
Rice Science ›› 2024, Vol. 31 ›› Issue (1): 103-117.DOI: 10.1016/j.rsci.2023.10.001
• Research Papers • Previous Articles Next Articles
Liu Dan1,2,#, Zhao Huibo1,3,#, Wang Zi’an1,2, Xu Jing1, Liu Yiting1,2, Wang Jiajia1, Chen Minmin1, Liu Xiong1, Zhang Zhihai1, Cen Jiangsu1, Zhu Li1,3, Hu Jiang1, Ren Deyong1, Gao Zhenyu1, Dong Guojun1, Zhang Qiang1, Shen Lan1, Li Qing1, Qian Qian1,3( ), Hu Songping2(
), Hu Songping2( ), Zhang Guangheng1,3(
), Zhang Guangheng1,3( )
)
Received:2023-08-07
Accepted:2023-10-31
Online:2024-01-28
Published:2024-02-06
Contact:
Zhang Guangheng (About author:First author contact:#These authors contributed equally to this work
Liu Dan, Zhao Huibo, Wang Zi’an, Xu Jing, Liu Yiting, Wang Jiajia, Chen Minmin, Liu Xiong, Zhang Zhihai, Cen Jiangsu, Zhu Li, Hu Jiang, Ren Deyong, Gao Zhenyu, Dong Guojun, Zhang Qiang, Shen Lan, Li Qing, Qian Qian, Hu Songping, Zhang Guangheng. Leaf Morphology Genes SRL1 and RENL1 Co-Regulate Cellulose Synthesis and Affect Rice Drought Tolerance[J]. Rice Science, 2024, 31(1): 103-117.
Add to citation manager EndNote|Ris|BibTeX
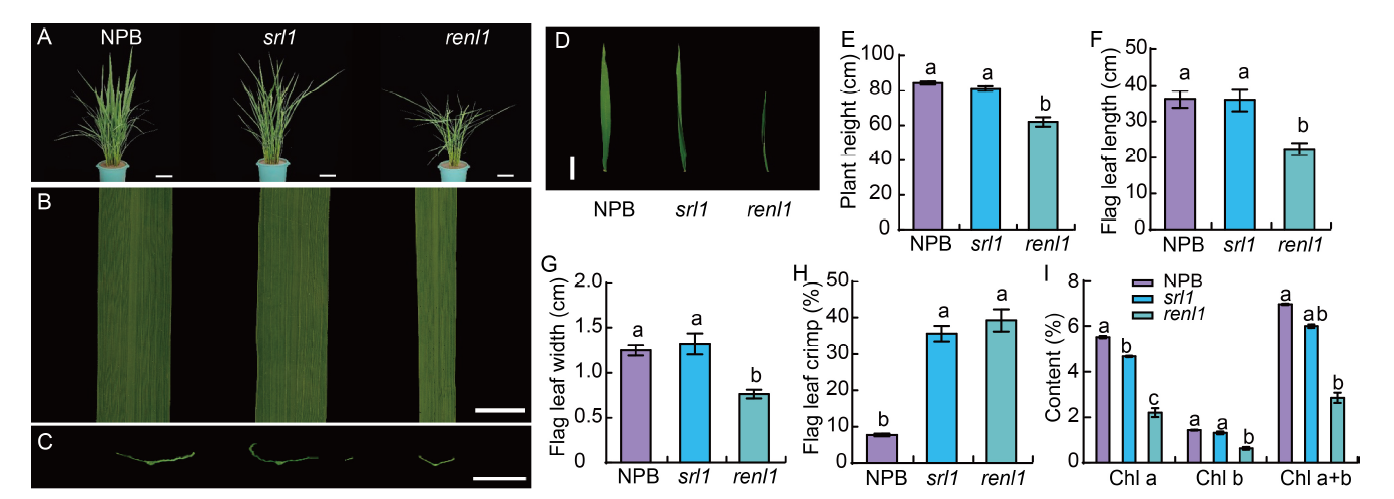
Fig. 1. Phenotypic characterization of Nipponbare (NPB) and mutants (srl1 and renl1). A-D, Phenotypes of tillering plant (A, scale bars are 10 cm), local flag leaf (B, scale bar is 1 cm), blade cross section (C, scale bar is 1 cm), and flag leaf morphology (D, scale bar is 10 cm). E-I, Comparisons of plant height (E), flag leaf length (F), flag leaf width (G), flag leaf crimp (H), and chlorophyll content (I) between wild type (NPB) and mutants (srl1 and renl1). Data are Mean ± SD (n = 3), and the lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.

