
Rice Science ›› 2026, Vol. 33 ›› Issue (1): 39-58.DOI: 10.1016/j.rsci.2025.10.010
• Reviews • Previous Articles Next Articles
Huang Qina1( ), Wu Lijuan1, Jiang Hongrui1, He Yan2, Liu Song3, Yang Changdeng1, Liang Yan1(
), Wu Lijuan1, Jiang Hongrui1, He Yan2, Liu Song3, Yang Changdeng1, Liang Yan1( )
)
Received:2025-08-01
Accepted:2025-09-25
Online:2026-01-28
Published:2026-02-03
Contact:
Huang Qina (About author:First author contact:# These authors contributed equally to this work
Huang Qina, Wu Lijuan, Jiang Hongrui, He Yan, Liu Song, Yang Changdeng, Liang Yan. NRAMPs: Versatile Transporters Involved in Metal Ion Homeostasis and Their Applications in Rice Breeding[J]. Rice Science, 2026, 33(1): 39-58.
Add to citation manager EndNote|Ris|BibTeX

Fig. 1. Phylogenetic relationships, gene structures, predicted phosphorylation sites, and promoter elements of OsNRAMP family. A, Evolutionary relationships among OsNRAMP transporters were inferred using the neighbor-joining method without distance correction (1 000 bootstrap replicates). Two sister groups were identified: one containing OsNRAMP1 and OsNRAMP5, and the other containing OsNRAMP2 and OsNRAMP7, both sharing a common ancestor. OsNRAMP3, OsNRAMP4, and OsNRAMP6 were found to share an ancestral lineage with OsNRAMP1 and OsNRAMP5. CDS, Coding sequence; UTR, Untranslated region. B, The highest predicted phosphorylation levels in OsNRAMP1, OsNRAMP5, and OsNRAMP6 occur between amino acid positions 251 and 350. In contrast, OsNRAMP2, OsNRAMP3, and OsNRAMP7 show peak phosphorylation within the first 100 amino acids (positions 1‒100 aa). C, Multiple functional cis-regulatory elements are present, including hormone-responsive motifs such as ABRE (abscisic acid), CGTCA/TGACG (methyl jasmonate), TCA-element (salicylic acid), GARE/P-box (gibberellic acid), and TGA-element (auxin). Stress-related elements include STRE (stress), WUN-motif (wound), LTR (cold), MYB/MYC (drought), and ARE (anaerobic induction).
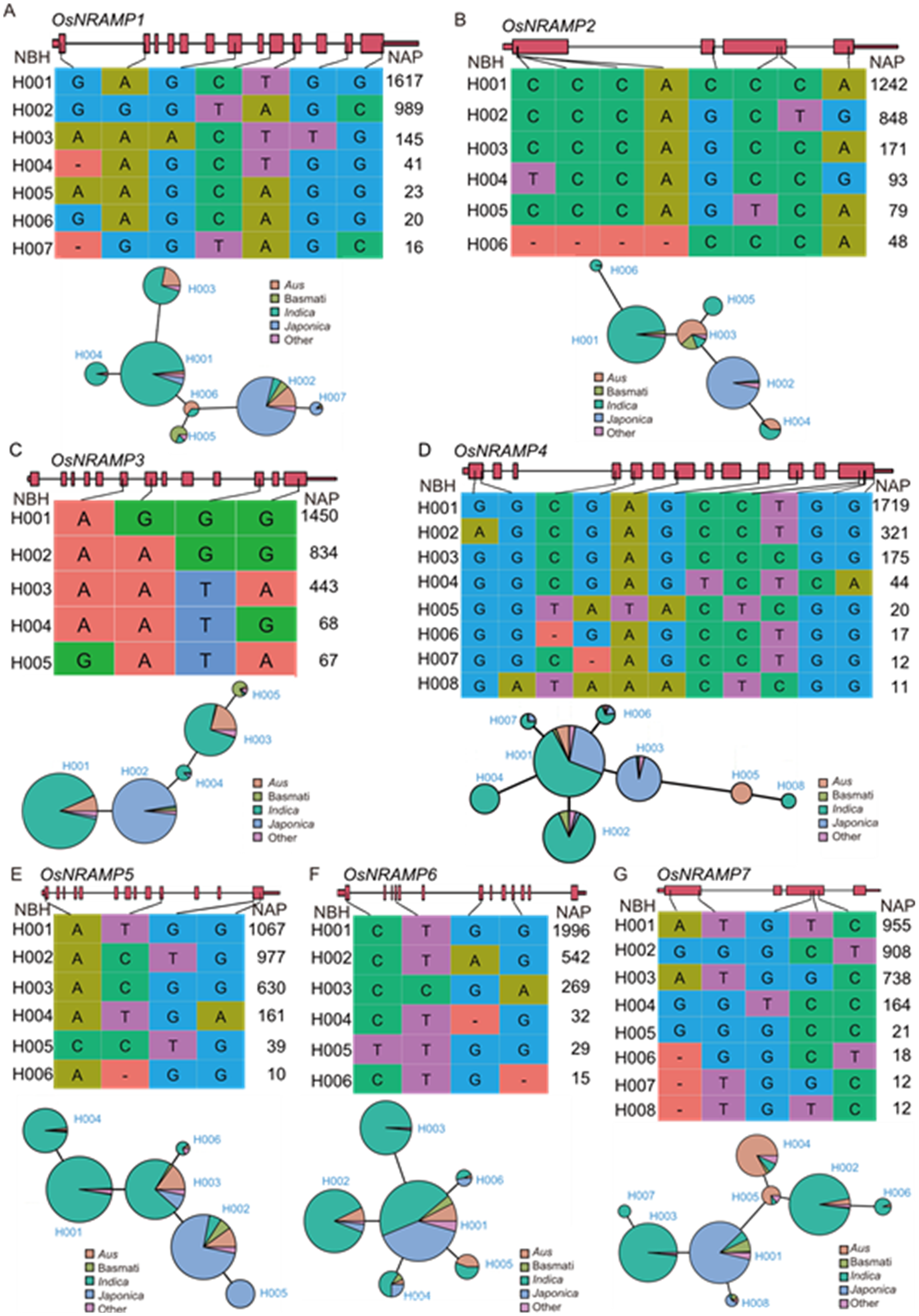
Fig. 2. Haplotype analysis of OsNRAMP1‒7 across 3K Rice Genome (RG) panel. A, OsNRAMP1, a key gene involved in multi-ion transport, exhibits seven haplotypes in the 3K RG panel, showing extensive phenotypic variation. B, E, and F, OsNRAMP2, OsNRAMP5, and OsNRAMP6, which are involved in iron (Fe) and manganese (Mn) metabolism, each display six haplotypes. C, OsNRAMP3, which facilitates Mn2+ translocation, has five haplotypes, with 1 450 accessions carrying the OsNRAMP3-H001 haplotype. D and G, OsNRAMP4 and OsNRAMP7 each show eight haplotypes. NBH, Nucleotide-based haplotype; NAP, Number of accessions in the 3K RG panel. ‘-’ indicates a base deletion.
