plants. Plant Sci, 154(1): 1-11.
PMID |
| [16] |
Gardemann A, Schimkat D, Heldt H W. 1986. Control of CO2 fixation regulation of stromal fructose-1,6-bisphosphatase in spinach by pH and Mg2+ concentration. Planta, 168(4): 536-545.
PMID |
| [17] | Gebbing T, Schnyder H, Kühbauch W. 1999. The utilization of pre-anthesis reserves in grain filling of wheat: Assessment by steady-state 13CO2/12CO2 labelling. Plant Cell Environ, 22(7): 851-858. |
| [18] |
Goodenough U, Heiss A A, Roth R, Rusch J, Lee J H. 2019. Acidocalcisomes: Ultrastructure, biogenesis, and distribution in microbial eukaryotes. Protist, 170(3): 287-313.
PMID |
| [19] | Hirose T, Zhang Z J, Miyao A, Hirochika H, Ohsugi R, Terao T. 2010. Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. J Exp Bot, 61(13): 3639-3646. |
| [20] | Holford I C R. 1997. Soil phosphorus: Its measurement, and its uptake by plants. Soil Res, 35(2): 227. |
| [21] | Huang L Y, Sun F, Yuan S, Peng S B, Wang F. 2018. Different mechanisms underlying the yield advantage of ordinary hybrid and super hybrid rice over inbred rice under low and moderate N input conditions. Field Crops Res, 216: 150-157. |
| [22] | Ishimura K, Hirose T, Aoki N, Takahashi S, Ono K, Yamamoto S, Wu J, Saji S, Baba T, Ugaki M, Matsumoto T, Ohsugi R. 2001. Antisense expression of a rice sucrose transporter OsSUT1 in rice (Oryza sativa L.). Plant Cell Physiol, 42(10): 1181-1185. |
| [23] | Jia H F, Ren H Y, Gu M, Zhao J N, Sun S B, Zhang X, Chen J Y, Wu P, Xu G H. 2011. The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol, 156(3): 1164-1175. |
| [24] |
Julius B T, Leach K A, Tran T M, Mertz R A, Braun D M. 2017. Sugar transporters in plants: New insights and discoveries. Plant Cell Physiol, 58(9): 1442-1460.
PMID |
| [25] |
Komine Y, Eggink L L, Park H, Hoober J K. 2000. Vacuolar granules in Chlamydomonas reinhardtii: Polyphosphate and a 70-kDa polypeptide as major components. Planta, 210(6): 897-905.
PMID |
| [26] | Lal A, Ku M S, Edwards G E. 1996. Analysis of inhibition of photosynthesis due to water stress in the C3 species Hordeum vulgare and Vicia faba: Electron transport, CO2 fixation and carboxylation capacity. Photosynth Res, 49(1): 57-69. |
| [27] |
Li G H, Zhou C Y, Yang Z J, Zhang C H, Dai Q G, Huo Z Y, Xu K. 2022. Low nitrogen enhances apoplastic phloem loading and improves the translocation of photoassimilates in rice leaves and stems. Plant Cell Physiol, 63(7): 991-1007.
PMID |
| [28] | Li H, Huang G, Meng Q, Ma L, Yuan L, Wang F, Zhang W, Cui Z, Shen J, Chen X, Jiang R, Zhang F. 2011. Integrated soil and plant phosphorus management for crop and environment in China: A review. Plant Soil, 349(1/2): 157-167. |
| [29] | Liesche J. 2017. Sucrose transporters and plasmodesmal regulation in passive phloem loading. J Integr Plant Biol, 59(5): 311-321. |
| [30] | Lim J D, Cho J I, Park Y I, Hahn T R, Choi S B, Jeon J S. 2006. Sucrose transport from source to sink seeds in rice. Physiol Plant, 126(4): 572-584. |
| [31] | Liu W F, Yang H, Ciais P, Stamm C, Zhao X, Williams J R, Abbaspour K C, Schulin R. 2018. Integrative crop-soil-management modeling to assess global phosphorus losses from major crop cultivations. Global Biogeochem Cycles, 32(7): 1074-1086. |
| [32] |
Long S P, Bernacchi C J. 2003. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot, 54: 2393-2401.
