
Rice Science ›› 2025, Vol. 32 ›› Issue (2): 160-176.DOI: 10.1016/j.rsci.2025.01.002
• Reviews • Previous Articles Next Articles
Sanchika Snehi1, Ravi Kiran Kt2, Sanket Rathi1, Sameer Upadhyay1, Suneetha Kota3, Satish Kumar Sanwal4, Lokeshkumar Bm4, Arun Balasubramaniam1, Nitish Ranjan Prakash4( ), Pawan Kumar Singh1(
), Pawan Kumar Singh1( )
)
Received:2024-09-12
Accepted:2024-12-18
Online:2025-03-28
Published:2025-04-14
Contact:
Nitish Ranjan Prakash (nitishranjan240@gmail.com); Pawan Kumar Singh (pks.gpb@bhu.ac.in)
Sanchika Snehi, Ravi Kiran Kt, Sanket Rathi, Sameer Upadhyay, Suneetha Kota, Satish Kumar Sanwal, Lokeshkumar Bm, Arun Balasubramaniam, Nitish Ranjan Prakash, Pawan Kumar Singh. Discerning Genes to Deliver Varieties: Enhancing Vegetative- and Reproductive-Stage Flooding Tolerance in Rice[J]. Rice Science, 2025, 32(2): 160-176.
Add to citation manager EndNote|Ris|BibTeX
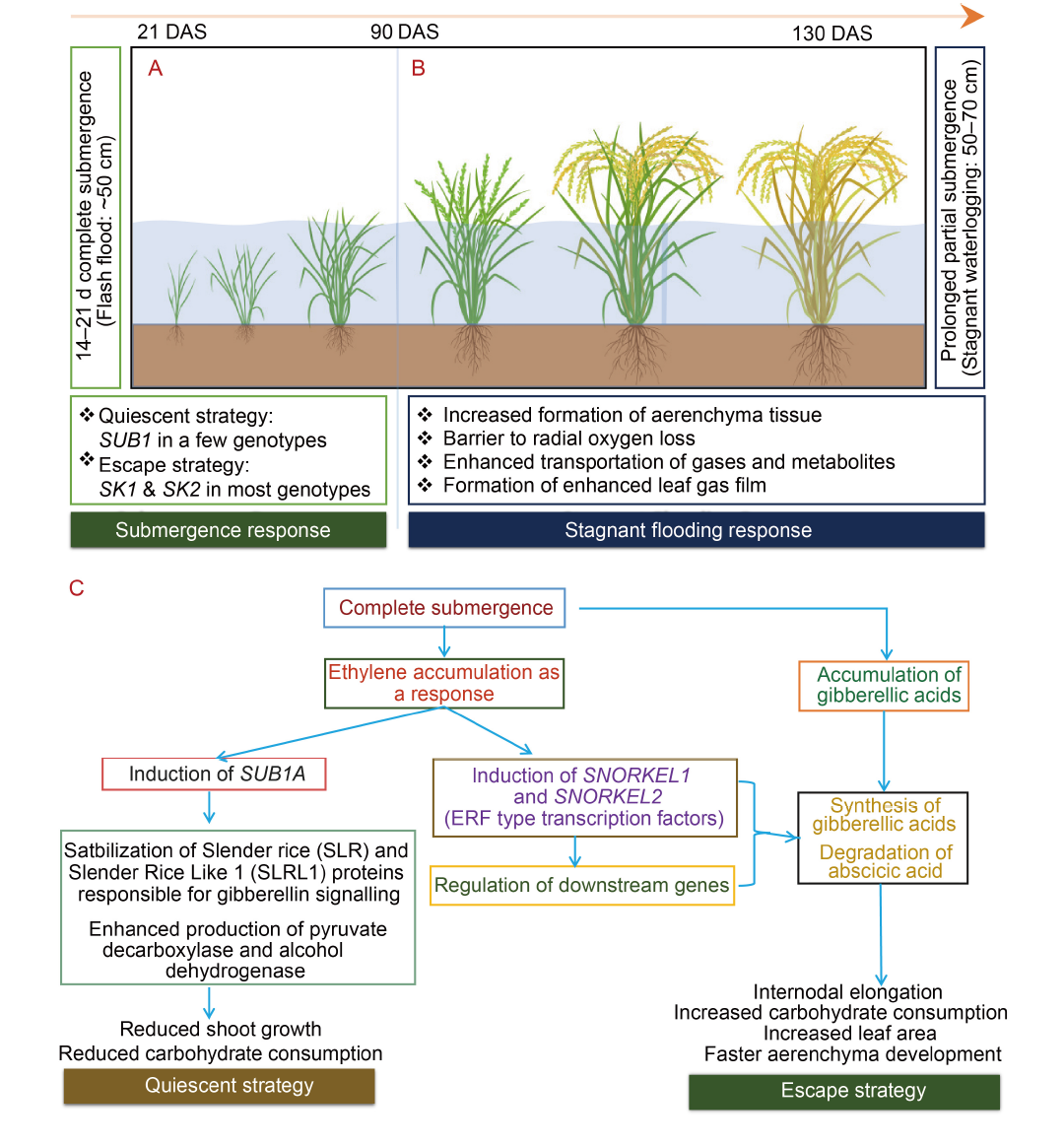
Fig. 1. Overall physiological strategy for flooding stress tolerance in rice. A, Complete submergence of rice plants under flash floods for 14‒21 d at the seedling and tillering stages (vegetative stages). Rice employs one of two strategies, viz. the quiescent strategy or the escape strategy, to manage hypoxic conditions. B, Prolonged stagnant flooding (with vegetative parts submerged) during the booting to grain-filling stages. Rice can manage hypoxic conditions in submerged plant parts and enhance its survival through increased aerenchyma formation, creating barriers to radial oxygen loss, leak-proof internal transport of gases and metabolites, and the formation of gas films. C, Response of rice plants to complete submergence. The quiescent strategy reduces growth and metabolism to save energy and metabolites for post-stress recovery, while the escape strategy enhances growth by internode elongation and faster aerenchyma development to maintain metabolism. ERF, Ethylene response factor. DAS, Days after sowing.