| Protein | Subcellular localization | Spatiotemporal expression | Transport metal ion | Inducible expressiona | Suppressed expressiona | Function | Reference |
|---|---|---|---|---|---|---|---|
| OsNRAMP1 | Plasma membrane | Mainly expressed in roots and leaves | Mn2+, Ni2+, Cd2+, Pb2+, etc. | -Fe, +Cd, +As, Infection of bacterial and fungal pathogens | JA, ABA, SA, BRs | Participate in regulation of basic resistance to pathogens, and maintain reactive oxygen species homeostasis | Takahashi |
| Contribute to uptake and transport of Cd2+ and Mn2+, but not for Fe2+ or As3+ | et al, | ||||||
| et al, | |||||||
| OsNRAMP2 | Tonoplast | Mainly expressed in leaves | Cd2+, Fe2+ | JA, ABA, ethephon, citric acid, high-Mn exposure, pathogen infection | SA | Transport iron from vacuole to | Li et al, |
| cytoplasm | |||||||
| Play a key role in seed germination | |||||||
| OsNRAMP3 | Plasma membrane | Specifically expressed in vascular bundles | Mn2+, Cd2+, Ni2+ | -Fe, ABA, fungal infection, citric acid | +Cd | Participate in Mn2+-influx | Yamaji et al, |
| Involve in Mn distribution and contribute to remobilization of Mn from old to young leaves | et al, | ||||||
| Act as a switch in response to | |||||||
| environmental Mn availability | |||||||
| OsNRAMP4 | Plasma membrane | Mainly expressed in roots | Al3+, Mn2+, Cd2+ | +Al | / | Isolate Al3+ into vacuoles to remove Al toxicity | Xia et al, |
| Transport Mn2+ from root to | Hao et al, | ||||||
| aboveground tissues | |||||||
| Alter Cd accumulation in grains via | |||||||
| affecting cellular Cd2+ distribution | |||||||
| OsNRAMP5 | Plasma membrane | Constitutively expressed in mature roots, hulls, stems, and leaves | Mn2+, Fe2+ Pb2+, Cd2+, Co2+ | -Fe, -Zn, high-Mn application, soil pH | +Cd | Involve in the absorption of external Mn2+, Cd2+, Co2+, and Fe2+ | Ishimaru et al, |
| Responsible for uptake and transport of metal ions from roots to shoots | et al, | ||||||
| Maintain ion homeostasis | |||||||
| OsNRAMP6 | Plasma membrane | Specific expression in early-stage foliage | Fe2+, Mn2+, Cd2+ | Fungal infection | / | As a transporter of Fe and Mn | Yang, |
| Negative regulation of rice immunity | et al, | ||||||
| Participate in Cd absorption and transport | |||||||
| OsNRAMP7 | Plasma membrane | Root, node I, and spikelet at the flowering stage | Fe2+, Zn2+, Na+ | -Fe, drought, salt, BRs, JA | CTK | Exhibit significant correlations with | Wu et al, |
| Fe2+, Zn2+ distribution | Li J J et al, | ||||||
| Involve in Na+ transport and ABA-regulated drought tolerance pathway |
Table 1. Functional analysis of OsNRAMPs.
