
Rice Science ›› 2023, Vol. 30 ›› Issue (1): 58-69.DOI: 10.1016/j.rsci.2022.07.009
• Research Paper • Previous Articles Next Articles
Ernieca Lyngdoh Nongbri, Sudip Das, Karma Landup Bhutia, Aleimo G. Momin, Mayank Rai, Wricha Tyagi( )
)
Received:2022-02-06
Accepted:2022-07-06
Online:2023-01-28
Published:2022-11-11
Contact:
Wricha Tyagi
About author:First author contact:This is an open access article under the CC BY-NC-ND license (
Peer review under responsibility of China National Rice Research Institute
Ernieca Lyngdoh Nongbri, Sudip Das, Karma Landup Bhutia, Aleimo G. Momin, Mayank Rai, Wricha Tyagi. Differential Expression of Iron Deficiency Responsive Rice Genes under Low Phosphorus and Iron Toxicity Conditions and Association of OsIRO3 with Yield in Acidic Soils[J]. Rice Science, 2023, 30(1): 58-69.
Add to citation manager EndNote|Ris|BibTeX
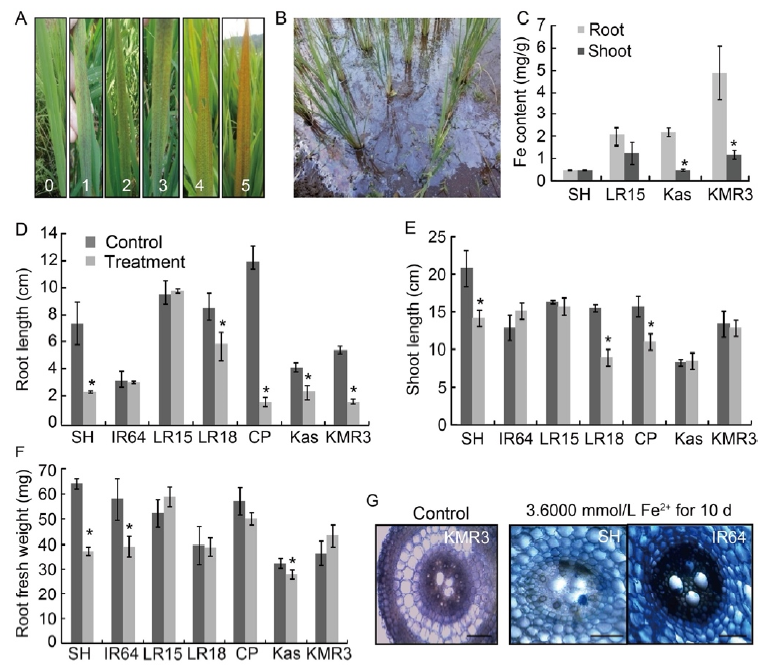
Fig. 1. Screening under acidic lowland soils and hydroponic conditions. A, Leaf bronzing scores (0?5, from left to right) given to rice genotypes grown in the lowland fields depending on the intensity of leaf bronzing symptoms on foliage. B, Fe plaque accumulation in an experimental field. C, Fe content evaluated on selected representative genotypes harvested from lowland fields. Data are Mean ± SD (n = 20). Statistically significant differences between the root and shoot were determined by the Student’s t-test (*, P < 0.05). D?F, Root length (D), shoot length (E) and root fresh weight (F) under hydroponic conditions. The histograms represent average phenotypic values under control (0.0284 mmol/L Fe2+ and 0.3500 mmol/L Pi) and treatment (3.6000 mmol/L Fe2+ and 0.3500 mmol/L Pi) conditions. Data are Mean ± SD (n = 10). Statistically significant differences between the control and treatment were determined by the Student’s t-test (*, P < 0.05). G, Cross-section of roots after staining with Perl’s blue stain viewed under a Leica DM750 microscope. Scale bars, 1 mm. SH, Shasharang; LR15, Priya; LR18, Paijong; CP, Chakhao Poirieton; Kas, Kasalath.