Fig. 2. Leaf cytological morphology analysis. A and B, Frozen sections of main veins (A, scale bars are 50 μm), and large and small veins of Nipponbare (NPB), srl1, and renl1 (B, scale bars are 100 μm). BC, Bulliform form. C-E, Numbers of large (C) and small (D) vascular bundles, and bulliform cells (E). Data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.
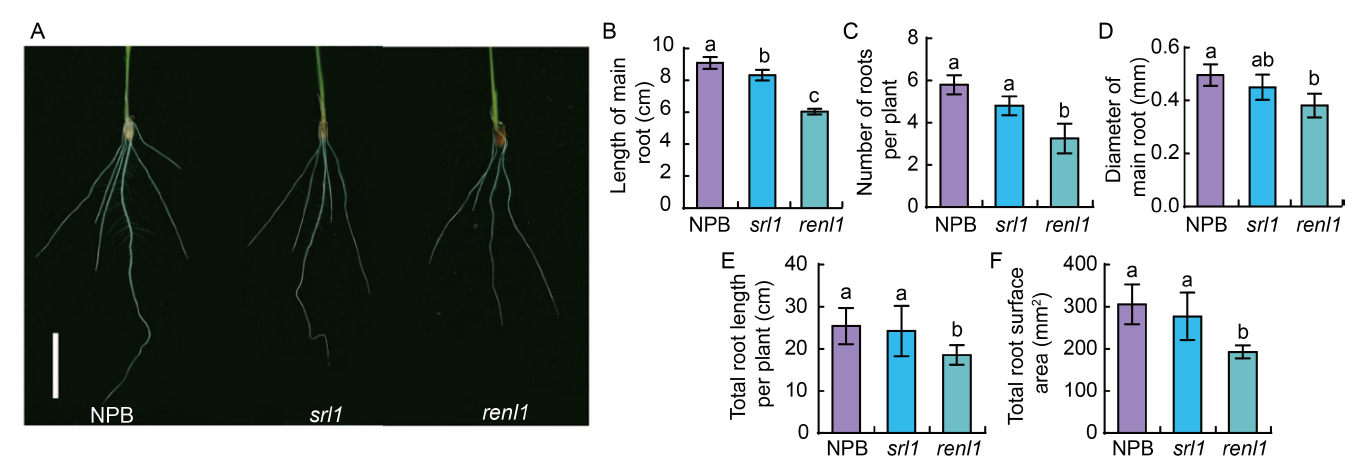
Fig. 3. RENL1 affects rice root development. A, Phenotypes of seedling root. Scale bar is 2 cm. B-F, Length of main root (B), the number of roots per plant (C), diameter of main root (D), total root length per plant (E), and total root surface area per plant (F) in Nipponbare (NPB) and mutants (srl1 and renl1). Data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.