| Protein | Subcellular localization | Spatiotemporal expression | Transport metal ion | Inducible expressiona | Suppressed expressiona | Function | Reference |
|---|---|---|---|---|---|---|---|
| OsNRAMP1 | Plasma membrane | Mainly expressed in roots and leaves | Mn2+, Ni2+, Cd2+, Pb2+, etc. | -Fe, +Cd, +As, Infection of bacterial and fungal pathogens | JA, ABA, SA, BRs | Participate in regulation of basic resistance to pathogens, and maintain reactive oxygen species homeostasis | Takahashi |
| Contribute to uptake and transport of Cd2+ and Mn2+, but not for Fe2+ or As3+ | et al, | ||||||
| et al, | |||||||
| OsNRAMP2 | Tonoplast | Mainly expressed in leaves | Cd2+, Fe2+ | JA, ABA, ethephon, citric acid, high-Mn exposure, pathogen infection | SA | Transport iron from vacuole to | Li et al, |
| cytoplasm | |||||||
| Play a key role in seed germination | |||||||
| OsNRAMP3 | Plasma membrane | Specifically expressed in vascular bundles | Mn2+, Cd2+, Ni2+ | -Fe, ABA, fungal infection, citric acid | +Cd | Participate in Mn2+-influx | Yamaji et al, |
| Involve in Mn distribution and contribute to remobilization of Mn from old to young leaves | et al, | ||||||
| Act as a switch in response to | |||||||
| environmental Mn availability | |||||||
| OsNRAMP4 | Plasma membrane | Mainly expressed in roots | Al3+, Mn2+, Cd2+ | +Al | / | Isolate Al3+ into vacuoles to remove Al toxicity | Xia et al, |
| Transport Mn2+ from root to | Hao et al, | ||||||
| aboveground tissues | |||||||
| Alter Cd accumulation in grains via | |||||||
| affecting cellular Cd2+ distribution | |||||||
| OsNRAMP5 | Plasma membrane | Constitutively expressed in mature roots, hulls, stems, and leaves | Mn2+, Fe2+ Pb2+, Cd2+, Co2+ | -Fe, -Zn, high-Mn application, soil pH | +Cd | Involve in the absorption of external Mn2+, Cd2+, Co2+, and Fe2+ | Ishimaru et al, |
| Responsible for uptake and transport of metal ions from roots to shoots | et al, | ||||||
| Maintain ion homeostasis | |||||||
| OsNRAMP6 | Plasma membrane | Specific expression in early-stage foliage | Fe2+, Mn2+, Cd2+ | Fungal infection | / | As a transporter of Fe and Mn | Yang, |
| Negative regulation of rice immunity | et al, | ||||||
| Participate in Cd absorption and transport | |||||||
| OsNRAMP7 | Plasma membrane | Root, node I, and spikelet at the flowering stage | Fe2+, Zn2+, Na+ | -Fe, drought, salt, BRs, JA | CTK | Exhibit significant correlations with | Wu et al, |
| Fe2+, Zn2+ distribution | Li J J et al, | ||||||
| Involve in Na+ transport and ABA-regulated drought tolerance pathway |
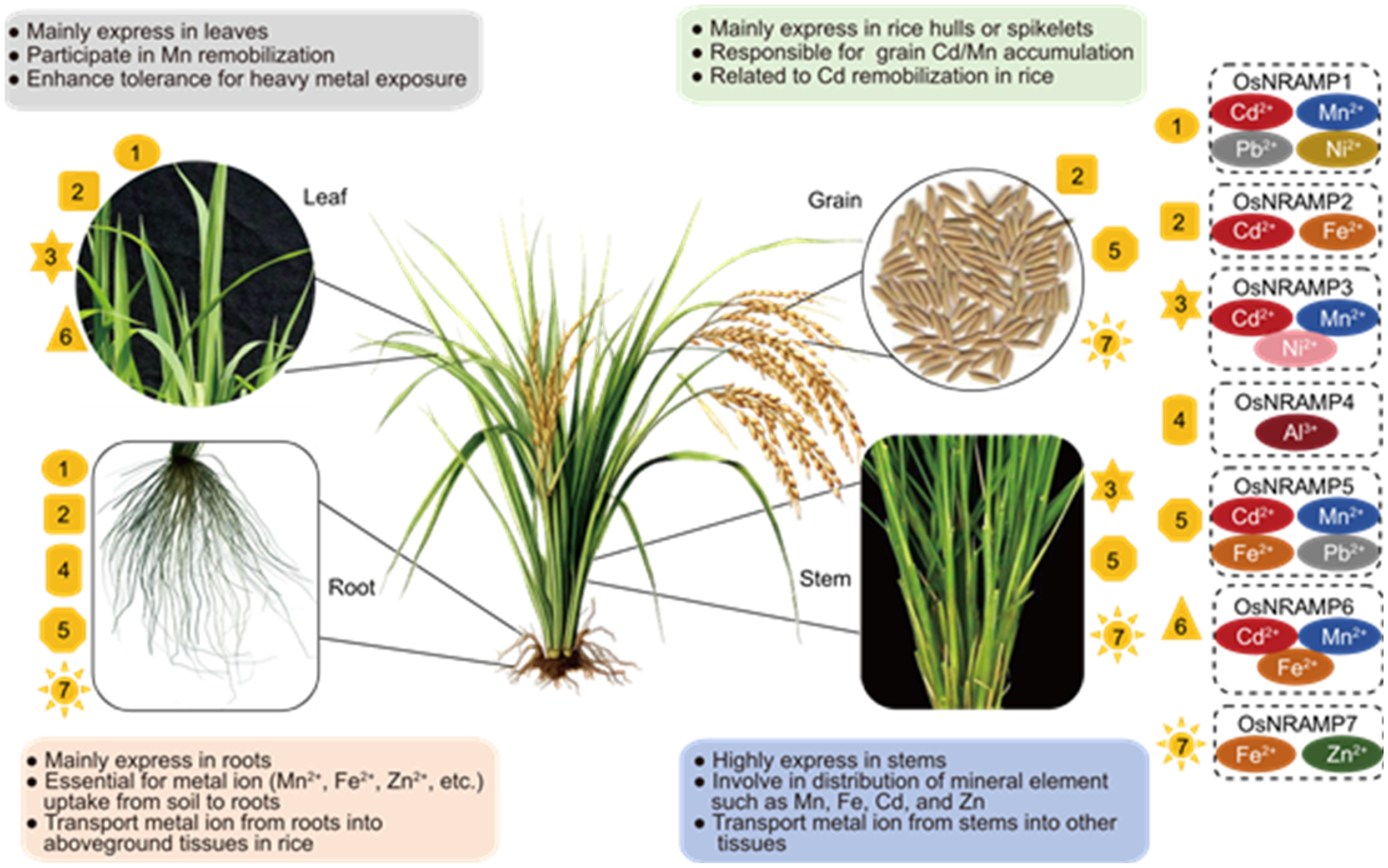
Fig. 3. Expression patterns of OsNRAMP family members and their functions in ion transport. OsNRAMP1, OsNRAMP2, OsNRAMP4, OsNRAMP5, and OsNRAMP7 are mainly expressed in rice roots, where they facilitate the uptake and transport of metal ions such as Mn2+, Fe2+, and Zn2+ from soil into the plant. OsNRAMP3, OsNRAMP5, and OsNRAMP7 are highly active in the stem, supporting the distribution of Mn, Fe, Zn, and Cd to various tissues. In leaves, OsNRAMP1, OsNRAMP2, OsNRAMP3, and OsNRAMP6 contribute to Mn distribution and (re)mobilization, with notably higher expression levels. Moreover, OsNRAMP2 and OsNRAMP5 are key transporters involved in Cd and Mn accumulation in grains. Notably, OsNRAMP5 plays a central role in reducing Cd toxicity and provides important insights for developing low-Cd rice varieties.