PMID |
| [33] | Mandala V S, Loh D M, Shepard S M, Geeson M B, Sergeyev I V, Nocera D G, Cummins C C, Hong M. 2020. Bacterial phosphate granules contain cyclic polyphosphates: Evidence from 31P solid-state NMR. J Am Chem Soc, 142(43): 18407-18421. |
| [34] |
Matsukura C A, Saitoh T, Hirose T, Ohsugi R, Perata P, Yamaguchi J. 2000. Sugar uptake and transport in rice embryo: Expression of companion cell-specific sucrose transporter (OsSUT1) induced by sugar and light. Plant Physiol, 124(1): 85-94.
PMID |
| [35] |
Michael C D, Anna S, Mariah S C, Claudia I C, Joshua A M, Andrew D R, Rodrigo V. 2014. Nonstructural carbon in woody plants. Annu Rev Plant Biol, 65: 667-687.
PMID |
| [36] | Nanamori M, Shinano T, Wasaki J, Yamamura T, Rao I M, Osaki M. 2004. Low phosphorus tolerance mechanisms: Phosphorus recycling and photosynthate partitioning in the tropical forage grass, Brachiaria hybrid cultivar Mulato compared with rice. Plant Cell Physiol, 45(4): 460-469. |
| [37] | Pandey M, Srivastava A K, D’Souza S F, Penna S. 2013. Thiourea, a ROS scavenger, regulates source-to-sink relationship to enhance crop yield and oil content in Brassica juncea (L.). PLoS One, 8(9): e73921. |
| [38] | Pokhrel A, Lingo J C, Wolschendorf F, Gray M J. 2019. Assaying for inorganic polyphosphate in bacteria. J Vis Exp, e58818. |
| [39] | Quick W P, Chaves M M, Wendler R, David M, Rodrigues M L, Passaharinho J A, Pereira J S, Adcock M D, Leegood R C, Stitt M. 1992. The effect of water stress on photosynthetic carbon metabolism in four species grown under field conditions. Plant Cell Environ, 15(1): 25-35. |
| [40] |
Rao N N, Gómez-García M R, Kornberg A. 2009. Inorganic polyphosphate: Essential for growth and survival. Annu Rev Biochem, 78: 605-647.
PMID |
| [41] | Rose T J, Pariasca-Tanaka J, Rose M T, Fukuta Y, Wissuwa M. 2010. Genotypic variation in grain phosphorus concentration, and opportunities to improve P-use efficiency in rice. Field Crops Res, 119(1): 154-160. |
| [42] |
Sasaki T, Burr B. 2000. International rice genome sequencing project: The effort to completely sequence the rice genome. Curr Opin Plant Biol, 3(2): 138-141.
PMID |
| [43] |
Sattari S Z, Bouwman A F, Giller K E,van Ittersum M K. 2012. Residual soil phosphorus as the missing piece in the global phosphorus crisis puzzle. Proc Natl Acad Sci USA, 109(16): 6348-6353.
PMID |
| [44] |
Scofield G N, Hirose T, Gaudron J A, Furbank R T, Upadhyaya N M, Ohsugi R. 2002. Antisense suppression of the rice transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct Plant Biol, 29(7): 815.
PMID |
| [45] |
Scofield G N, Aoki N, Hirose T, Takano M, Jenkins C L D, Furbank R T. 2007. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. J Exp Bot, 58(3): 483-495.
PMID |
| [46] | Seufferheld M J, Alvarez H M, Farias M E. 2008. Role of polyphosphates in microbial adaptation to extreme environments. Appl Environ Microbiol, 74(19): 5867-5874. |
| [47] |
Stein O, Granot D. 2019. An overview of sucrose synthases in plants. Front Plant Sci, 10: 95.
PMID |
| [48] | Sun M, Li P C, Wang N, Zheng C S, Sun X Z, Dong H L, Han H M, Feng W N, Shao J J, Zhang Y F. 2022. Soil available phosphorus deficiency reduces boll biomass and lint yield by affecting sucrose metabolism in cotton-boll subtending leaves. Agronomy, 12(5): 1065. |
| [49] |
Sun Y, Lin Z, Reinders A, Ward J M. 2012. Functionally important amino acids in rice sucrose transporter OsSUT1. Biochemistry, 51(15): 3284-3291.
PMID |
| [50] | van Voorthuysen T, Regierer B, Springer F, Dijkema C, Vreugdenhil D, Kossmann J. 2000. Introduction of polyphosphate as a novel phosphate pool in the chloroplast of transgenic potato plants modifies carbohydrate partitioning. J Biotechnol, 77(1): 65-80. |
| [51] |
Wang X, Wang X M, Hui K M, Wei W, Zhang W, Miao A J, Xiao L, Yang L Y. 2018. Highly effective polyphosphate synthesis, phosphate removal, and concentration using engineered environmental bacteria based on a simple solo medium-copy plasmid strategy. Environ Sci Technol, 52(1): 214-222.