| QTL | Chr | Trait | SD | Linked marker | LOD | PVE (%) | Parent | MP | MT | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| qSUB6.1 | 6 | Submergence tolerance | S1 | P1/M3-1‒P2/M1-11 | 4.7 | 26.5 | IR74 × FR13A | RIL | AFLP & RFLP | Nandi et al, |
| qSUB7.1 | 7 | Submergence tolerance | S1 | P2/M1-5‒P2/M8-5 | 3.6 | 21.4 | ||||
| qSUB9.1 | 9 | Submergence tolerance | S1 | RZ698 | ||||||
| qSUB11.1 | 11 | Submergence tolerance | S1 | P1/M5-2‒P1/M5-13 | 3.2 | 19.4 | ||||
| qSUB12.1 | 12 | Submergence tolerance | S1 | P3/M4-5‒P3/M1-7 | 3.2 | 21.4 | ||||
| qLNE1.1 | 1 | Internode increment | S2 | RG109‒sd-1 | 18.9 | 33.1 | IR74 × Jalmagna | RIL | RFLP & AFLP | Sripongpangkul et al, |
| qLNE4.1 | 4 | Internode increment | S2 | P3M1-5‒P3M5-1 | 10.7 | 36.7 | ||||
| qPPS5.1 | 5 | Survival rate, tolerance score | S3 | R1553 | 11.2 | 34.1 | IR49830 × CT6241 | DH | RFLP & SSLP | Toojinda et al, |
| qPPS9.1 | 9 | Survival rate, tolerance score | S3 | RZ698 | 17.3 | 48.3 | ||||
| qEEA12.1 | 12 | Early elongation ability | S4 | RM5479‒RM6953 | 18.2 | 41.0 | Habibganj Aman VIII × Patnai 23 | F2 | RFLP & SSR | Tang et al, |
| qTIL2.1 | 2 | Internode elongation ability | S4 | RM208 | 4.9 | 14.4 | NIL1-3-12 × C9285 | F2 | SSR | Nagai et al, |
| qSUB1.1 | 1 | Submergence tolerance | S5 | MDC17‒RM12168 | 9.4 | 41.9 | IR72 × Madabaru | F2:3 | SSR & InDel | Septiningsih et al, 2012 |
| qSUB2.1 | 2 | Submergence tolerance | S5 | RM6318‒RM2578 | 3.8 | 19.6 | ||||
| qSUB9.1 | 9 | Submergence tolerance | S5 | RM23911‒RM23966 | 3.6 | 18.6 | ||||
| qSUB12.1 | 12 | Submergence tolerance | S5 | RM511‒RM463 | 4.2 | 21.5 | ||||
| qSUB1.1 | 1 | Submergence tolerance | S1 | id1000556‒id1003559 | 5.04 | 20.2 | FR13A × IR42 | RIL | SSR & SNP | Gonzaga et al, |
| qSUB9.1 | 9 | Submergence tolerance | S1 | id9001352‒SC3 | 16.89 | 53.0 | ||||
| qSUB8.1 | 8 | Submergence tolerance | S1 | 8608433-8686009 | 10.26 | 25.7 | Ciherang-SUB1 × IR10F365 | RIL | 6kSNP chip | Gonzaga et al, |
| qDTF3.1 | 3 | Days to 50% flowering | S6 | 2499734‒2560888 | 12.5 | 26.0 | Ciherang-SUB1 × IR10F365 | RIL | 6kSNP chip | Singh et al, |
| qFLW3.1 | 3 | Flag leaf width | S6 | 2499734‒2560888 | 4.0 | 11.3 | ||||
| qGY3.1 | 3 | Grain yield | S6 | 2499734‒2560888 | 6.2 | 14.3 | ||||
| qGY5.1 | 5 | Grain yield | S6 | id5003312‒ud5000983 | 3.66 | 12.2 | ||||
| qSER5.1 | 5 | Shoot elongation rate | S6 | ud5000983‒id5013231 | 12.5 | 32.0 | ||||
| qFLL5.1 | 5 | Flag leaf length | S6 | ud5000983‒5747652 | 6.18 | 14.0 | ||||
| qPL9.1 | 9 | Panicle length | S6 | 9641863‒9869869 | 5.47 | 22.0 | ||||
| qGW10.1 | 10 | Grain weight | S6 | 10603169‒10703329 | 6.3 | 13.1 | ||||
| qSTI-EL3.1 | 3 | Stem elongation | S6 | SNP130‒SNP112 | 2.36 | 23.4 | Rashpanjor × Swarna | RIL | GBS | Chattopadhyay et al, 2021 |
| qSTI-GW10.1 | 10 | Stem elongation | S6 | SNP389‒SNP407 | 2.15 | 54.9 | ||||
| qSTI-EL12.1 | 12 | Stem elongation | S6 | SNP488‒SNP498 | 2.05 | 15.5 | ||||
| qSUB2.1 | 2 | Submergence tolerance | S1 | 10.78 | 10.8 | TOS6454 × (Each of FARO44, FARO52, and FARO60) | RIL | DArT | Akintayo et al, |
Table 1. List of major QTLs (> 10% PVE) on flooding tolerance at vegetative-stage submergence and stagnant flooding in rice.
| QTL | Chr | Trait | SD | Linked marker | LOD | PVE (%) | Parent | MP | MT | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| qSUB6.1 | 6 | Submergence tolerance | S1 | P1/M3-1‒P2/M1-11 | 4.7 | 26.5 | IR74 × FR13A | RIL | AFLP & RFLP | Nandi et al, |
| qSUB7.1 | 7 | Submergence tolerance | S1 | P2/M1-5‒P2/M8-5 | 3.6 | 21.4 | ||||
| qSUB9.1 | 9 | Submergence tolerance | S1 | RZ698 | ||||||
| qSUB11.1 | 11 | Submergence tolerance | S1 | P1/M5-2‒P1/M5-13 | 3.2 | 19.4 | ||||
| qSUB12.1 | 12 | Submergence tolerance | S1 | P3/M4-5‒P3/M1-7 | 3.2 | 21.4 | ||||
| qLNE1.1 | 1 | Internode increment | S2 | RG109‒sd-1 | 18.9 | 33.1 | IR74 × Jalmagna | RIL | RFLP & AFLP | Sripongpangkul et al, |
| qLNE4.1 | 4 | Internode increment | S2 | P3M1-5‒P3M5-1 | 10.7 | 36.7 | ||||
| qPPS5.1 | 5 | Survival rate, tolerance score | S3 | R1553 | 11.2 | 34.1 | IR49830 × CT6241 | DH | RFLP & SSLP | Toojinda et al, |
| qPPS9.1 | 9 | Survival rate, tolerance score | S3 | RZ698 | 17.3 | 48.3 | ||||
| qEEA12.1 | 12 | Early elongation ability | S4 | RM5479‒RM6953 | 18.2 | 41.0 | Habibganj Aman VIII × Patnai 23 | F2 | RFLP & SSR | Tang et al, |
| qTIL2.1 | 2 | Internode elongation ability | S4 | RM208 | 4.9 | 14.4 | NIL1-3-12 × C9285 | F2 | SSR | Nagai et al, |
| qSUB1.1 | 1 | Submergence tolerance | S5 | MDC17‒RM12168 | 9.4 | 41.9 | IR72 × Madabaru | F2:3 | SSR & InDel | Septiningsih et al, 2012 |
| qSUB2.1 | 2 | Submergence tolerance | S5 | RM6318‒RM2578 | 3.8 | 19.6 | ||||
| qSUB9.1 | 9 | Submergence tolerance | S5 | RM23911‒RM23966 | 3.6 | 18.6 | ||||
| qSUB12.1 | 12 | Submergence tolerance | S5 | RM511‒RM463 | 4.2 | 21.5 | ||||
| qSUB1.1 | 1 | Submergence tolerance | S1 | id1000556‒id1003559 | 5.04 | 20.2 | FR13A × IR42 | RIL | SSR & SNP | Gonzaga et al, |
| qSUB9.1 | 9 | Submergence tolerance | S1 | id9001352‒SC3 | 16.89 | 53.0 | ||||
| qSUB8.1 | 8 | Submergence tolerance | S1 | 8608433-8686009 | 10.26 | 25.7 | Ciherang-SUB1 × IR10F365 | RIL | 6kSNP chip | Gonzaga et al, |
| qDTF3.1 | 3 | Days to 50% flowering | S6 | 2499734‒2560888 | 12.5 | 26.0 | Ciherang-SUB1 × IR10F365 | RIL | 6kSNP chip | Singh et al, |
| qFLW3.1 | 3 | Flag leaf width | S6 | 2499734‒2560888 | 4.0 | 11.3 | ||||
| qGY3.1 | 3 | Grain yield | S6 | 2499734‒2560888 | 6.2 | 14.3 | ||||
| qGY5.1 | 5 | Grain yield | S6 | id5003312‒ud5000983 | 3.66 | 12.2 | ||||
| qSER5.1 | 5 | Shoot elongation rate | S6 | ud5000983‒id5013231 | 12.5 | 32.0 | ||||
| qFLL5.1 | 5 | Flag leaf length | S6 | ud5000983‒5747652 | 6.18 | 14.0 | ||||
| qPL9.1 | 9 | Panicle length | S6 | 9641863‒9869869 | 5.47 | 22.0 | ||||
| qGW10.1 | 10 | Grain weight | S6 | 10603169‒10703329 | 6.3 | 13.1 | ||||
| qSTI-EL3.1 | 3 | Stem elongation | S6 | SNP130‒SNP112 | 2.36 | 23.4 | Rashpanjor × Swarna | RIL | GBS | Chattopadhyay et al, 2021 |
| qSTI-GW10.1 | 10 | Stem elongation | S6 | SNP389‒SNP407 | 2.15 | 54.9 | ||||
| qSTI-EL12.1 | 12 | Stem elongation | S6 | SNP488‒SNP498 | 2.05 | 15.5 | ||||
| qSUB2.1 | 2 | Submergence tolerance | S1 | 10.78 | 10.8 | TOS6454 × (Each of FARO44, FARO52, and FARO60) | RIL | DArT | Akintayo et al, |
| Technique used | Genotype | Stress level | DEG | Class of genes | Reference |
|---|---|---|---|---|---|
| 44k Agilent microarray | FR13A and Goda Heenati (both carrying SUB1 but differ in submergence tolerance) | 14-day-old seedlings were submerged for 3 d | FR13A (692 up-regulated and 819 down-regulated); Goda Heenati (1 281 up-regulated and 1 507 down-regulated) | Antioxidant/ROS scavenging genes are important for better performance of shoots of FR13A during submergence | Xiong et al, |
| RT-PCR | M202 (susceptible) and M202-SUB1 (tolerant) | Submergence of 14- day-old seedlings | DWARF4 (DWF4); DWARF1 (DWF1) | Brassinosteroid synthesis, transport, and responsive genes are differentially regulated and modulated gibberellic acid signaling and homeostasis | Schmitz et al, 2013 |