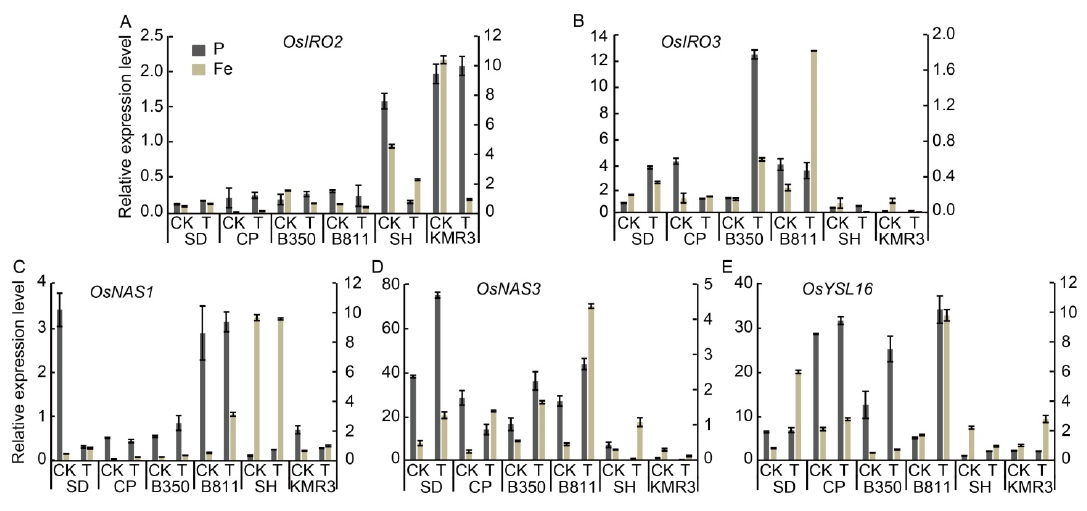
Fig. 2. Transcript levels of five rice genes involved in Fe deficiency responses in shoots of six rice genotypes after treatments of low P and high Fe for 24 h. A?E, Expression levels of OsIRO2 (A), OsIRO3 (B), OsNAS1 (C), OsNAS3 (D) and OsYSL16 (E) under low P and high Fe conditions for 24 h. Plants were grown using sand culture supplemented with Yoshida solution in control, low P and excess Fe treatments at pH 5.4. Shoots were harvested and used for qRT-PCR. Rice β-tubulin transcript level was used for normalization and transcript abundance was expressed as a ratio relative to the levels in control (Mean ± SD, n = 4). Y axes on the left and right represent normalized relative expression in the P and Fe experiments, respectively. CK, Control (0.3500 mmol/L Pi and 0.0284 mmol/L Fe2+); T, Treatment (low Pi: 0.0150 mmol/L Pi and 0.0284 mmol/L Fe2+; high Fe2+: 0.3500 mmol/L Pi and 3.6000 mmol/L Fe2+). SD, Sahbhagi Dhan; CP, Chakhao Poirieton; B350, BAM350; B811, BAM811; SH, Shasharang.
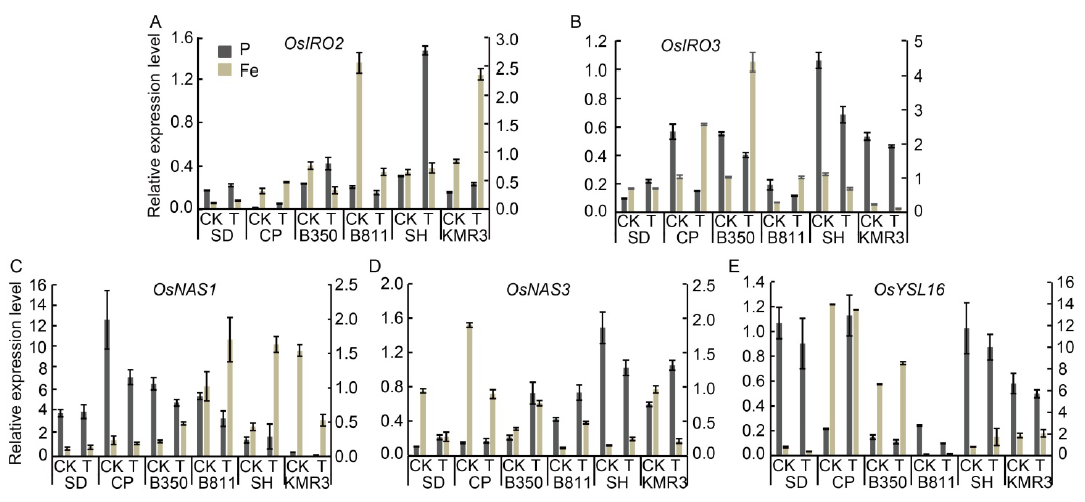
Fig. 3. Transcript levels of five rice genes involved in Fe deficiency responses in shoots of six rice genotypes after treatments of low P and high Fe for 48 h. A?E, Expression levels of OsIRO2 (A), OsIRO3 (B), OsNAS1 (C), OsNAS3 (D) and OsYSL16 (E) under low P and high Fe conditions for 48 h. Plants were grown using sand culture supplemented with Yoshida solution in