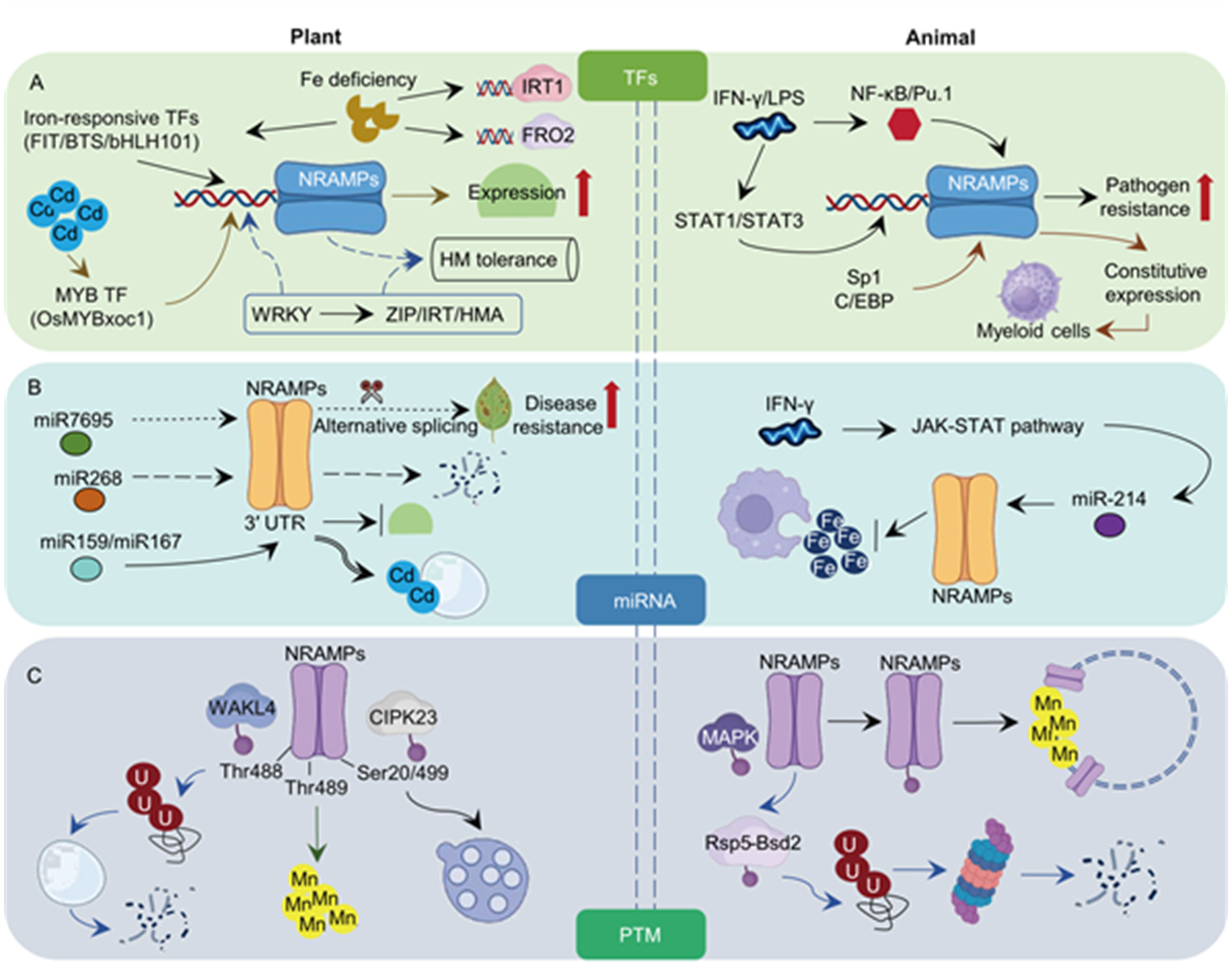
Fig. 4. Comparative regulatory networks of NRAMP transporters in plants and animals. A, Transcriptional regulation of NRAMPs. In plants (left), iron-responsive transcription factors (TFs) (FIT, BTS, bHLH101) and MYB TFs control NRAMP expression to balance metal uptake (Fe, Mn, Zn) and Cd detoxification. Interactions with other transporters (ZIP/IRT/HMA) further regulate metal homeostasis. In animals (right), pro-inflammatory signals (IFN-γ/LPS) activate myeloid-specific TFs (NF-κB, Pu.1, STAT1/3), inducing NRAMP1 (SLC11A1) expression and enhancing pathogen resistance. Basal expression is maintained by constitutive regulators (Sp1 and C/EBP). B, MicroRNA (miRNA)-mediated post-transcriptional control. In plants, Cd-induced miRNAs (e.g., osa-miR268, osa-miR7695) degrade OsNRAMP mRNAs or alter splicing, limiting Cd movement while preserving essential metals. In animals, immune-related miRNAs (e.g., miR-214) target a conserved sequence of NRAMPs, linking iron metabolism to antimicrobial defense. C, Post-translational modifications (PTMs). In plants, phosphorylation by WAKL4/CIPK23 modulates NRAMP stability and activity. In animals, MAPK-driven or Rsp5-Bsd2-mediated phosphorylation regulates NRAMP localization.
| [1] | Abe T, Kuramata M, Igura M, et al. 2017. ‘Koshihikari Kan No. 1’, a new rice variety with nearly cadmium-free in grains. Breed Res, 19(3): 109-115. |
| [2] | Alejandro S, Cailliatte R, Alcon C, et al. 2017. Intracellular distribution of manganese by the trans-Golgi network transporter NRAMP2 is critical for photosynthesis and cellular redox homeostasis. Plant Cell, 29(12): 3068-3084. |
| [3] | Bao H, Kommadath A, Liang G X, et al. 2015. Genome-wide whole blood microRNAome and transcriptome analyses reveal miRNA-mRNA regulated host response to foodborne pathogen Salmonella infection in swine. Sci Rep, 5: 12620. |
| [4] | Belgareh-Touzé N, Léon S, Erpapazoglou Z, et al. 2008. Versatile role of the yeast ubiquitin ligase Rsp5p in intracellular trafficking. Biochem Soc Trans, 36: 791-796. |
| [5] | Belouchi A, Kwan T, Gros P. 1997. Cloning and characterization of the OsNramp family from Oryza sativa, a new family of membrane proteins possibly implicated in the transport of metal ions. Plant Mol Biol, 33(6): 1085-1092. |
| [6] | Bozzi A T, Gaudet R. 2021. Molecular mechanism of Nramp-family transition metal transport. J Mol Biol, 433(16): 166991. |
| [7] | Bozzi A T, Bane L B, Weihofen W A, et al. 2016. Crystal structure and conformational change mechanism of a bacterial Nramp-family divalent metal transporter. Structure, 24(12): 2102-2114. |
| [8] | Cai Y M, Wang M E, Chen B D, et al. 2020. Effects of external Mn2+ activities on OsNRAMP5 expression level and Cd accumulation in indica rice. Environ Pollut, 260: 113941. |
| [9] | Cailliatte R, Lapeyre B, Briat J F, et al. 2009. The NRAMP6 metal transporter contributes to cadmium toxicity. Biochem J, 422(2): 217-228. |