| qRT-PCR | FR13A (tolerant) and Tung Lu 3 (sensitive) | Submergence of 10-day-old seedlings | sucrose synthase 1 and alcohol dehydrogenase 1 | Higher expression of both genes, while expression is much higher in Tung Lu 3 | Yang et al, |
| qRT-PCR for TF gene family | IR64 (susceptible) and IR64-SUB1 (tolerant) | 75-day-old plants were submerged for 30 h | One common down-regulated in both; IR64 (27 down-regulated); IR64-SUB1 (13 up-regulated and 7 down-regulated) | DEGs belonging to NAC, MYB, TIFY, and Zn-finger TFs, are down-regulated upon submergence; DEGs in regulating hormonal pathways, i.e. gibberellins, abscisic acid, and jasmonic acid, apart from ethylene, are up-regulated | Sharma et al, |
| qRT-PCR for WRKY gene family | Nipponbare, Epagri 108, and BR IRGA 409 | 14-day-old seedlings were submerged for 6, 12, 24, and 48 h | 100-fold higher expression of OsWRKY11 and OsWRKY56 under submergence | WRKY transcription factors are known for their role in stress responses and in aerenchyma development | Viana et al, |
| RNA-seq | Oryza coarctata | Submergence up to 12 h | 15 158 DEGs | Stress-responsive transcription factors of bHLH, MYB, AP2-EREBP, WRKY, NAC, and bZIP class are differentially regulated | Garg et al, |
| RNA-seq | M202 (susceptible) and M202-SUB1 (tolerant) | 3 d submergence of 14-day-old seedlings | 703 genes up-regulated; 307 genes down-regulated | Carbohydrate metabolism, peroxisome (ROS scavenging), growth, and development are up‐regulated in M202-SUB1 | Locke et al, |
| RNA-seq | Nampyeongbyeo | Submergence at 14 d after heading and sampled after 4 d | 106 genes up-regulated; 30 genes down-regulated | Starch and sucrose synthesis, glycolysis, and carbon fixation are important in submergence response; genes for each step related to starch and d-glucose synthesis are down-regulated in the seeds and leaves but up-regulated in the stems | Lee et al, |
| RNA-seq | Yuefu (lowland) and IRAT109 (upland rice) | 28-day-old seedlings were exposed to anoxic condition at root | Yuefu (667 DEGs); IRAT109 (448 DEGs) | Phytohormone signalling (auxin, jasmonic acid, and ethylene), energy metabolism, aerenchyma formation, ROS, and cell wall modification are common among DEGs | Liu et al, |
Table 2. Transcriptomic results of flooding tolerance at the vegetative stage and stagnant flooding at the reproductive stage in rice.
| Technique used | Genotype | Stress level | DEG | Class of genes | Reference |
|---|---|---|---|---|---|
| 44k Agilent microarray | FR13A and Goda Heenati (both carrying SUB1 but differ in submergence tolerance) | 14-day-old seedlings were submerged for 3 d | FR13A (692 up-regulated and 819 down-regulated); Goda Heenati (1 281 up-regulated and 1 507 down-regulated) | Antioxidant/ROS scavenging genes are important for better performance of shoots of FR13A during submergence | Xiong et al, |
| RT-PCR | M202 (susceptible) and M202-SUB1 (tolerant) | Submergence of 14- day-old seedlings | DWARF4 (DWF4); DWARF1 (DWF1) | Brassinosteroid synthesis, transport, and responsive genes are differentially regulated and modulated gibberellic acid signaling and homeostasis | Schmitz et al, 2013 |
| qRT-PCR | FR13A (tolerant) and Tung Lu 3 (sensitive) | Submergence of 10-day-old seedlings | sucrose synthase 1 and alcohol dehydrogenase 1 | Higher expression of both genes, while expression is much higher in Tung Lu 3 | Yang et al, |
| qRT-PCR for TF gene family | IR64 (susceptible) and IR64-SUB1 (tolerant) | 75-day-old plants were submerged for 30 h | One common down-regulated in both; IR64 (27 down-regulated); IR64-SUB1 (13 up-regulated and 7 down-regulated) | DEGs belonging to NAC, MYB, TIFY, and Zn-finger TFs, are down-regulated upon submergence; DEGs in regulating hormonal pathways, i.e. gibberellins, abscisic acid, and jasmonic acid, apart from ethylene, are up-regulated | Sharma et al, |
| qRT-PCR for WRKY gene family | Nipponbare, Epagri 108, and BR IRGA 409 | 14-day-old seedlings were submerged for 6, 12, 24, and 48 h | 100-fold higher expression of OsWRKY11 and OsWRKY56 under submergence | WRKY transcription factors are known for their role in stress responses and in aerenchyma development | Viana et al, |
| RNA-seq | Oryza coarctata | Submergence up to 12 h | 15 158 DEGs | Stress-responsive transcription factors of bHLH, MYB, AP2-EREBP, WRKY, NAC, and bZIP class are differentially regulated | Garg et al, |
| RNA-seq | M202 (susceptible) and M202-SUB1 (tolerant) | 3 d submergence of 14-day-old seedlings | 703 genes up-regulated; 307 genes down-regulated | Carbohydrate metabolism, peroxisome (ROS scavenging), growth, and development are up‐regulated in M202-SUB1 | Locke et al, |
| RNA-seq | Nampyeongbyeo | Submergence at 14 d after heading and sampled after 4 d | 106 genes up-regulated; 30 genes down-regulated | Starch and sucrose synthesis, glycolysis, and carbon fixation are important in submergence response; genes for each step related to starch and d-glucose synthesis are down-regulated in the seeds and leaves but up-regulated in the stems | Lee et al, |
| RNA-seq | Yuefu (lowland) and IRAT109 (upland rice) | 28-day-old seedlings were exposed to anoxic condition at root | Yuefu (667 DEGs); IRAT109 (448 DEGs) | Phytohormone signalling (auxin, jasmonic acid, and ethylene), energy metabolism, aerenchyma formation, ROS, and cell wall modification are common among DEGs | Liu et al, |
| Analysis performed | Genotype | Stress level | Metabolite induced/detected | Reference |
|---|---|---|---|---|
| GC-MS and NMR | M202 (sensitive); M202-SUB1 (tolerant) | Seedlings at the 3-leaf stage were submerged for 0, 1, 2, and 3 d and then de-submerged for 1 d | GC-MS analysis exclusively identifies metabolites such as amino acids phenylalanine, proline, pyroglutamate, as well as TCA cycle intermediates like citrate, malate, and succinate. It also detects organic acids including GABA, glycerate, oxalate, shikimate, and threonate | Barding et al, |
| NMR, RPIP-UPLC-MS, GC-MS | M202 (sensitive); M202-SUB1 (tolerant) | 14-day-old seedlings were submerged for 3 d | The influence of SUB1 on metabolite levels, especially free amino acids, glucose, and sucrose, during the recovery period also affects the dynamics of trehalose-6-phosphate and the expression of mRNAs that encode crucial enzymes and signaling proteins | Locke et al, |
| LC-MS | Wufengyou 286 | 8 d submergence after panicle differentiation stage | On rice spikelets, 113 differential metabolites are detected when comparing submergence and control conditions, with 55 metabolites showing an increase and 58 metabolites decrease under submergence. The stress caused by submergence disrupted the electron transfer chain, leading to a reduction in photosynthetic rate. Additionally, the plant’s antioxidant system is triggered to manage reactive oxygen species and regulate their metabolisms | Xiong et al, |
Table 3. Metabolomic studies on flooding tolerance at the vegetative stage and stagnant flooding at the reproductive stage in rice.