control, low P and excess Fe treatments at pH 5.4. Shoots were harvested and used for qRT-PCR. Rice β-tubulin transcript level was used for normalization and transcript abundance was expressed as a ratio relative to the levels in control (Mean ± SD, n = 4). Y axes on the left and right represent normalized relative expression in the P and Fe experiments, respectively. CK, Control (0.3500 mmol/L Pi and 0.0284 mmol/L Fe2+); T, Treatment (low Pi: 0.0150 mmol/L Pi and 0.0284 mmol/L Fe2+; high Fe2+: 0.3500 mmol/L Pi and 3.6000 mmol/L Fe2+). SD, Sahbhagi Dhan; CP, Chakhao Poirieton; B350, BAM350; B811, BAM811; SH, Shasharang.
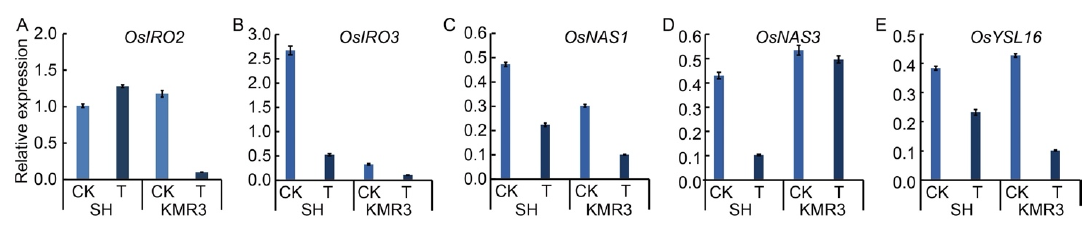
Fig. 4. Transcript levels of five rice genes involved in Fe deficiency responses in shoots of two genotypes after exposure to 7 d of high Fe treatment. A?E, Expression levels of OsIRO2 (A), OsIRO3 (B), OsNAS1 (C), OsNAS3 (D) and OsYSL16 (E) under high Fe conditions for 7 d. Plants were grown using sand culture supplemented with Yoshida solution in control (CK, 0.3500 mmol/L Pi and 0.0284 mmol/L Fe2+) and excess Fe (T, 0.3500 mmol/L Pi and 3.6000 mmol/L Fe2+) treatments at pH 5.4. Shoots were harvested and used for qRT-PCR. Rice β-tubulin transcript level was used for normalization and transcript abundance was expressed as a ratio relative to the levels in control (Mean ± SD, n = 4). Y axis on the left represents normalized relative expression under Fe experiment. SH, Shasharang.