| [10] | Cailliatte R, Schikora A, Briat J F, et al. 2010. High-affinity manganese uptake by the metal transporter NRAMP1 is essential for Arabidopsis growth in low manganese conditions. Plant Cell, 22(3): 904-917. |
| [11] | Campo S, Peris-Peris C, Siré C, et al. 2013. Identification of a novel microRNA (miRNA) from rice that targets an alternatively spliced transcript of the Nramp6 (Natural resistance-associated macrophage protein 6) gene involved in pathogen resistance. New Phytol, 199(1): 212-227. |
| [12] | Cao Z Z, Lin X Y, Yang Y J, et al. 2019. Gene identification and transcriptome analysis of low cadmium accumulation rice mutant (lcd1) in response to cadmium stress using MutMap and RNA-seq. BMC Plant Biol, 19(1): 250. |
| [13] | Castaings L, Alcon C, Kosuth T, et al. 2021. Manganese triggers phosphorylation-mediated endocytosis of the Arabidopsis metal transporter NRAMP1. Plant J, 106(5): 1328-1337. |
| [14] | Cellier M, Govoni G, Vidal S, et al. 1994. Human natural resistance-associated macrophage protein: cDNA cloning, chromosomal mapping, genomic organization, and tissue-specific expression. J Exp Med, 180: 1741-1752. |
| [15] | Cellier M F M. 2017. Developmental control of NRAMP1 (SLC11A1) expression in professional phagocytes. Biology, 6(2): 28. |
| [16] | Chaney R L. 2015. How does contamination of rice soils with Cd and Zn cause high incidence of human Cd disease in subsistence rice farmers. Curr Pollut Rep, 1(1): 13-22. |
| [17] | Chang J D, Huang S, Yamaji N, et al. 2020a. OsNRAMP1 transporter contributes to cadmium and manganese uptake in rice. Plant Cell Environ, 43(10): 2476-2491. |
| [18] | Chang J D, Huang S, Konishi N, et al. 2020b. Overexpression of the manganese/cadmium transporter OsNRAMP5 reduces cadmium accumulation in rice grain. J Exp Bot, 71(18): 5705-5715. |
| [19] | Chang J D, Gao W P, Wang P, et al. 2022a. OsNRAMP5 is a major transporter for lead uptake in rice. Environ Sci Technol, 56(23): 17481-17490. |
| [20] | Chang J D, Xie Y, Zhang H H, et al. 2022b. The vacuolar transporter OsNRAMP2 mediates Fe remobilization during germination and affects Cd distribution to rice grain. Plant Soil, 476(1): 79-95. |
| [21] | Chen S S, Han X J, Fang J, et al. 2017. Sedum alfredii SaNramp6 metal transporter contributes to cadmium accumulation in transgenic Arabidopsis thaliana Sci Rep, 7(1): 13318. |
| [22] | Chu C L, Huang R Y, Liu L P, et al. 2022. The rice heavy-metal transporter OsNRAMP1 regulates disease resistance by modulating ROS homoeostasis. Plant Cell Environ, 45(4): 1109-1126. |
| [23] | Ding Y F, Wang Y, Jiang Z H, et al. 2017. MicroRNA268 over-expression affects rice seedling growth under cadmium stress. J Agric Food Chem, 65: 5860-5867. |
| [24] | Dong J F, Chen K, Chen L, et al. 2025. OsNRAMP7 positively regulates heat tolerance at seedling and reproductive stages in rice. Plant Stress, 16: 100870. |
| [25] | Ehrnstorfer I A, Geertsma E R, Pardon E, et al. 2014. Crystal structure of a SLC11 (NRAMP) transporter reveals the basis for transition-metal ion transport. Nat Struct Mol Biol, 21(11): 990-996. |
| [26] | Fu D L, Zhang Z Q, Wallrad L, et al. 2022. Ca2+-dependent phosphorylation of NRAMP1 by CPK21 and CPK23 facilitates manganese uptake and homeostasis in Arabidopsis Proc Natl Acad Sci USA, 119: e2204574119. |