| Analysis performed | Genotype | Stress level | Metabolite induced/detected | Reference |
|---|---|---|---|---|
| GC-MS and NMR | M202 (sensitive); M202-SUB1 (tolerant) | Seedlings at the 3-leaf stage were submerged for 0, 1, 2, and 3 d and then de-submerged for 1 d | GC-MS analysis exclusively identifies metabolites such as amino acids phenylalanine, proline, pyroglutamate, as well as TCA cycle intermediates like citrate, malate, and succinate. It also detects organic acids including GABA, glycerate, oxalate, shikimate, and threonate | Barding et al, |
| NMR, RPIP-UPLC-MS, GC-MS | M202 (sensitive); M202-SUB1 (tolerant) | 14-day-old seedlings were submerged for 3 d | The influence of SUB1 on metabolite levels, especially free amino acids, glucose, and sucrose, during the recovery period also affects the dynamics of trehalose-6-phosphate and the expression of mRNAs that encode crucial enzymes and signaling proteins | Locke et al, |
| LC-MS | Wufengyou 286 | 8 d submergence after panicle differentiation stage | On rice spikelets, 113 differential metabolites are detected when comparing submergence and control conditions, with 55 metabolites showing an increase and 58 metabolites decrease under submergence. The stress caused by submergence disrupted the electron transfer chain, leading to a reduction in photosynthetic rate. Additionally, the plant’s antioxidant system is triggered to manage reactive oxygen species and regulate their metabolisms | Xiong et al, |

Fig. 2. Germplasm bases include landraces native to submergence- prone regions, deep-water rice landraces (donors for stagnant flooding tolerance traits), released varieties (for submergence, semi- deep-water, and deep-water ecologies), wild relatives, and respective donors for component traits. A, Overall breeding strategy to develop vegetative-stage submergence and reproductive-stage stagnant flooding tolerant rice varieties involves the physiological, morphological, and molecular characterization of traits to identify component traits (easy to track across generations and simple to score) governing flooding responses. A high-throughput phenotyping platform can dissect component traits and use hyperspectral signatures to monitor stress and post-stress recovery. SUB1-dependent and -independent pathways can be explored to identify candidate genes/QTLs, which can then be used in marker-assisted backcross breeding (e.g., transferring SUB1A-1 alleles), gene pyramiding, haplotype-based selection, and marker- assisted introgression of alleles/genes from wild species. Reproductive-stage stagnant flood tolerance is influenced by multiple physiological traits; hence, a genomic selection (GS)- based breeding strategy (augmented with haplotype-assisted selection and environmental characterization) is deemed suitable. B, Proposed breeding strategy includes the evaluation of the germplasm base and the identification of donors for traits governing vegetative-stage sub- mergence tolerance and reproductive- stage stagnant flooding tolerance. Elite lines can be crossed with donors, and the accumulation of beneficial alleles can be achieved using a haplotype-based GS strategy (targeting Sub1-dependent and -independent pathways) for vegetative-stage sub- mergence and a multi-trait GS approach for reproductive-stage stagnant flooding tolerance. The identified elite lines can be used for field evaluation under stress in multi- location trials. GA, Gibberellic acid; GWAS, Genome- wide association study; ROS, Reactive oxygen species.
| Gene | RAP-DB ID | Function | Reference |
|---|---|---|---|
| OsRboh1 | Os01g0360200 | NADPH oxidase | Wu and Yang, |
| OsFLZ2 | Os01g0593200 | FCS-like zinc finger protein 2 | Ma et al, |
| OsACS5 | Os01g0192900 | 1-Aminocyclopropane-1-carboxylic acid synthase 5 | Zhou et al, |
| ALDH2a | Os02g0730000 | Mitochondrial aldehyde dehydrogenase | Nakazono et al, |
| OsCTP | Os02g0465900 | Vacuolar antiporter-regulating protein | Qi et al, |
| OsMPK3 | Os02g0148100 | TEY-type mitogen-activated protein kinase | Singh and Sinha, |
| OsABA8ox1 | Os02g0703600 | Similar to abscisic acid 8ʹ-hydroxylase 1; Response to submergence | Saika et al, |
| OsDWF4 | Os03g0227700 | Cytochrome P450 90B2, brassinosteroid C-22 hydroxylase | Schmitz et al, |
| OsERF66 | Os03g0341000 | ETHYLENE RESPONSE FACTOR 66 | Hsiao et al, |
| OsGAPDH | Os04g0486600 | Cytosolic glyceraldehyde-3-phosphate dehydrogenase 2 | Arumugam Pillai et al, |
| OsGID1 | Os05g0407500 | GIBBERELLIN-INSENSITIVE DWARF 1 (GID1) | Du et al, |
| OsERF67 | Os07g0674800 | ETHYLENE RESPONSE FACTOR 67 | Hsiao et al, |
| OsGGT | Os10g0555100 | Glycogenin glucosyltransferase | Qi et al, |
| OsARD1 | Os10g0419400 | Acireductone dioxygenase; SUBMERGENCE-INDUCED PROTEIN 2 | Liang et al, |
| OsDWF1 | Os10g0397400 | BRASSINOSTEROID DEFICIENT DWARF 2 | Schmitz et al, |
| OsMT1a | Os11g0704500 | Down-regulation promotes aerenchyma formation | Yamauchi et al, |
| OsSUB1A-1 | Absent in Reference ‘Nipponbare’ | Group VII ethylene response factors | Xu et al, |
Table 4. Cloned and characterized genes that can be targeted for haplotype-based selection and gene editing to generate vegetative-stage submergence tolerance in rice.