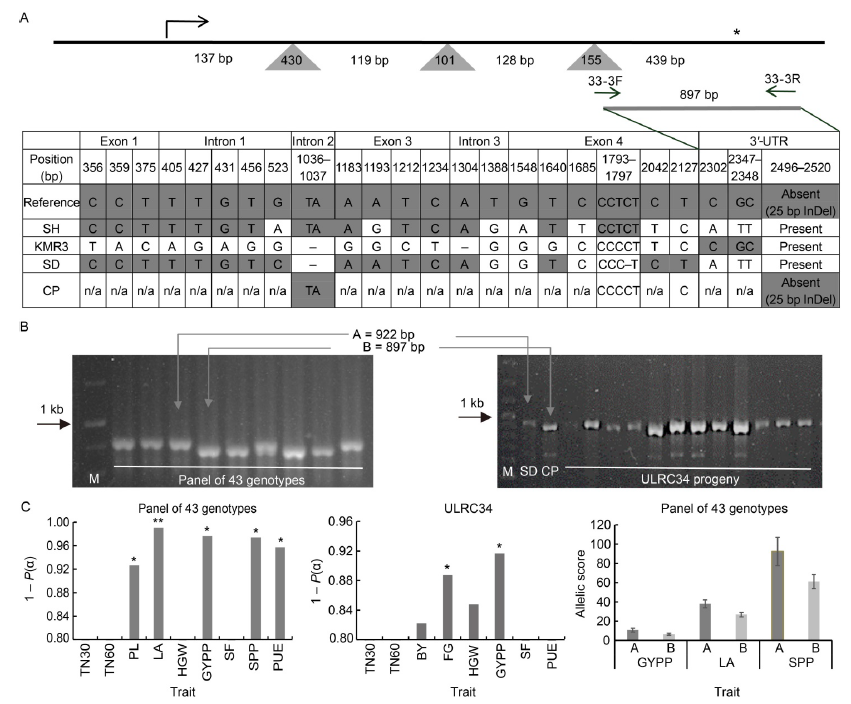
Fig. 5. Marker development and validation of OsIRO3 insertion/deletion (InDel) marker. A, A 25-bp InDel in 3°-UTR (untranslated region) of OsIRO3 was targeted for marker development. Schematic diagram of OsIRO3 gene along with primers designed to amplify the 3°-UTR region is shown. Dark grey triangles and lines indicate introns and exons, respectively. The arrow and star in the top indicate transcription start and stop positions, respectively. The positions of forward and reverse primers (33-3F/R) are given below the gene diagram. Allelic polymorphisms across OsIRO3 gene for five rice genotypes are given in the bottom. Dark grey and white colours indicate reference and novel alleles, respectively. B, Representative polymorphisms observed in a panel of 43 diverse rice genotypes and ULRC34 recombinant inbred lines. C, Associations of OsIRO3 InDel in two populations with major traits of interest in Fe toxic acidic lowland field. The difference in the phenotypic means of the two allelic classes was tested using the t-test. Bars in the first two graphs represent significance of differences. Single and double asterisks on the top of bars indicate significance of phenotypic difference between the two allelic classes at 0.1 and 0.01 levels of significance. Bars in the third graph indicate phenotypic means of genotypes carrying SD and CP type alleles in the panel of 43 diverse rice genotypes. Error bars indicate confidence interval. TN30, Tiller number at 30 d after transplanting; TN60, Tiller number at 60 d after transplanting; PL, Panicle length (cm); LA, Leaf area (cm2); HGW, 100-grain weight (g); GYPP, Grain yield per panicle (g); SF, Spikelet fertility rate (%); SPP, Spikelet number per panicle; PUE, Phosphorus use efficiency at harvest (%); BY, Biological yield (g); FG, Filled grain number per plant. M, Marker; SH, Shasharang; SD, Sahbhagi Dhan; CP, Chakhao Poirieton.
| [1] | Álvarez-Fernández A, Díaz-Benito P, Abadía A, López-Millán A F, Abadía J. 2014. Metal species involved in long distance metal transport in plants. Front Plant Sci, 5: 105. |
| [2] |
Aoyama T, Kobayashi T, Takahashi M, Nagasaka S, Usuda K, Kakei Y, Ishimaru Y, Nakanishi H, Mori S, Nishizawa N K. 2009. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol, 70(6): 681-692.
PMID |
| [3] | Audebert A, Fofana M. 2009. Rice yield gap due to iron toxicity in West Africa. J Agron Crop Sci, 7: 66-76. |
| [4] | Audebert A, Sahrawat K L. 2000. Mechanisms for iron toxicity tolerance in lowland rice. J Plant Nutr, 23(11/12): 1877-1885. |
| [5] | Aung M S, Masuda H, Nozoye T, Kobayashi T, Jeon J S, An G, Nishizawa N K. 2019. Nicotianamine synthesis by OsNAS3 is important for mitigating iron excess stress in rice. Front Plant Sci, 10: 660. |
| [6] | Bashir K, Ishimaru Y, Nishizawa N K. 2010. Iron uptake and loading into rice grains. Rice, 3(2/3): 122-130. |
| [7] |
Bashir K, Hanada K, Shimizu M, Seki M, Nakanishi H, Nishizawa N K. 2014. Transcriptomic analysis of rice in response to iron deficiency and excess. Rice, 7(1): 18.