| [27] | Gao H L, Xie W X, Yang C H, et al. 2018. NRAMP2, a trans-Golgi network-localized manganese transporter, is required for Arabidopsis root growth under manganese deficiency. New Phytol, 217(1): 179-193. |
| [28] | Gomez M A, Alisaraie L, Shio M T, et al. 2010. Protein tyrosine phosphatases are regulated by mononuclear iron dicitrate. J Biol Chem, 285: 24620-24628. |
| [29] | Gu L, Hou Y Y, Sun Y Y, et al. 2024. The maize WRKY transcription factor ZmWRKY64 confers cadmium tolerance in Arabidopsis and maize (Zea mays L.). Plant Cell Rep, 43(2): 44. |
| [30] | Hao X H, Mo Y F, Ji W J, et al. 2022. The OsNramp4 aluminum transporter is involved in cadmium accumulation in rice grains. Reprod Breed, 2(4): 125-132. |
| [31] | Hettema E H, Valdez-Taubas J, Pelham H R B. 2004. Bsd2 binds the ubiquitin ligase Rsp5 and mediates the ubiquitination of transmembrane proteins. EMBO J, 23(6): 1279-1288. |
| [32] | Huang C F. 2022. Ca2+ signaling in plant manganese uptake: CPK21/23 kinases phosphorylate and activate manganese transporter NRAMP1. Stress Biol, 2(1): 43. |
| [33] | Huang H L, Yamaji N, Huang S, et al. 2025. Uptake and accumulation of cobalt is mediated by OsNramp 5 in rice. Plant Cell Environ, 48(1): 3-14. |
| [34] | Huang S, Konishi N, Yamaji N, et al. 2024. Local distribution of manganese to leaf sheath is mediated by OsNramp 5 in rice. New Phytol, 241(4): 1708-1719. |
| [35] | Ishikawa S, Ishimaru Y, Igura M, et al. 2012. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc Natl Acad Sci USA, 109: 19166-19171. |
| [36] | Ishimaru Y, Takahashi R, Bashir K, et al. 2012. Characterizing the role of rice NRAMP5 in manganese, iron and cadmium transport. Sci Rep, 2: 286. |
| [37] | Ji X Y, Wang Y C, Liu G F. 2012. Expression analysis of MYC genes from Tamarix hispida in response to different abiotic stresses. Int J Mol Sci, 13(2): 1300-1313. |
| [38] | Kanwal F, Riaz A, Ali S, et al. 2024. NRAMPs and manganese: Magic keys to reduce cadmium toxicity and accumulation in plants. Sci Total Environ, 921: 171005. |
| [39] | Kosuth T, Leskova A, Ródenas R, et al. 2023. Phosphorylation by CIPK23 regulates the high-affinity Mn transporter NRAMP1 in Arabidopsis. FEBS Lett, 597(16): 2048-2058. |
| [40] | Kumari K, Kumar P, Sharma V K, et al. 2019. Genomic marker assisted identification of genetic loci and genes associated with variation of grain zinc concentration in rice. J Genet, 98: 111. |
| [41] | Kuramata M, Abe T, Tanikawa H, et al. 2022. A weak allele of OsNRAMP5 confers moderate cadmium uptake while avoiding manganese deficiency in rice. J Exp Bot, 73(18): 6475-6489. |
| [42] | Lafuse W P, Alvarez G R, Zwilling B S. 2000. Regulation of Nramp1 mRNA stability by oxidants and protein kinase C in RAW264.7 macrophages expressing Nramp1Gly169. Biochem J, 351(Pt3): 687-696. |
| [43] | Lanquar V, Ramos M S, Lelièvre F, et al. 2010. Export of vacuolar manganese by AtNRAMP3 and AtNRAMP4 is required for optimal photosynthesis and growth under manganese deficiency. Plant Physiol, 152(4): 1986-1999. |
| [44] | Li J J, Liu Y Y, Kong L H, et al. 2023. An intracellular transporter OsNRAMP7 is required for distribution and accumulation of iron into rice grains. Plant Sci, 336: 111831. |