| Gene | RAP-DB ID | Function | Reference |
|---|---|---|---|
| OsRboh1 | Os01g0360200 | NADPH oxidase | Wu and Yang, |
| OsFLZ2 | Os01g0593200 | FCS-like zinc finger protein 2 | Ma et al, |
| OsACS5 | Os01g0192900 | 1-Aminocyclopropane-1-carboxylic acid synthase 5 | Zhou et al, |
| ALDH2a | Os02g0730000 | Mitochondrial aldehyde dehydrogenase | Nakazono et al, |
| OsCTP | Os02g0465900 | Vacuolar antiporter-regulating protein | Qi et al, |
| OsMPK3 | Os02g0148100 | TEY-type mitogen-activated protein kinase | Singh and Sinha, |
| OsABA8ox1 | Os02g0703600 | Similar to abscisic acid 8ʹ-hydroxylase 1; Response to submergence | Saika et al, |
| OsDWF4 | Os03g0227700 | Cytochrome P450 90B2, brassinosteroid C-22 hydroxylase | Schmitz et al, |
| OsERF66 | Os03g0341000 | ETHYLENE RESPONSE FACTOR 66 | Hsiao et al, |
| OsGAPDH | Os04g0486600 | Cytosolic glyceraldehyde-3-phosphate dehydrogenase 2 | Arumugam Pillai et al, |
| OsGID1 | Os05g0407500 | GIBBERELLIN-INSENSITIVE DWARF 1 (GID1) | Du et al, |
| OsERF67 | Os07g0674800 | ETHYLENE RESPONSE FACTOR 67 | Hsiao et al, |
| OsGGT | Os10g0555100 | Glycogenin glucosyltransferase | Qi et al, |
| OsARD1 | Os10g0419400 | Acireductone dioxygenase; SUBMERGENCE-INDUCED PROTEIN 2 | Liang et al, |
| OsDWF1 | Os10g0397400 | BRASSINOSTEROID DEFICIENT DWARF 2 | Schmitz et al, |
| OsMT1a | Os11g0704500 | Down-regulation promotes aerenchyma formation | Yamauchi et al, |
| OsSUB1A-1 | Absent in Reference ‘Nipponbare’ | Group VII ethylene response factors | Xu et al, |
| [1] | Adak M K, Ghosh N, Dasgupta D K, et al. 2011. Impeded carbohydrate metabolism in rice plants under submergence stress. Rice Sci, 18(2): 116-126. |
| [2] | Akintayo O, Daniel I, Afeez S, et al. 2023. qSUB2: A novel QTL with positive epistasis with SUB1 locus enhances submergence tolerance in rice. Crop Sci, 63(3): 1246-1256. |
| [3] | Anandan A, Kumar Pradhan S, Kumar Das S, et al. 2015. Differential responses of rice genotypes and physiological mechanism under prolonged deepwater flooding. Field Crops Res, 172: 153-163. |
| [4] | Arumugam Pillai M, Lihuang Z, Akiyama T. 2002. Molecular cloning, characterization, expression and chromosomal location of OsGAPDH, a submergence responsive gene in rice (Oryza sativa L.). Theor Appl Genet, 105(1): 34-42. |
| [5] | Atwell B J, Wang H, Scafaro A P. 2014. Could abiotic stress tolerance in wild relatives of rice be used to improve Oryza sativa? Plant Sci, 215/216: 48-58. |
| [6] | Ayano M, Kani T, Kojima M, et al. 2014. Gibberellin biosynthesis and signal transduction is essential for internode elongation in deepwater rice. Plant Cell Environ, 37(10): 2313-2324. |
| [7] | Azharudheen T P, Sah R P, Moharana D, et al. 2021. Genetic improvement of rice for lowlands. In: Advances in Rice Breeding: Stress Tolerance, Climate Resilience, Quality & High Yield. Odisha, India: ICAR-National Rice Research Institute: 63-85. |
| [8] | Bailey-Serres J, Fukao T, Ronald P, et al. 2010. Submergence tolerant rice: SUB1’s journey from landrace to modern cultivar. Rice, 3(2): 138-147. |
| [9] | Baishakhy S D, Islam M A, Kamruzzaman M. 2023. Overcoming barriers to adapt rice farming to recurring flash floods in haor wetlands of Bangladesh. Heliyon, 9(3): e14011. |
| [10] | Barding Jr G A, Béni S, Fukao T, et al. 2013. Comparison of GC-MS and NMR for metabolite profiling of rice subjected to submergence stress. J Proteome Res, 12(2): 898-909. |
| [11] | Barik J, Kumar V, Lenka S K, et al. 2020. Assessment of variation in morpho-physiological traits and genetic diversity in relation to submergence tolerance of five indigenous lowland rice landraces. Rice Sci, 27(1): 32-43. |
| [12] | Basu S, Kumar G, Kumari N, et al. 2020. Reactive oxygen species and reactive nitrogen species induce lysigenous aerenchyma formation through programmed cell death in rice roots under submergence. Environ Exp Bot, 177: 104118. |
| [13] | Basu S, Monika, Kumari S, et al. 2024. Sub1 QTL confers submergence tolerance in rice through nitro-oxidative regulation and phytohormonal signaling. Plant Physiol Biochem, 211: 108682. |
| [14] | Chakraborty K, Ray S, Vijayan J, et al. 2021. Preformed aerenchyma determines the differential tolerance response under partial submergence imposed by fresh and saline water flooding in rice. Physiol Plant, 173(4): 1597-1615. |
| [15] | Chattopadhyay K, Chakraborty K, Samal P, et al. 2021. Identification of QTLs for stagnant flooding tolerance in rice employing genotyping by sequencing of a RIL population derived from Swarna × Rashpanjor. Physiol Mol Biol Plants, 27(12): 2893-2909. |
| [16] | Chen Y, Chen Y H, Zhang Y J, et al. 2021. Heterotrimeric G protein γ subunit DEP1 is involved in hydrogen peroxide signaling and promotes aerenchyma formation in rice roots. Plant Signal Behav, 16(5): 1889251. |
| [17] | Cho S H, Yoo S C, Zhang H T, et al. 2014. Rice NARROW LEAF1 regulates leaf and adventitious root development. Plant Mol Biol Rep, 32(1): 270-281. |
| [18] | Colmer T D, Kotula L, Malik A I, et al. 2019. Rice acclimation to soil flooding: Low concentrations of organic acids can trigger a barrier to radial oxygen loss in roots. Plant Cell Environ, 42(7): 2183-2197. |
| [19] | Coumou D, Rahmstorf S. 2012. A decade of weather extremes. Nat Clim Chang, 2(7): 491-496. |
| [20] | Das K K, Panda D, Sarkar R K, et al. 2009. Submergence tolerance in relation to variable floodwater conditions in rice. Environ Exp Bot, 66(3): 425-434. |
| [21] | Du H, Chang Y, Huang F, et al. 2015. GID1 modulates stomatal response and submergence tolerance involving abscisic acid and gibberellic acid signaling in rice. J Integr Plant Biol, 57(11): 954-968. |
| [22] | Ejiri M, Fukao T, Miyashita T, et al. 2021. A barrier to radial oxygen loss helps the root system cope with waterlogging-induced hypoxia. Breed Sci, 71(1): 40-50. |
| [23] | Fukao T, Bailey-Serres J. 2008. Submergence tolerance conferred by Sub1A is mediated by SLR1 and SLRL1 restriction of gibberellin responses in rice. Proc Natl Acad Sci USA, 105(43): 16814-16819. |
| [24] | Fukao T, Yeung E, Bailey-Serres J. 2011. The submergence tolerance regulator SUB1A mediates crosstalk between submergence and drought tolerance in rice. Plant Cell, 23(1): 412-427. |
| [25] | Ganie S A, Ahammed G J, Wani S H. 2020. Vascular plant one zinc-finger (VOZ) transcription factors: Novel regulators of abiotic stress tolerance in rice (Oryza sativa L.). Genet Resour Crop Evol, 67(4): 799-807. |
| [26] | Gao C M, Ding L, Li Y R, et al. 2017. Nitrate increases ethylene production and aerenchyma formation in roots of lowland rice plants under water stress. Funct Plant Biol, 44(4): 430-442. |
| [27] | Garg R, Verma M, Agrawal S, et al. 2014. Deep transcriptome sequencing of wild halophyte rice, Porteresia coarctata, provides novel insights into the salinity and submergence tolerance factors. DNA Res, 21( 1): 69-84. |
| [28] | Gonzaga Z J C, Carandang J, Sanchez D L, et al. 2016. Mapping additional QTLs from FR13A to increase submergence tolerance in rice beyond SUB1. Euphytica, 209(3): 627-636. |
| [29] | Gonzaga Z J C, Carandang J, Singh A, et al. 2017. Mapping QTLs for submergence tolerance in rice using a population fixed for SUB1A tolerant allele. Mol Breed, 37(4): 47. |