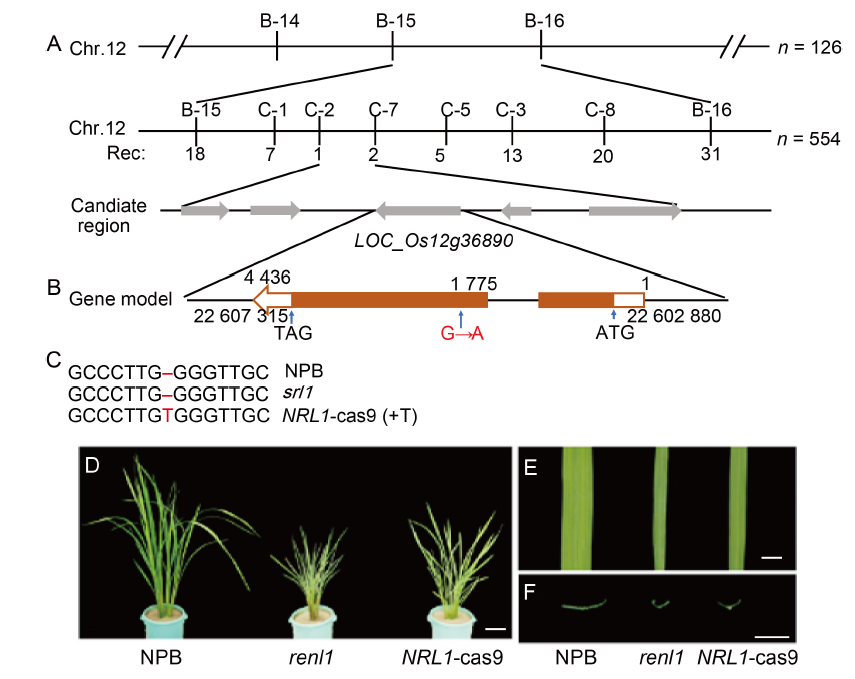
Fig. 4. Map-based cloning of RENL1. A, RENL1 gene was mapped to an interval between molecular markers B-15 and B-16 on chromosome (Chr.) 12 and further delimited to a 12.7 kb genomic region between markers C-2 and C-7. The numbers below the markers indicate the number of recombinants (Rec). B, Structure of RENL1 gene, a G→A base substitution occurred at position 1 775 of the open reading fragment. C, Gene knockout site detection. ‘T’ in red represents the site knocked by CRISPR/Cas9. D, Phenotypes of Nipponbare (NPB), renl1, and NRL1-cas9 at the tillering stage. Scale bar is 10 cm. E, Flag leaf type of NPB, renl1, and NRL1-cas9. Scale bar is 1 cm. F, Cross section of flag leaves of NPB, renl1, and NRL1-cas9. Scale bar is 1 cm.

Fig. 5. Morphology, physiology, and scanning electron microscope observation of rice leaves in wild type (Nipponbare, NPB) and mutants (srl1, nrl1, and renl1). A and B, Flag leaf fluorescence section under blue fluorescence with a light-emitting diode (LED) wavelength of 470 nm (A) and under green fluorescence with an LED wavelength of 530 nm (B). Scale bars are 100 μm. C-E, Scanning electron microscope observation of abaxial epidermis. Cork-silica cell pairs are represented by red boxes (C), papillae and nodular papillae are indicated with arrows (D), and stomata on the abaxial epidermis (E). Bars in C, D, and E are 20 μm, 10 μm, and 2 μm, respectively. F-M, Blade stomatal width (F), blade stomatal length (G), leaf epidermal stomatal density (H), net photosynthetic rate (I), stomatal conductance (J), transpiration rate (K), intercellular CO2 concentration (L), and proportion of mesophyll cells to leaf cells (M) in wild type and mutants. Data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.