PMID |
| [8] |
Bashir K, Nozoye T, Nagasaka S, Rasheed S, Miyauchi N, Seki M, Nakanishi H, Nishizawa N K. 2017. Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice. J Exp Bot, 68(7): 1785-1795.
PMID |
| [9] | Bayuelo-Jiménez J S, Gallardo-Valdéz M, Pérez-Decelis V A, Magdaleno-Armas L, Ochoa I, Lynch J P. 2011. Genotypic variation for root traits of maize (Zea mays L.) from the Purhepecha Plateau under contrasting phosphorus availability. Field Crops Res, 121(3): 350-362. |
| [10] | Becker M, Asch F. 2005. Iron toxicity in rice: Conditions and management concepts. J Plant Nutr Soil Sci, 168: 558-573. |
| [11] |
Bhutia K L, Nongbri E L, Gympad E, Rai M, Tyagi W. 2020. In silico characterization, and expression analysis of rice golden 2-like (OsGLK) members in response to low phosphorous. Mol Biol Rep, 47(4): 2529-2549.
PMID |
| [12] | Bhutia K L, Nongbri E L, Sharma T O, Rai M, Tyagi W. 2021. A 1.84-Mb region on rice chromosome 2 carrying SPL4, SPL5 and MLO8 genes is associated with higher yield under phosphorus- deficient acidic soil. J Appl Genet, 62(2): 207-222. |
| [13] | Curie C, Panaviene Z, Loulergue C, Dellaporta S L, Briat J F, Walker E L. 2001. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature, 409: 346-349. |
| [14] | da Silveira V C, de Oliveira A P, Sperotto R A, Espindola L S, Amaral L, Dias J F, da Cunha J B, Fett J P. 2007. Influence of iron on mineral status of two rice (Oryza sativa L.) cultivars. Braz J Plant Physiol, 19(2): 127-139. |
| [15] | Das S, Tyagi W, Rai M, Yumnam J S. 2017a. Understanding Fe2+toxicity and P deficiency tolerance in rice for enhancing productivity under acidic soils. Biotechnol Genet Eng Rev, 33(1): 97-117. |
| [16] | Das S, Tyagi W, Rai M, Debnath A. 2017b. Identification of potential genotype influencing stress tolerance to Fe toxicity and P deficiency under low land acidic soils condition of north eastern rice, ‘Shasarang’. Int J Bio-resour Stress Manag, 8(6): 838-845. |
| [17] |
Deng D, Wu S C, Wu F Y, Deng H, Wong M H. 2010. Effects of root anatomy and Fe plaque on arsenic uptake by rice seedlings grown in solution culture. Environ Pollut, 158(8): 2589-2595.
PMID |
| [18] | Dufey I, Hakizimana P, Draye X, Lutts S, Bertin P. 2009. QTL mapping for biomass and physiological parameters linked to resistance mechanisms to ferrous iron toxicity in rice. Euphytica, 167(2): 143-160. |
| [19] | Dufey I, Mathieu A S, Draye X, Lutts S, Bertin P. 2015. Construction of an integrated map through comparative studies allows the identification of candidate regions for resistance to ferrous iron toxicity in rice. Euphytica, 203(1): 59-69. |
| [20] | Elec V, Quimio C A, Mendoza R, Sajise A G C, Beebout S E J, Gregorio G B, Singh R K. 2013. Maintaining elevated Fe2+ concentration in solution culture for the development of a rapid and repeatable screening technique for iron toxicity tolerance in rice (Oryza sativa L.). Plant Soil, 372(1/2): 253-264. |
| [21] | Engel K, Asch F, Becker M. 2012. Classification of rice genotypes based on their mechanisms of adaptation to iron toxicity. J Plant Nutr Soil Sci, 175(6): 871-881. |
| [22] | Fang W C, Wang J W, Lin C C, Kao C H. 2001. Iron induction of lipid peroxidation and effects on antioxidative enzyme activities in rice leaves. Plant Growth Regul, 35(1): 75-80. |
| [23] | Hall T. 1999. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser, 41: 95-98. |
| [24] |
Higuchi K, Suzuki K, Nakanishi H, Yamaguchi H, Nishizawa N K, Mori S. 1999. Cloning of nicotianamine synthase genes, novel genes involved in the biosynthesis of phytosiderophores. Plant Physiol, 119(2): 471-480.