| [30] | Gopala Krishnan S, Vinod K K, Bhowmick P K, et al. 2022. Rice breeding. In: Fundamentals of Field Crop Breeding. Singapore: Springer Nature Singapore: 113-220. |
| [31] | Goswami S, Kar R K, Paul A, et al. 2017. Genetic potentiality of indigenous rice genotypes from Eastern India with reference to submergence tolerance and deepwater traits. Curr Plant Biol, 11: 23-32. |
| [32] | Gujjar R S, Supaibulwatana K, Srivastava S, et al. 2024. Regulation of stress-responsive transcription factors of rice by CPPU, a synthetic cytokinin, during water deficit stress at protein level. Cereal Res Commun, https://doi.org/10.1007/s42976-024-00540-4. |
| [33] | Haque M A, Rafii M Y, Yusoff M M, et al. 2023. Flooding tolerance in rice: Adaptive mechanism and marker-assisted selection breeding approaches. Mol Biol Rep, 50(3): 2795-2812. |
| [34] | Hattori Y, Nagai K, Furukawa S, et al. 2009. The ethylene response factors SNORKEL1 and SNORKEL2 allow rice to adapt to deep water. Nature, 460: 1026-1030. |
| [35] | Hattori Y, Nagai K, Ashikari M. 2011. Rice growth adapting to deepwater. Curr Opin Plant Biol, 14(1): 100-105. |
| [36] | Hossain M, Biswas P, Islam M R. 2023. Cold-tolerant and short- duration rice (Oryza sativa L.) for sustainable food security of the flash flood-prone haor wetlands of Bangladesh. Sustainability, 15(24): 16873. |
| [37] | Hsiao P Y, Zeng C Y, Shih M C. 2024. Group VII ethylene response factors forming distinct regulatory loops mediate submergence responses. Plant Physiol, 194(3): 1745-1763. |
| [38] | Hussain W, Anumalla M, Ismail A M, et al. 2024. Revisiting FR13A for submergence tolerance: Beyond the SUB1A gene. J Exp Bot, 75(18): 5477-5483. |
| [39] | Iftekharuddaula K M, Ghosal S, Gonzaga Z J, et al. 2016. Allelic diversity of newly characterized submergence-tolerant rice (Oryza sativa L.) germplasm from Bangladesh. Genet Resour Crop Evol, 63(5): 859-867. |
| [40] | Ismail A M, Johnson D E, Ella E S, et al. 2012. Adaptation to flooding during emergence and seedling growth in rice and weeds, and implications for crop establishment. AoB Plants, 2012: pls019. |
| [41] | Ismail A M, Singh U S, Singh S, et al. 2013. The contribution of submergence-tolerant (Sub1) rice varieties to food security in flood-prone rainfed lowland areas in Asia. Field Crops Res, 152: 83-93. |
| [42] | Kato Y, Collard B C Y, Septiningsih E M, et al. 2019. Increasing flooding tolerance in rice: Combining tolerance of submergence and of stagnant flooding. Ann Bot, 124(7): 1199-1209. |
| [43] | Kawano N, Ito O, Sakagami J I. 2009. Morphological and physiological responses of rice seedlings to complete submergence (flash flooding). Ann Bot, 103(2): 161-169. |
| [44] | Kende H, van der Knaap E, Cho H T. 1998. Deepwater rice: A model plant to study stem elongation. Plant Physiol, 118(4): 1105-1110. |
| [45] | Khasna E N, Ardana I K K G, Zakiyah A S, et al. 2020. Sub1A gene screening for submergence stress in Indonesian local rice varieties. AIP Conf Proc, 2260(1): 060012. |
| [46] | Kuanar S R, Ray A, Sethi S K, et al. 2017. Physiological basis of stagnant flooding tolerance in rice. Rice Sci, 24(2): 73-84. |
| [47] | Kuanar S R, Molla K A, Chattopadhyay K, et al. 2019. Introgression of Sub1 (SUB1) QTL in mega rice cultivars increases ethylene production to the detriment of grain-filling under stagnant flooding. Sci Rep, 9(1): 18567. |
| [48] | Kulichikhin K, Yamauchi T, Watanabe K, et al. 2014. Biochemical and molecular characterization of rice (Oryza sativa L.) roots forming a barrier to radial oxygen loss. Plant Cell Environ, 37(10): 2406-2420. |
| [49] | Kumar A, Nayak A K, Hanjagi P S, et al. 2021. Submergence stress in rice: Adaptive mechanisms, coping strategies and future research needs. Environ Exp Bot, 186: 104448. |
| [50] | Kuroha T, Ashikari M. 2020. Molecular mechanisms and future improvement of submergence tolerance in rice. Mol Breed, 40(4): 41. |
| [51] | Lee H S, Hwang W H, Jeong J H, et al. 2019. Analysis of the distribution of assimilation products and the characteristics of transcriptomes in rice by submergence during the ripening stage. BMC Genomics, 20(1): 18. |
| [52] | Lian B Y, Wu A M, Wu H M, et al. 2024. GhVOZ1-AVP1 module positively regulates salt tolerance in upland cotton (Gossypium hirsutum L.). Int J Biol Macromol, 258(Pt 2): 129116. |
| [53] | Liang S S, Xiong W, Yin C C, et al. 2019. Overexpression of OsARD1 improves submergence, drought, and salt tolerances of seedling through the enhancement of ethylene synthesis in rice. Front Plant Sci, 10: 1088. |
| [54] | Lin C, Sauter M. 2019. Polar auxin transport determines adventitious root emergence and growth in rice. Front Plant Sci, 10: 444. |
| [55] | Lin C C, Lee W J, Zeng C Y, et al. 2023. SUB1A-1 anchors a regulatory cascade for epigenetic and transcriptional controls of submergence tolerance in rice. PNAS Nexus, 2(7): pgad229. |
| [56] | Liu H J, Wang S F, Yu X B, et al. 2005. ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J, 43(1): 47-56. |
| [57] | Liu J, Hasanuzzaman M, Sun H Z, et al. 2020. Comparative morphological and transcriptomic responses of lowland and upland rice to root-zone hypoxia. Environ Exp Bot, 169: 103916. |
| [58] | Liu S P, Wang J R, Wang L, et al. 2009. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res, 19(9): 1110-1119. |
| [59] | Locke A M, Barding Jr G A, Sathnur S, et al. 2018. Rice SUB1A constrains remodelling of the transcriptome and metabolome during submergence to facilitate post-submergence recovery. Plant Cell Environ, 41(4): 721-736. |
| [60] | Ma Y M, Zhao J L, Fu H, et al. 2021. Genome-wide identification, expression and functional analysis reveal the involvement of FCS-like zinc finger gene family in submergence response in rice. Rice, 14(1): 76. |
| [61] | Mhimdi M, Pérez-Pérez J M. 2020. Understanding of adventitious root formation: What can we learn from comparative genetics? Front Plant Sci, 11: 582020. |
| [62] | Mittal L, Tayyeba S, Sinha A K. 2022. Finding a breather for Oryza sativa: Understanding hormone signalling pathways involved in rice plants to submergence stress. Plant Cell Environ, 45(2): 279-295. |
| [63] | Mohammed U, Caine R S, Atkinson J A, et al. 2019. Rice plants overexpressing OsEPF1 show reduced stomatal density and increased root cortical aerenchyma formation. Sci Rep, 9(1): 5584. |
| [64] | Mohanty H K, Mallik S, Grover A. 2000. Prospects of improving flooding tolerance in lowland rice varieties by conventional breeding and genetic engineering. Curr Sci, 78(2): 132-137. |
| [65] | Mondal S, Khan M I R, Dixit S, et al. 2020. Growth, productivity and grain quality of AG1 and AG2 QTLs introgression lines under flooding in direct-seeded rice system. Field Crops Res, 248: 107713. |
| [66] | Mori Y, Kurokawa Y, Koike M, et al. 2019. Diel O2 dynamics in partially and completely submerged deepwater rice: Leaf gas films enhance internodal O2 status, influence gene expression and accelerate stem elongation for ‘snorkelling’ during submergence. Plant Cell Physiol, 60(5): 973-985. |