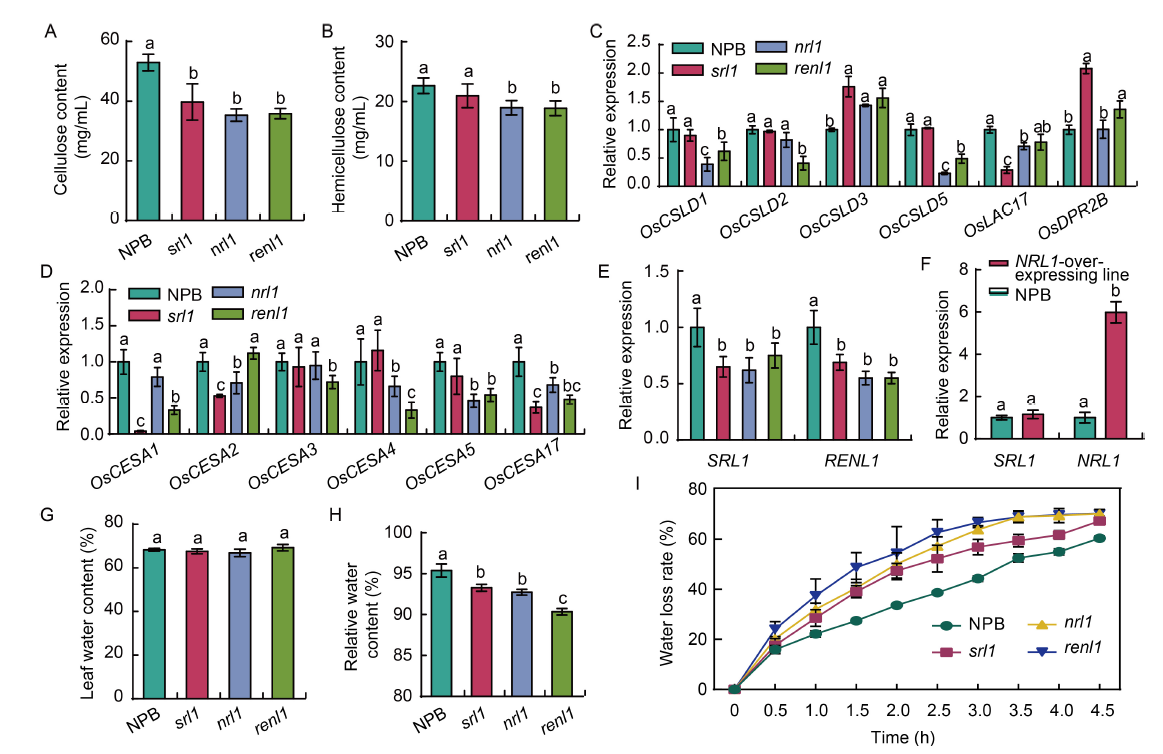
Fig. 6. Measurements of physiological indices and relative expression levels of genes in rice leaves. A and B, Cellulose (A) and hemicellulose (B) contents of rice leaves in wild type (Nipponbare, NPB) and mutants (srl1, nrl1, and renl1). C and D, qRT-PCR analysis of cell wall cellulose-related synthetic genes (C for OsCSLD family and D for OsCESA family). E and F, Analysis of the expression levels of NRL1 and SRL1 in mature leaves (E) and in NPB and NRL1-overexpressing lines (F). G-I, Leaf water content (G), relative water content (H), and water loss rate in leaf (I) in NPB, srl1, nrl1, and renl1. Data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.
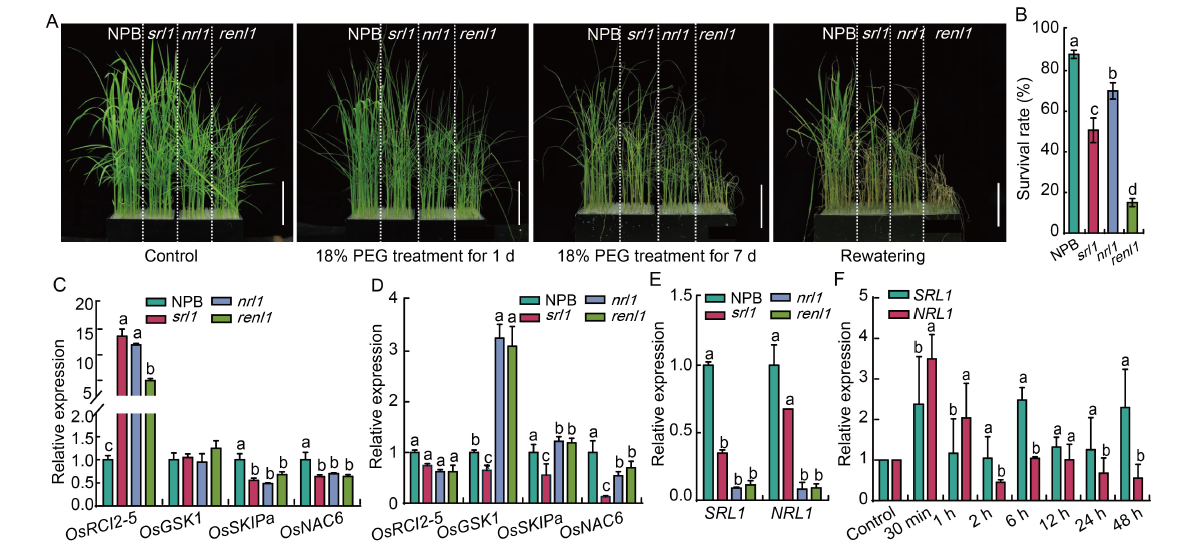
Fig. 7. RENL1 and SRL1 affect drought tolerance of rice. A, Drought tolerance of wild type (Nipponbare, NPB) and mutants (srl1, nrl1, and renl1) under 18% polyethylene glycol (PEG) stress treatment. Scale bars are 10 cm. B, Survival rates of NPB, srl1, nrl1, and renl1 after rewatering. C and D, Relative expression levels of drought tolerant genes in NPB, srl1, nrl1, and renl1 before (C) and after (D) 18% PEG stress for uninterrupted 48 h. E, Relative expression of SRL1 and NRL1 genes in NPB, srl1, nrl1, and renl1 at the seedling stage. F, Relative expression of drought-induced genes after 18% PEG treatment for different times. In B to F, data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.