PMID |
| [25] |
Higuchi K, Watanabe S, Takahashi M, Kawasaki S, Nakanishi H, Nishizawa N K, Mori S. 2001. Nicotianamine synthase gene expression differs in barley and rice under Fe-deficient conditions. Plant J, 25(2): 159-167.
PMID |
| [26] | Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2003. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long- distance transport of iron and differentially regulated by iron. Plant J, 36(3): 366-381. |
| [27] | Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2004. A rice FRD3-like (OsFRDL1) gene is expressed in the cells involved in long-distance transport. Soil Sci Plant Nutr, 50(7): 1133-1140. |
| [28] |
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa N K. 2009. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem, 284(6): 3470-3479.
PMID |
| [29] |
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J, 45(3): 335-346.
PMID |
| [30] | Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa N K. 2010. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J, 62(3): 379-390. |
| [31] |
Kakei Y, Ishimaru Y, Kobayashi T, Yamakawa T, Nakanishi H, Nishizawa N K. 2012. OsYSL16 plays a role in the allocation of iron. Plant Mol Biol, 79(6): 583-594.
PMID |
| [32] |
Kobayashi T, Suzuki M, Inoue H, Itai R N, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2005. Expression of iron-acquisition-related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot, 56(415): 1305-1316.
PMID |
| [33] |
Kobayashi T, Ogo Y, Itai R N, Nakanishi H, Takahashi M, Mori S, Nishizawa N K. 2007. The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Natl Acad Sci USA, 104(48): 19150-19155.
PMID |
| [34] |
Kobayashi T, Nakanishi Itai R, Nishizawa N K. 2014. Iron deficiency responses in rice roots. Rice, 7(1): 27.
PMID |
| [35] |
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2004. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J, 39(3): 415-424.
PMID |
| [36] |
Lee S, Chiecko J C, Kim S A, Walker E L, Lee Y, Guerinot M L, An G. 2009. Disruption of OsYSL15 leads to iron inefficiency in rice plants. Plant Physiol, 150(2): 786-800.
PMID |
| [37] | Li L, Ye L X, Kong Q H, Shou H X. 2019. A vacuolar membrane ferric-chelate reductase, OsFRO1, alleviates Fe toxicity in rice (Oryza sativa L.). Front Plant Sci, 10: 700. |
| [38] |
Matthus E, Wu L B, Ueda Y, Höller S, Becker M, Frei M. 2015. Loci, genes, and mechanisms associated with tolerance to ferrous iron toxicity in rice (Oryza sativa L.). Theor Appl Genet, 128(10): 2085-2098.
PMID |
| [39] | Meng L J, Wang B X, Zhao X Q, Ponce K, Qian Q, Ye G Y. 2017. Association mapping of ferrous, zinc, and aluminum tolerance at the seedling stage in indica rice using MAGIC populations. Front Plant Sci, 8: 1822. |
| [40] |
Moore K L, Chen Y, van de Meene A M L, Hughes L, Liu W J, Geraki T, Mosselmans F, McGrath S P, Grovenor C, Zhao F J. 2014. Combined NanoSIMS and synchrotron X-ray fluorescence reveal distinct cellular and subcellular distribution patterns of trace elements in rice tissues. New Phytol, 201(1): 104-115.
PMID |
| [41] |
Murray M G, Thompson W F. 1980. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res, 8: 4321-4325.
PMID |
| [42] | Müller C, Kuki K N, Pinheiro D T, Souza L R, Siqueira Silva A I, Loureiro M E, Oliva M A, Almeida A M. 2015. Differential physiological responses in rice upon exposure to excess distinct iron forms. Plant Soil, 391(1/2): 123-138. |
| [43] |
Ogo Y, Itai R N, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa N K. 2006. Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot, 57(11): 2867-2878.