| [67] | Mwakyusa L, Dixit S, Herzog M, et al. 2023. Flood-tolerant rice for enhanced production and livelihood of smallholder farmers of Africa. Front Sustain Food Syst, 7: 1244460. |
| [68] | Nagai K, Hattori Y, Ashikari M. 2010. Stunt or elongate? Two opposite strategies by which rice adapts to floods. J Plant Res, 123(3): 303-309. |
| [69] | Nagai K, Kuroha T, Ayano M, et al. 2012. Two novel QTLs regulate internode elongation in deepwater rice during the early vegetative stage. Breed Sci, 62(2): 178-185. |
| [70] | Nagai K, Mori Y, Ishikawa S, et al. 2020. Antagonistic regulation of the gibberellic acid response during stem growth in rice. Nature, 584: 109-114. |
| [71] | Nakazono M, Tsuji H, Li Y, et al. 2000. Expression of a gene encoding mitochondrial aldehyde dehydrogenase in rice increases under submerged conditions. Plant Physiol, 124(2): 587-598. |
| [72] | Nandi S, Subudhi P K, Senadhira D, et al. 1997. Mapping QTLs for submergence tolerance in rice by AFLP analysis and selective genotyping. Mol Gen Genet, 255(1): 1-8. |
| [73] | Niroula R K, Pucciariello C, Ho V T, et al. 2012. SUB1A-dependent and -independent mechanisms are involved in the flooding tolerance of wild rice species. Plant J, 72(2): 282-293. |
| [74] | Nishiuchi S, Watanabe K, Sato S, et al. 2021. Expression analysis of genes for cytochrome P450 CYP86 and glycerol-3-phosphate acyltransferase related to suberin biosynthesis in rice roots under stagnant deoxygenated conditions. Plant Root, 15: 19-35. |
| [75] | Oe S, Sasayama D, Luo Q S, et al. 2022. Growth responses of seedlings under complete submergence in rice cultivars carrying both the submergence-tolerance gene SUB1A-1 and the floating genes SNORKELs. Plant Prod Sci, 25(1): 70-77. |
| [76] | Oladosu Y, Rafii M Y, Arolu F, et al. 2020. Submergence tolerance in rice: Review of mechanism, breeding and, future prospects. Sustainability, 12(4): 1632. |
| [77] | Panda D, Barik J. 2021. Flooding tolerance in rice: Focus on mechanisms and approaches. Rice Sci, 28(1): 43-57. |
| [78] | Panja S, Mondal K, Kar R K, et al. 2023. Exploration of ready-to- eat soft Bora rice genotypes of Assam for submergence tolerance. J Crop Sci Biotechnol, 26(1): 87-95. |
| [79] | Parlanti S, Kudahettige N P, Lombardi L, et al. 2011. Distinct mechanisms for aerenchyma formation in leaf sheaths of rice genotypes displaying a quiescence or escape strategy for flooding tolerance. Ann Bot, 107(8): 1335-1343. |
| [80] | Pedersen O, Rich S M, Colmer T D. 2009. Surviving floods: Leaf gas films improve O2 and CO2 exchange, root aeration, and growth of completely submerged rice. Plant J, 58(1): 147-156. |
| [81] | Phukan U J, Jindal S, Laldinsangi C, et al. 2024. A microscopic scenario on recovery mechanisms under waterlogging and submergence stress in rice. Planta, 259(1): 9. |
| [82] | Prakash N R, Lokeshkumar B M, Rathor S, et al. 2024. Mechanisms of saline and submergence tolerance in rice for coastal ecology. In: Singh R K, Prakash M, Gautam R K, et al. Genetic Improvement of Rice for Salt Tolerance. Singapore: Springer Nature Singapore: 231-256. |
| [83] | Puckridge D W, Kupkanchanakul T, Palaklang W, et al. 2001. Production of rice and associated crops in deeply flooded areas of Chao Phraya Delta. In: Proceedings of the 2000 International Conference: The Chao Phraya Delta: Historical Development, Dynamics and Challenges of Thailand Rice Bowl. December 12-15. Bangkok, Thailand: Kasetsart University: 51-85. |
| [84] | Qi Y H, Yamauchi Y, Ling J Q, et al. 2005a. The submergence- induced gene OsCTP in rice (Oryza sativa L.) is similar to Escherichia coli cation transport protein ChaC. Plant Sci, 168(1): 15-22. |
| [85] | Qi Y H, Kawano N, Yamauchi Y, et al. 2005b. Identification and cloning of a submergence-induced gene OsGGT (glycogenin glucosyltransferase) from rice (Oryza sativa L.) by suppression subtractive hybridization. Planta, 221(3): 437-445. |
| [86] | Reddy J N, Sarkar R K, Patnaik S S C, et al. 2009. Improvement of rice germplasm for rainfed lowlands of eastern India. SABRAO J Breed Genet, 41: 1-5. |
| [87] | Rohilla M, Mazumder A, Chowdhury D, et al. 2024. Understanding natural genetic variation for nutritional quality in grain and identification of superior haplotypes in deepwater rice genotypes of Assam, India. Gene, 928: 148801. |
| [88] | Saika H, Okamoto M, Miyoshi K, et al. 2007. Ethylene promotes submergence-induced expression of OsABA8ox1, a gene that encodes ABA 8ʹ-hydroxylase in rice. Plant Cell Physiol, 48(2): 287-298. |
| [89] | Samal R, Roy P S, Sahoo A, et al. 2018. Morphological and molecular dissection of wild rices from eastern India suggests distinct speciation between O. rufipogon and O. nivara populations. Sci Rep, 8(1): 2773. |
| [90] | Samanta P, Ganie S A, Chakraborty A, et al. 2021. Study on regulation of carbohydrate usage in a heterogeneous rice population under submergence. J Plant Biochem Biotechnol, 30(1): 138-146. |
| [91] | Samanta P, Chakrabarti A, Dey N. 2022. Study on physiological responses with allelic diversity of Sub1A and SK loci in rice seedlings under complete submergence. Plant Physiol Rep, 27(2): 275-281. |
| [92] | Sarić R, Nguyen V D, Burge T, et al. 2022. Applications of hyperspectral imaging in plant phenotyping. Trends Plant Sci, 27(3): 301-315. |
| [93] | Sarkar R K, Bhattacharjee B. 2011. Rice genotypes with SUB1 QTL differ in submergence tolerance, elongation ability during submergence and re-generation growth at re-emergence. Rice, 5(1): 7. |
| [94] | Sarkar R K, Reddy J N, Das S R. 2021. Molecular breeding for improving flooding tolerance in rice: Recent progress and future perspectives. In: Hassain M A, Hassan L, Md Ifterkharuddaula K, et al. Molecular Breeding for Rice Abiotic Stress Tolerance and Nutritional Quality. Chichester, West Sussex, UK: Wilery Blackwell: 75-91. |
| [95] | Schmitz A J, Folsom J J, Jikamaru Y, et al. 2013. SUB1A-mediated submergence tolerance response in rice involves differential regulation of the brassinosteroid pathway. New Phytol, 198(4): 1060-1070. |
| [96] | Septiningsih E M, Pamplona A M, Sanchez D L, et al. 2009. Development of submergence-tolerant rice cultivars: The Sub1 locus and beyond. Ann Bot, 103(2): 151-160. |
| [97] | Septiningsih E M, Sanchez D L, Singh N, et al. 2012. Identifying novel QTLs for submergence tolerance in rice cultivars IR72 and Madabaru. Theor Appl Genet, 124(5): 867-874. |
| [98] | Sharma N, Dang T M, Singh N, et al. 2018. Allelic variants of OsSUB1A cause differential expression of transcription factor genes in response to submergence in rice. Rice, 11(1): 2. |
| [99] | Singh A, Septiningsih E M, Balyan H S, et al. 2017a. Genetics, physiological mechanisms and breeding of flood-tolerant rice (Oryza sativa L.). Plant Cell Physiol, 58(2): 185-197. |
| [100] | Singh A, Carandang J, Gonzaga Z J C, et al. 2017b. Identification of QTLs for yield and agronomic traits in rice under stagnant flooding conditions. Rice, 10(1): 15. |
| [101] | Singh A, Singh Y, Mahato A K, et al. 2020. Allelic sequence variation in the Sub1A, Sub1B and Sub1C genes among diverse rice cultivars and its association with submergence tolerance. Sci Rep, 10(1): 8621. |