Fig. 8. Drought stress on antioxidant enzyme activities of rice leaves in wild type (Nipponbare, NPB) and mutants (srl1, nrl1, and renl1). A-E, Changes of H2O2 content (A), malonaldehyde (MDA) content (B), superoxide (SOD) activity (C), peroxidase (POD) activity (D), and catalase (CAT) activity (E) in NPB, srl1, nrl1, and renl1 under drought stress. Data are Mean ± SD (n = 3). The lowercase letters above the bars represent the significant differences at the 0.05 level by one-way analysis of variance.
| [1] | Barrs H D, Weatherley P E. 1962. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust J Biol Sci, 15(3): 413-428. |
| [2] | Bernal A J, Jensen J K, Harholt J, Sørensen S, Moller I, Blaukopf C, Johansen B, de Lotto R, Pauly M, Scheller H V, Willats W G T. 2007. Disruption of ATCSLD5results in reduced growth, reduced xylan and homogalacturonan synthase activity and altered xylan occurrence in Arabidopsis. Plant J, 52(5): 791-802. |
| [3] | Bernal A J, Yoo C M, Mutwil M, Jensen J K, Hou G C, Blaukopf C, Sørensen I, Blancaflor E B, Scheller H V, Willats W G T. 2008. Functional analysis of the cellulose synthase-like genes CSLD1, CSLD2, and CSLD4 in tip-growing Arabidopsis cells. Plant Physiol, 148(3): 1238-1253. |
| [4] | Bertolino L T, Caine R S, Gray J E. 2019. Impact of stomatal density and morphology on water-use efficiency in a changing world. Front Plant Sci, 10: 225. |
| [5] | Braybrook S A, Kuhlemeier C. 2010. How a plant builds leaves. Plant Cell, 22(4): 1006-1018. |
| [6] | Carpita N C, Gibeaut D M. 1993. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J, 3(1): 1-30. |
| [7] | Chen Z Z, Hong X H, Zhang H R, Wang Y Q, Li X, Zhu J K, Gong Z Z. 2005. Disruption of the cellulose synthase gene, AtCesA8/IRX1, enhances drought and osmotic stress tolerance in Arabidopsis. Plant J, 43(2): 273-283. |
| [8] | Deng Q W, Liu Z X, Zhang Q, Gao Y, Jiang Y J, Zheng Y X, Hu W. 2020. Fluorescence microscopic observation and free-hand section techniques for rice leaves. J Zhongkai Univ Agric Eng, 33(1): 24-27. (in Chinese with English abstract) |
| [9] | Dey A, Samanta M K, Gayen S, Maiti M K. 2016. The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression. BMC Plant Biol, 16: 158. |
| [10] | El-Kereamy A, Bi Y M, Ranathunge K, Beatty P H, Good A G, Rothstein S J. 2012. The rice R2R3-MYB transcription factor OsMYB55 is involved in the tolerance to high temperature and modulates amino acid metabolism. PLoS One, 7(12): e52030. |
| [11] | Fang L K, Zhao F M, Cong Y F, Sang X C, Du Q, Wang D Z, Li Y F, Ling Y H, Yang Z L, He G H. 2012. Rolling-leaf14 is a 2OG-Fe (II) oxygenase family protein that modulates rice leaf rolling by affecting secondary cell wall formation in leaves. Plant Biotechnol J, 10(5): 524-532. |
| [12] | Finkel T, Holbrook N J. 2000. Oxidants, oxidative stress and the biology of ageing. Nature, 408: 239-247. |
| [13] | Ghosh D, Xu J. 2014. Abiotic stress responses in plant roots: A proteomics perspective. Front Plant Sci, 5: 6. |
| [14] | Hu J, Zhu L, Zeng D L, Gao Z Y, Guo L B, Fang Y X, Zhang G H, Dong G J, Yan M X, Liu J, Qian Q. 2010. Identification and characterization of NARROW ANDROLLED LEAF 1, a novel gene regulating leaf morphology and plant architecture in rice. Plant Mol Biol, 73(3): 283-292. |