PMID |
| [44] |
Ogo Y, Itai R N, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa N K. 2007. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J, 51(3): 366-377.
PMID |
| [45] |
Rai V, Sanagala R, Sinilal B, Yadav S, Sarkar A K, Dantu P K, Jain A. 2015. Iron availability affects phosphate deficiency-mediated responses, and evidence of cross-talk with auxin and zinc in Arabidopsis. Plant Cell Physiol, 56(6): 1107-1123.
PMID |
| [46] |
Roschzttardtz H, Conéjéro G, Curie C, Mari S. 2010. Straight- forward histochemical staining of Fe by the adaptation of an old- school technique: Identification of the endodermal vacuole as the site of Fe storage in Arabidopsis embryos. Plant Signal Behav, 5(1): 56-57.
PMID |
| [47] |
Saengwilai P, Tian X L, Lynch J P. 2014. Low crown root number enhances nitrogen acquisition from low-nitrogen soils in maize. Plant Physiol, 166(2): 581-589.
PMID |
| [48] | Sahrawat K L. 2004. Iron toxicity in wetland rice and the role of other nutrients. J Plant Nutr, 27(8): 1471-1504. |
| [49] |
Takahashi M, Yamaguchi H, Nakanishi H, Shioiri T, Nishizawa N K, Mori S. 1999. Cloning two genes for nicotianamine amino- transferase, a critical enzyme in iron acquisition (Strategy II)in graminaceous plants. Plant Physiol, 121(3): 947-956.
PMID |
| [50] |
Takahashi M, Nakanishi H, Kawasaki S, Nishizawa N K, Mori S. 2001. Enhanced tolerance of rice to low iron availability in alkaline soils using barley nicotianamine aminotransferase genes. Nat Biotechnol, 19(5): 466-469.
PMID |
| [51] | Tiwari K K, Singh A, Pattnaik S, Sandhu M, Kaur S, Jain S, Tiwari S, Mehrotra S, Anumalla M, Samal R, Bhardwaj J, Dubey N, Sahu V, Kharshing G A, Zeliang P K, Sreenivasan K, Kumar P, Parida S K, Mithra S V A, Rai V, Tyagi W, Agrawal P K, Rao A R, Pattanayak A, Chandel G, Singh A K, Bisht I S, Bhat K V, Rao G J N, Khurana J P, Singh N K, Mohapatra T. 2015. Identification of a diverse mini-core panel of Indian rice germplasm based on genotyping using microsatellite markers. Plant Breed, 134(2): 164-171. |
| [52] | Tyagi W, Rai M. 2017. Root transcriptomes of two acidic soil adapted indica rice genotypes suggest diverse and complex mechanism of low phosphorus tolerance. Protoplasma, 254(2): 725-736. |
| [53] |
Tyagi W, Yumnam J S, Sen D, Rai M. 2020. Root transcriptome reveals efficient cell signaling and energy conservation key to aluminum toxicity tolerance in acidic soil adapted rice genotype. Sci Rep, 10(1): 4580.
PMID |
| [54] | Wissuwa M, Ae N. 2001. Further characterization of two QTLs that increase phosphorus uptake of rice (Oryza sativa L.) under phosphorus deficiency. Plant Soil, 237(2): 275-286. |
| [55] | Wu L B, Shhadi M Y, Gregorio G, Matthus E, Becker M, Frei M. 2014. Genetic and physiological analysis of tolerance to acute iron toxicity in rice. Rice, 7(1): 8. |
| [56] | Wu L B, Ueda Y, Lai S K, Frei M. 2017. Shoot tolerance mechanisms to iron toxicity in rice (Oryza sativa L.). Plant Cell Environ, 40(4): 570-584. |
| [57] |
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma J F. 2009. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol, 149(1): 297-305.
PMID |
| [58] |
Yokosho K, Yamaji N, Ma J F. 2016. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J Exp Bot, 67(18): 5485-5494.