PMID |
| [52] | Wang X F, Wang Y F, Piñeros M A, Wang Z Y, Wang W X, Li C Y, Wu Z C, Kochian L V, Wu P. 2014. Phosphate transporters OsPHT1;9 and OsPHT1;10 are involved in phosphate uptake in rice. Plant Cell Environ, 37(5): 1159-1170. |
| [53] | Wei R P, Wang X, Zhang W, Shen J N, Zhang H F, Gao Y, Yang L Y. 2020. The improved phosphorus utilization and reduced phosphorus consumption of ppk-expressing transgenic rice. Field Crops Res, 248: 107715. |
| [54] | Winter H, Huber S C. 2000. Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit Rev Biochem Mol Biol, 35(4): 253-289. |
| [55] | Xin L. 2015. Effects of potassium application on carbohydrate formation, accumulation and yield of japonica rice in cold region. Harbin, Heilongjiang, China: Northeast Agricultural University. (in Chinese with English abstract) |
| [56] |
Xu Q Y, Chen S Y, Ren Y J, Chen S L, Liesche J. 2018. Regulation of sucrose transporters and phloem loading in response to environmental cues. Plant Physiol, 176(1): 930-945.
PMID |
| [57] |
Yadav U P, Ayre B G, Bush D R. 2015. Transgenic approaches to altering carbon and nitrogen partitioning in whole plants: Assessing the potential to improve crop yields and nutritional quality. Front Plant Sci, 6: 275.
PMID |
| [58] | Yaseen M, Malhi S S. 2009. Variation in yield, phosphorus uptake, and physiological efficiency of wheat genotypes at adequate and stress phosphorus levels in soil. Commun Soil Sci Plant Anal, 40(19/20): 3104-3120. |
| [59] | Yoshida S, Forno D A, Cock J. 1971. Laboratory Manual for Physiological Studies of Rice. Manila, the Philippines: International Rice Research Institute. |
| [60] | Zhang F, Wu X N, Zhou H M, Wang D F, Jiang T T, Sun Y F, Cao Y, Pei W X, Sun S B, Xu G H. 2014. Overexpression of rice phosphate transporter gene OsPT6 enhances phosphate uptake and accumulation in transgenic rice plants. Plant Soil, 384(1/2): 259-270. |
| [61] | Zhang J S, Li D F, Xu X, Ziska L H, Zhu J G, Liu G, Zhu C W. 2020. The potential role of sucrose transport gene expression in the photosynthetic and yield response of rice cultivars to future CO2 concentration. Physiol Plant, 168(1): 218-226. |
| [62] |
Zhang X Q, Li K C, Xing R E, Liu S, Li P C. 2017. Metabolite profiling of wheat seedlings induced by chitosan: Revelation of the enhanced carbon and nitrogen metabolism. Front Plant Sci, 8: 2017.
PMID |
| [63] |
Zhou J, Jiao F C, Wu Z C, Li Y Y, Wang X M, He X W, Zhong W Q, Wu P. 2008. OsPHR2 is involved in phosphate-starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol, 146(4): 1673-1686.
PMID |
| [64] |
Zhu Y J, Komor E, Moore P H. 1997. Sucrose accumulation in the sugarcane stem is regulated by the difference between the activities of soluble acid invertase and sucrose phosphate synthase. Plant Physiol, 115(2): 609-616.
PMID |
| [1] | Mondal Satyen, Jamil Hasan M., Ahmed Tofayel, Giashuddin Miah M., C. Sta Cruz Pompe, M. Ismail Abdel. Effects of AG1 and AG2 QTLs on Nonstructural Carbohydrate and Seed Management Options for Rice Seedling Growth and Establishment under Flooding Stress [J]. Rice Science, 2020, 27(6): 515-528. |
| [2] | Kumari Manisha, Asthir Bavita. Transformation of Sucrose to Starch and Protein in Rice Leaves and Grains under Two Establishment Methods [J]. Rice Science, 2016, 23(5): 255-265. |
| [3] | DONG Chen-fei, CAI Qing-sheng, WANG Cai-lin, Jiro HARADA, Keisuke NEMOTO, SHEN Yi-xin. QTL Analysis for Traits Associated with Feeding Value of Straw in Rice (Oryza sativa L.) [J]. RICE SCIENCE, 2008, 15(3): 195-200 . |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||