| [15] | Huang L C, Chen L, Wang L, Yang Y L, Rao Y C, Ren D Y, Dai L P, Gao Y H, Zou W W, Lu X L, Zhang G H, Zhu L, Hu J, Chen G, Shen L, Dong G J, Gao Z Y, Guo L B, Qian Q, Zeng D L. 2019. A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice. Plant J, 98(5): 884-897. |
| [16] | Jenks M A, Andersen L, Teusink R S, Williams M H. 2001. Leaf cuticular waxes of potted rose cultivars as affected by plant development, drought and paclobutrazol treatments. Physiol Plant, 112(1): 62-70. |
| [17] | Kadioglu A, Terzi R. 2007. A dehydration avoidance mechanism: Leaf rolling. Bot Rev, 73(4): 290-302. |
| [18] | Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko K R, Marsch- Martinez N, Krishnan A, Nataraja K N, Udayakumar M, Pereira A. 2007. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci USA, 104(39): 15270-15275. |
| [19] | Kim C M, Park S H, Je B I, Park S H, Park S J, Piao H L, Eun M Y, Dolan L, Han C D. 2007. OsCSLD1, a cellulose synthase-like D1 gene, is required for root hair morphogenesis in rice. Plant Physiol, 143(3): 1220-1230. |
| [20] | Lerouxel O, Cavalier D M, Liepman A H, Keegstra K. 2006. Biosynthesis of plant cell wall polysaccharides: A complex process. Curr Opin Plant Biol, 9(6): 621-630. |
| [21] | Li M, Xiong G Y, Li R, Cui J J, Tang D, Zhang B C, Pauly M, Cheng Z K, Zhou Y H. 2009. Rice cellulose synthase-like D4 is essential for normal cell-wall biosynthesis and plant growth. Plant J, 60(6): 1055-1069. |
| [22] | Li W Q, Zhang M J, Gan P F, Qiao L, Yang S Q, Miao H, Wang G F, Zhang M M, Liu W T, Li H F, Shi C H, Chen K M. 2017. CLD1/SRL1 modulates leaf rolling by affecting cell wall formation, epidermis integrity and water homeostasis in rice. Plant J, 92(5): 904-923. |
| [23] | Li X M, Han H P, Chen M, Yang W, Liu L, Li N, Ding X H, Chu Z H. 2017. Overexpression of OsDT11, which encodes a novel cysteine-rich peptide, enhances drought tolerance and increases ABA concentration in rice. Plant Mol Biol, 93(1/2): 21-34. |
| [24] | Liang J Y, Guo S Y, Sun B, Liu Q, Chen X H, Peng H F, Zhang Z M, Xie Q J. 2018. Constitutive expression of REL1 confers the rice response to drought stress and abscisic acid. Rice, 11(1): 59. |
| [25] | Maruyama K, Urano K, Yoshiwara K, Morishita Y, Sakurai N, Suzuki H, Kojima M, Sakakibara H, Shibata D, Saito K, Shinozaki K, Yamaguchi-Shinozaki K. 2014. Integrated analysis of the effects of cold and dehydration on rice metabolites, phytohormones, and gene transcripts. Plant Physiol, 164(4): 1759-1771. |
| [26] | McFarlane H E, Döring A, Persson S. 2014. The cell biology of cellulose synthesis. Annu Rev Plant Biol, 65: 69-94. |
| [27] | Nakashima K, Tran L S P, van Nguyen D, Fujita M, Maruyama K, Todaka D, Ito Y, Hayashi N, Shinozaki K, Yamaguchi-Shinozaki K. 2007. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J, 51(4): 617-630. |
| [28] | Ning Y S, Jantasuriyarat C, Zhao Q Z, Zhang H W, Chen S B, Liu J L, Liu L J, Tang S Y, Park C H, Wang X J, Liu X L, Dai L Y, Xie Q, Wang G L. 2011. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol, 157(1): 242-255. |
| [29] | Richmond T A, Somerville C R. 2000. The cellulose synthase superfamily. Plant Physiol, 124(2): 495-498. |