| [45] | Li J Y, Liu J P, Dong D K, et al. 2014. Natural variation underlies alterations in Nramp aluminum transporter (NRAT1) expression and function that play a key role in rice aluminum tolerance. Proc Natl Acad Sci USA, 111(17): 6503-6508. |
| [46] | Li J Y, Wang Y R, Zheng L, et al. 2019. The intracellular transporter AtNRAMP6 is involved in Fe homeostasis in Arabidopsis Front Plant Sci, 10: 1124. |
| [47] | Li L, Zhu Z Z, Liao Y H, et al. 2022. NRAMP6 and NRAMP1 cooperatively regulate root growth and manganese translocation under manganese deficiency in Arabidopsis. Plant J, 110(6): 1564-1577. |
| [48] | Li S Z, Mo Y F, Hao X H, et al. 2023. Functional analysis of OsNramp4 on manganese ion uptake and translocation in rice. Life Sci Res, 27(1): 42-48. (in Chinese with English abstract) |
| [49] | Li Y, Li J J, Yu Y H, et al. 2021. The tonoplast-localized transporter OsNRAMP2 is involved in iron homeostasis and affects seed germination in rice. J Exp Bot, 72(13): 4839-4852. |
| [50] | Lim S D, Hwang J G, Han A R, et al. 2014. Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol Biol, 85(4/5): 365-379. |
| [51] | Liu C L, Chen G, Li Y Y, et al. 2017. Characterization of a major QTL for manganese accumulation in rice grain. Sci Rep, 7(1): 17704. |
| [52] | Liu C L, Ding S L, Zhang A P, et al. 2020. Development of nutritious rice with high zinc/selenium and low cadmium in grains through QTL pyramiding. J Integr Plant Biol, 62(3): 349-359. |
| [53] | Liu P, Jiang L, Long P, et al. 2023. A genome-wide co-expression network analysis revealed ZmNRAMP6-mediated regulatory pathway involved in maize tolerance to lead stress. Theor Appl Genet, 136(5): 122. |
| [54] | Lu M X, Wang Z G, Fu S, et al. 2017. Functional characterization of the SbNrat1 gene in sorghum. Plant Sci, 262: 18-23. |
| [55] | Lu Z C, Chen S S, Han X J, et al. 2020. A single amino acid change in Nramp6 from Sedum Alfredii Hance affects cadmium accumulation. Int J Mol Sci, 21(9): 3169. |
| [56] | Lv Q M, Li W G, Sun Z Z, et al. 2020. Resequencing of 1, 143 indica rice accessions reveals important genetic variations and different heterosis patterns. Nat Commun, 11(1): 4778. |
| [57] | Ma X C, Yang H B, Bu Y F, et al. 2023. Genome-wide identification of the NRAMP gene family in Populus trichocarpa and their function as heavy metal transporters. Ecotoxicol Environ Saf, 261: 115110. |
| [58] | Majumdar S, Sachdev S, Kundu R. 2020. Salicylic acid mediated reduction in grain cadmium accumulation and amelioration of toxicity in Oryza sativa L. cv Bandana. Ecotoxicol Environ Saf, 205: 111167. |
| [59] | Mani A, Sankaranarayanan K. 2018. In silico analysis of natural resistance-associated macrophage protein (NRAMP) family of transporters in rice. Protein J, 37(3): 237-247. |
| [60] | Mani A, Sankaranarayanan K. 2022. Natural resistance-associated macrophage proteins (NRAMPs):Functional significance of metal transport in plants. In: Plant Metal and Metalloid Transporters. Singapore: Springer Nature Singapore: 91-107. |