PMID |
| [59] | Yoneyama T, Ishikawa S, Fujimaki S. 2015. Route and regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: Metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. Int J Mol Sci, 16(8): 19111-19129. |
| [60] | Yoshida S, Forno D A, Cock J. 1976. Laboratory Manual for Physiological Studies of Rice. 3rd edn. Manila, the Philippines: International Rice Research Institute. |
| [61] | Yumnam J S, Rai M, Tyagi W. 2017. Allele mining across two low-P tolerant genes PSTOL1 and PupK20-2 reveals novel haplotypes in rice genotypes adapted to acidic soils. Plant Genet Resour, 15(3): 221-229. |
| [62] | Zhang C M, Tanaka N, Dwiyanti M S, Shenton M, Maruyama H, Shinano T, Chu Q N, Xie J, Watanabe T. 2022. Ionomic profiling of rice genotypes and identification of varieties with elemental covariation effects. Rice Sci, 29(1): 76-88. |
| [63] | Zhang X K, Zhang F S, Dam M. 1999. Effect of iron plaque outside roots on nutrient uptake by rice (Oryza sativa L.): Phosphorus uptake. Plant Soil, 209(2): 187-192. |
| [64] | Zheng L Q, Cheng Z Q, Ai C X, Jiang X H, Bei X S, Zheng Y, Glahn R P, Welch R M, Miller D D, Lei X G, Shou H X. 2010. Nicotianamine, a novel enhancer of rice iron bioavailability to humans. PLoS One, 5(4): e10190. |
| [1] | Prathap V, Suresh KUMAR, Nand Lal MEENA, Chirag MAHESHWARI, Monika DALAL, Aruna TYAGI. Phosphorus Starvation Tolerance in Rice Through a Combined Physiological, Biochemical and Proteome Analysis [J]. Rice Science, 2023, 30(6): 8-. |
| [2] | Serena REGGI, Elisabetta ONELLI, Alessandra MOSCATELLI, Nadia STROPPA, Matteo Dell’ANNO, Kiril PERFANOV, Luciana ROSSI. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Rice Engineered Lines [J]. Rice Science, 2023, 30(6): 6-. |
| [3] | Sundus ZAFAR, XU Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 4-. |
| [4] | Kankunlanach KHAMPUANG, Nanthana CHAIWONG, Atilla YAZICI, Baris DEMIRER, Ismail CAKMAK, Chanakan PROM-U-THAI. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 9-. |
| [5] | FAN Fengfeng, CAI Meng, LUO Xiong, LIU Manman, YUAN Huanran, CHENG Mingxing, Ayaz AHMAD, LI Nengwu, LI Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Rice Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 14-. |
| [6] | LIN Shaodan, YAO Yue, LI Jiayi, LI Xiaobin, MA Jie, WENG Haiyong, CHENG Zuxin, YE Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 10-. |
| [7] | Md. Forshed DEWAN, Md. AHIDUZZAMAN, Md. Nahidul ISLAM, Habibul Bari SHOZIB. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and South-East Asia: A Review [J]. Rice Science, 2023, 30(6): 5-. |
| [8] | Raja CHAKRABORTY, Pratap KALITA, Saikat SEN. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Chakhao Poireiton (A Pigmented Black Rice) in High-Fat High-Sugar induced Rats [J]. Rice Science, 2023, 30(6): 11-. |
| [9] | LI Qianlong, FENG Qi, WANG Heqin, KANG Yunhai, ZHANG Conghe, DU Ming, ZHANG Yunhu, WANG Hui, CHEN Jinjie, HAN Bin, FANG Yu, WANG Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 7-. |
| [10] | JI Dongling, XIAO Wenhui, SUN Zhiwei, LIU Lijun, GU Junfei, ZHANG Hao, Tom Matthew HARRISON, LIU Ke, WANG Zhiqin, WANG Weilu, YANG Jianchang. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 12-. |
| [11] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [12] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [13] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| [14] | Lu Xuedan, Li Fan, Xiao Yunhua, Wang Feng, Zhang Guilian, Deng Huabing, Tang Wenbang. Grain Shape Genes: Shaping the Future of Rice Breeding [J]. Rice Science, 2023, 30(5): 379-404. |
| [15] | Zhang Guomei, Li Han, Liu Shanshan, Zhou Xuming, Lu Mingyang, Tang Liang, Sun Lihua. Water Extract of Rice False Smut Balls Activates Nrf2/HO-1 and Apoptosis Pathways, Causing Liver Injury [J]. Rice Science, 2023, 30(5): 473-485. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||