| [30] | Shen H S, Chen J C, Huang J H, Tang B S. 2005. Microstructure and distribution of silica bodies in rice epidermis. J Fujian Agric For Univ Nat Sci, 34(2): 137-140. (in Chinese with English abstract) |
| [31] | Song Y, Ai C R, Jing S J, Yu D Q. 2010. Research progress on functional analysis of rice WRKY genes. Rice Sci, 17(1): 60-72. |
| [32] | Tenhaken R. 2014. Cell wall remodeling under abiotic stress. Front Plant Sci, 5: 771. |
| [33] | Todaka D, Shinozaki K, Yamaguchi-Shinozaki K. 2015. Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci, 6: 84. |
| [34] | Udenfriend S, Kodukula K. 1995. How glycosyl-phosphatidylinositol- anchored membrane proteins are made. Annu Rev Biochem, 64: 563-591. |
| [35] | Wang X, Cnops G, Vanderhaeghen R, de Block S, van Montagu M, van Lijsebettens M. 2001. AtCSLD3, a cellulose synthase-like gene important for root hair growth in Arabidopsis. Plant Physiol, 126(2): 575-586. |
| [36] | Wellburn A R. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol, 144(3): 307-313. |
| [37] | Xiang J J, Zhang G H, Qian Q, Xue H W. 2012. Semi-rolled leaf1 encodes a putative glycosylphosphatidylinositol-anchored protein and modulates rice leaf rolling by regulating the formation of bulliform cells. Plant Physiol, 159(4): 1488-1500. |
| [38] | Xue D W, Zhang X Q, Lu X L, Chen G, Chen Z H. 2017. Molecular and evolutionary mechanisms of cuticular wax for plant drought tolerance. Front Plant Sci, 8: 621. |
| [39] | Yoo C Y, Pence H E, Jin J B, Miura K, Gosney M J, Hasegawa P M, Mickelbart M V. 2010. The Arabidopsis GTL1 transcription factor regulates water use efficiency and drought tolerance by modulating stomatal density via transrepression of SDD1. Plant Cell, 22(12): 4128-4141. |
| [40] | Yoshida S, Hasegawa S. 1982. The rice root system: Its development and function. In: International Rice Research Institute. Drought Resistance in Crops with Emphasis on Rice. Los Baños, the Philippines: International Rice Research Institute: 97-114. |
| [41] | Yoshikawa T, Eiguchi M, Hibara K I, Ito J I, Nagato Y. 2013. Rice SLENDER LEAF 1 gene encodes cellulose synthase-like D4 and is specifically expressed in M-phase cells to regulate cell proliferation. J Exp Bot, 64(7): 2049-2061. |
| [42] | Zhang G H, Hou X, Wang L, Xu J, Chen J, Fu X, Shen N W, Nian J Q, Jiang Z Z, Hu J, Zhu L, Rao Y C, Shi Y F, Ren D Y, Dong G J, Gao Z Y, Guo L B, Qian Q, Luan S. 2021. PHOTO- SENSITIVE LEAF ROLLING 1 encodes a polygalacturonase that modifies cell wall structure and drought tolerance in rice. New Phytol, 229(2): 890-901. |
| [43] | Zhong R Q, Ye Z H. 2007. Regulation of cell wall biosynthesis. Curr Opin Plant Biol, 10(6): 564-572. |
| [44] | Zhu J H, Lee B H, Dellinger M, Cui X P, Zhang C Q, Wu S, Nothnagel E A, Zhu J K. 2010. A cellulose synthase-like protein is required for osmotic stress tolerance in Arabidopsis. Plant J, 63(1): 128-140. |
| [45] | Zhu L, Hu J, Yan M X, Gao Z Y, Liu J, Qian Q, Guo L B. 2010. RNAi and expression analysis of a gene OsCSLD4, which controls plant narrow and rolled leaf in rice. J Nucl Agric Sci, 24(5): 873-880. (in Chinese with English abstract) |
| [46] | Zhu X Y, Xiong L Z. 2013. Putative megaenzyme DWA1 plays essential roles in drought resistance by regulating stress-induced wax deposition in rice. Proc Natl Acad Sci USA, 110(44): 17790-17795. |