| [102] | Singh P, Sinha A K. 2016. A positive feedback loop governed by SUB1A1 interaction with MITOGEN-ACTIVATED PROTEIN KINASE3 imparts submergence tolerance in rice. Plant Cell, 28(5): 1127-1143. |
| [103] | Singh R, Singh Y, Xalaxo S, et al. 2016. From QTL to variety- harnessing the benefits of QTLs for drought, flood and salt tolerance in mega rice varieties of India through a multi-institutional network. Plant Sci, 242: 278-287. |
| [104] | Sripongpangkul K, Posa G B T, Senadhira D W, et al. 2000. Genes/ QTLs affecting flood tolerance in rice. Theor Appl Genet, 101(7): 1074-1081. |
| [105] | Steffens B, Geske T, Sauter M. 2011. Aerenchyma formation in the rice stem and its promotion by H2O2. New Phytol, 190(2): 369-378. |
| [106] | Sun H W, Tao J Y, Hou M M, et al. 2015. A strigolactone signal is required for adventitious root formation in rice. Ann Bot, 115(7): 1155-1162. |
| [107] | Tang D Q, Kasai Y, Miyamoto N, et al. 2005. Comparison of QTLs for early elongation ability between two floating rice cultivars with a different phylogenetic origin. Breed Sci, 55(1): 1-5. |
| [108] | Toojinda T, Siangliw M, Tragoonrung S, et al. 2003. Molecular genetics of submergence tolerance in rice: QTL analysis of key traits. Ann Bot, 91(2): 243-253. |
| [109] | Viana V E, Marini N, Busanello C, et al. 2018. Regulation of rice responses to submergence by WRKY transcription factors. Biol Plant, 62(3): 551-560. |
| [110] | Villacastin A J, Adams K S, Boonjue R, et al. 2021. Dynamic differential evolution schemes of WRKY transcription factors in domesticated and wild rice. Sci Rep, 11: 14887. |
| [111] | Wang J, Han M Z, Huang Y X, et al. 2024. Flooding tolerance of rice: Regulatory pathways and adaptive mechanisms. Plants, 13(9): 1178. |
| [112] | Winkel A, Pedersen O, Ella E, et al. 2014. Gas film retention and underwater photosynthesis during field submergence of four contrasting rice genotypes. J Exp Bot, 65(12): 3225-3233. |
| [113] | Wu Y S, Yang C Y. 2016. Physiological responses and expression profile of NADPH oxidase in rice (Oryza sativa) seedlings under different levels of submergence. Rice, 9(1): 2. |
| [114] | Xiong H Y, Li Y, Yang J, et al. 2012. Comparative transcriptional profiling of two rice genotypes carrying SUB1A-1 but exhibiting differential tolerance to submergence. Funct Plant Biol, 39(6): 449-461. |
| [115] | Xiong Q Q, Cao C H, Shen T H, et al. 2019. Comprehensive metabolomic and proteomic analysis in biochemical metabolic pathways of rice spikes under drought and submergence stress. Biochim Biophys Acta Proteins Proteomics, 1867(3): 237-247. |
| [116] | Xu K N, Xu X, Fukao T, et al. 2006. Sub1A is an ethylene-response- factor-like gene that confers submergence tolerance to rice. Nature, 442: 705-708. |
| [117] | Xu M, Zhu L, Shou H X, et al. 2005. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol, 46(10): 1674-1681. |
| [118] | Yamauchi T, Fukazawa A, Nakazono M. 2017a. METALLOTHIONEIN genes encoding ROS scavenging enzymes are down-regulated in the root cortex during inducible aerenchyma formation in rice. Plant Signal Behav, 12(11): e1388976. |
| [119] | Yamauchi T, Yoshioka M, Fukazawa A, et al. 2017b. An NADPH oxidase RBOH functions in rice roots during lysigenous aerenchyma formation under oxygen-deficient conditions. Plant Cell, 29(4): 775-790. |
| [120] | Yang S Y, Wu Y S, Chen C T, et al. 2017. Physiological and molecular responses of seedlings of an upland rice (‘Tung Lu 3’) to total submergence compared to those of a submergence- tolerant lowland rice (‘FR13A’). Rice, 10(1): 42. |
| [121] | Zhou Z Y, Vriezen W, van Caeneghem W, et al. 2001. Rapid induction of a novel ACC synthase gene in deepwater rice seedlings upon complete submergence. Euphytica, 121(2): 137-143. |
| [122] | Zhu C H, Qi Q, Niu H J, et al. 2021. γ-Aminobutyric acid suppresses iron transportation from roots to shoots in rice seedlings by inducing aerenchyma formation. Int J Mol Sci, 22(1): 220. |
| [123] | Zhu W C, Li W F, Zhang H W, et al. 2024. Big data and artificial intelligence-aided crop breeding: Progress and prospects. J Integr Plant Biol, https://doi.org/10.1111/jipb.13791. |
| [124] | Zhu Y, Su H, Liu X X, et al. 2024. Identification of NADPH oxidase genes crucial for rice multiple disease resistance and yield traits. Rice, 17(1): 1. |
| [1] | Hao Zhiqi, Wang Tingyi, Chen Dongdong, Shen Lan, Zhang Guangheng, Qian Qian, Zhu Li. Leucine-Rich Repeat Protein Family Regulates Stress Tolerance and Development in Plants [J]. Rice Science, 2025, 32(1): 32-43. |
| [2] | Liang Liang, Wang Chenchang, Chen Tao. Advances in Understanding Cadmium Stress and Breeding of Cadmium-Tolerant Crops [J]. Rice Science, 2024, 31(5): 507-525. |
| [3] | Jiang Changjie, Liang Zhengwei, Xie Xianzhi. Priming for Saline-Alkaline Tolerance in Rice: Current Knowledge and Future Challenges [J]. Rice Science, 2023, 30(5): 417-425. |
| [4] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [5] | Hyeran Moon, Young-Ah Kim, Ryoung Shin, Chang-Jin Park. Nucleus-Encoded Thylakoid Protein, OsY3IP1, Confers Enhanced Tolerance to Saline and Alkaline Stresses in Rice [J]. Rice Science, 2022, 29(3): 225-236. |
| [6] | Dan Zeng, Chunchao Wang, Junpin Xie, Fan Zhang, Jialing Lu, Xiaorong Shi, Yingyao Shi, Yongli Zhou. Stress-Activated Protein Kinase OsSAPK7 Regulates Salt- Stress Tolerance by Modulating Diverse Stress-Defensive Responses in Rice [J]. Rice Science, 2021, 28(6): 547-556. |
| [7] | Panda Debabrata, Barik Jijnasa. Flooding Tolerance in Rice: Focus on Mechanisms and Approaches [J]. Rice Science, 2021, 28(1): 43-57. |
| [8] | Yanchang Luo, Tingchen Ma, Teo Joanne, Zhixiang Luo, Zefu Li, Jianbo Yang, Zhongchao Yin. Marker-Assisted Breeding of Thermo-Sensitive Genic Male Sterile Line 1892S for Disease Resistance and Submergence Tolerance [J]. Rice Science, 2021, 28(1): 89-98. |
| [9] | Ning Xiao, Yunyu Wu, Aihong Li. Strategy for Use of Rice Blast Resistance Genes in Rice Molecular Breeding [J]. Rice Science, 2020, 27(4): 263-277. |
| [10] | Barik Jijnasa, Kumar Vajinder, K. Lenka Sangram, Panda Debabrata. Assessment of Variation in Morpho-Physiological Traits and Genetic Diversity in Relation to Submergence Tolerance of Five Indigenous Lowland Rice Landraces [J]. Rice Science, 2020, 27(1): 32-43. |
| [11] | Md Iftekharuddaula Khandakar, Uddin Ahmed Helal, Ghosal Sharmistha, Rahman Moni Zakiah, Amin Al, Shamsher Ali Md. Development of New Submergence Tolerant Rice Variety for Bangladesh Using Marker-Assisted Backcrossing [J]. Rice Science, 2015, 22(1): 16-26. |
| [12] | CHEN Li-yun, XIAO Ying-hui, LEI Dong-yang. Mechanism of Sterility and Breeding Strategies for Photoperiod/Thermo- Sensitive Genic Male Sterile Rice [J]. RICE SCIENCE, 2010, 17(3): 161-167 . |
| [13] | JIANG Jing, ZHUANG Jie-yun, FAN Ye-yang, SHEN Bo. Mapping of QTLs for Leaf Malondialdehyde Content Associated with Stress Tolerance in Rice [J]. RICE SCIENCE, 2009, 16(1): 72-74 . |
| [14] | CHEN Li-yun, XIAO Ying-hui, TANG Wen-bang, LEI Dong-yang. Practices and Prospects of Super Hybrid Rice Breeding [J]. RICE SCIENCE, 2007, 14(2): 71-77 . |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||