| [1] | Wei Huanhe, Geng Xiaoyu, Zhang Xiang, Zhu Wang, Zhang Xubin, Chen Yinglong, Huo Zhongyang, Zhou Guisheng, Meng Tianyao, Dai Qigen. Grain Yield, Biomass Accumulation, and Leaf Photosynthetic Characteristics of Rice under Combined Salinity-Drought Stress [J]. Rice Science, 2024, 31(1): 118-128. |
| [2] | Masoumeh Kordi, Naser Farrokhi, Martin I. Pech-Canul, Asadollah Ahmadikhah. Rice Husk at a Glance: From Agro-Industrial to Modern Applications [J]. Rice Science, 2024, 31(1): 14-32. |
| [3] | Tian Yu, Sun Jing, Li Jiaxin, Wang Aixia, Nie Mengzi, Gong Xue, Wang Lili, Liu Liya, Wang Fengzhong, Tong Litao. Effects of Milling Methods on Rice Flour Properties and Rice Product Quality: A Review [J]. Rice Science, 2024, 31(1): 33-46. |
| [4] | Norhashila Hashim, Maimunah Mohd Ali, Muhammad Razif Mahadi, Ahmad Fikri Abdullah, Aimrun Wayayok, Muhamad Saufi Mohd Kassim, Askiah Jamaluddin. Smart Farming for Sustainable Rice Production: An Insight into Application, Challenge, and Future Prospect [J]. Rice Science, 2024, 31(1): 47-61. |
| [5] | Gao Ningning, Ye Shuifeng, Zhang Yu, Zhou Liguo, Ma Xiaosong, Yu Hanxi, Li Tianfei, Han Jing, Liu Zaochang, Luo Lijun. A β-Carotene Ketolase Gene NfcrtO from Subaerial Cyanobacteria Confers Drought Tolerance in Rice [J]. Rice Science, 2024, 31(1): 62-76. |
| [6] | Li Qianlong, Feng Qi, Wang Heqin, Kang Yunhai, Zhang Conghe, Du Ming, Zhang Yunhu, Wang Hui, Chen Jinjie, Han Bin, Fang Yu, Wang Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 552-565. |
| [7] | Ji Dongling, Xiao Wenhui, Sun Zhiwei, Liu Lijun, Gu Junfei, Zhang Hao, Matthew Tom Harrison, Liu Ke, Wang Zhiqin, Wang Weilu. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 598-612. |
| [8] | Prathap V, Suresh Kumar, Nand Lal Meena, Chirag Maheshwari, Monika Dalal, Aruna Tyagi. Phosphorus Starvation Tolerance in Rice Through Combined Physiological, Biochemical, and Proteome Analyses [J]. Rice Science, 2023, 30(6): 613-631. |
| [9] | Serena Reggi, Elisabetta Onelli, Alessandra Moscatelli, Nadia Stroppa, Matteo Dell’Anno, Kiril Perfanov, Luciana Rossi. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Engineered Rice Lines [J]. Rice Science, 2023, 30(6): 587-597. |
| [10] | Sundus Zafar, Xu Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 523-536. |
| [11] | Kankunlanach Khampuang, Nanthana Chaiwong, Atilla Yazici, Baris Demirer, Ismail Cakmak, Chanakan Prom-U-Thai. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 632-640. |
| [12] | Fan Fengfeng, Cai Meng, Luo Xiong, Liu Manman, Yuan Huanran, Cheng Mingxing, Ayaz Ahmad, Li Nengwu, Li Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 577-586. |
| [13] | Lin Shaodan, Yao Yue, Li Jiayi, Li Xiaobin, Ma Jie, Weng Haiyong, Cheng Zuxin, Ye Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 652-660. |
| [14] | Md. Forshed Dewan, Md. Ahiduzzaman, Md. Nahidul Islam, Habibul Bari Shozib. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and Southeast Asia: A Review [J]. Rice Science, 2023, 30(6): 537-551. |
| [15] | Raja Chakraborty, Pratap Kalita, Saikat Sen. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Pigmented Black Rice Variety Chakhao poireiton in High-Fat High-Sugar Induced Rats [J]. Rice Science, 2023, 30(6): 641-651. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||