| [61] | Meng J G, Zhang X D, Tan S K, et al. 2017. Genome-wide identification of Cd-responsive NRAMP transporter genes and analyzing expression of NRAMP 1 mediated by miR167 in Brassica napus. Biometals, 30(6): 917-931. |
| [62] | Mirza Z, Haque M M, Gupta M. 2022. WRKY transcription factors: A promising way to deal with arsenic stress in rice. Mol Biol Rep, 49(11): 10895-10904. |
| [63] | Moisan J, Thuraisingam T, Henault J, et al. 2006. Role of SLC11A1 (formerly NRAMP1) in regulation of signal transduction induced by Toll-like receptor 7 ligands. FEMS Immunol Med Microbiol, 47(1): 138-147. |
| [64] | Mukherjee A, Dwivedi S, Bhagavatula L, et al. 2023. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep, 42(5): 829-841. |
| [65] | Narayanan N N, Vasconcelos M W, Grusak M A. 2007. Expression profiling of Oryza sativa metal homeostasis genes in different rice cultivars using a cDNA macroarray. Plant Physiol Biochem, 45(5): 277-286. |
| [66] | Peris-Peris C, Serra-Cardona A, Sánchez-Sanuy F, et al. 2017. Two NRAMP6 isoforms function as iron and manganese transporters and contribute to disease resistance in rice. Mol Plant Microbe Interact, 30(5): 385-398. |
| [67] | Pottier M, Thi V A L, Primard-Brisset C, et al. 2022. Duplication of NRAMP3 gene in poplars generated two homologous transporters with distinct functions. Mol Biol Evol, 39(6): msac129. |
| [68] | Qiao Y, Giannopoulou E G, Chan C H, et al. 2013. Synergistic activation of inflammatory cytokine genes by interferon-γ-induced chromatin remodeling and toll-like receptor signaling. Immunity, 39(3): 454-469. |
| [69] | Qin L, Han P P, Chen L Y, et al. 2017. Genome-wide identification and expression analysis of NRAMP family genes in soybean (Glycine max L.). Front Plant Sci, 8: 1436. |
| [70] | Qu Z T, Nakanishi H. 2023. Amino acid residues of the metal transporter OsNRAMP5 responsible for cadmium absorption in rice. Plants, 12(24): 4182. |
| [71] | Ray S, Berry S P, Wilson E A, et al. 2023. High-resolution structures with bound Mn2+ and Cd2+ map the metal import pathway in an Nramp transporter. eLife, 12: e84006. |
| [72] | Richer E, Campion C G, Dabbas B, et al. 2008. Transcription factors Sp1 and C/EBP regulate NRAMP1 gene expression. FEBS J, 275: 5074-5089. |
| [73] | Sasaki A, Yamaji N, Yokosho K, et al. 2012. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell, 24(5): 2155-2167. |
| [74] | Shao J F, Yamaji N, Shen R F, et al. 2017. The key to Mn homeostasis in plants: Regulation of Mn transporters. Trends Plant Sci, 22(3): 215-224. |
| [75] | Shao Y, Peng Y, Mao B G, et al. 2022. M1TDS technology and creation of low-cadmium accumulation parents for hybrid rice breeding. Hybrid Rice, 37(1): 1-11. (in Chinese with English abstract) |
| [76] | Sui F Q, Chang J D, Tang Z, et al. 2018. Nramp5 expression and functionality likely explain higher cadmium uptake in rice than in wheat and maize. Plant Soil, 433(1): 377-389. |
| [77] | Takahashi R, Ishimaru Y, Nakanishi H, et al. 2011. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal Behav, 6(11): 1813-1816. |
| [78] | Tang L, Dong J Y, Qu M M, et al. 2022. Knockout of OsNRAMP5 enhances rice tolerance to cadmium toxicity in response to varying external cadmium concentrations via distinct mechanisms. Sci Total Environ, 832: 155006. |
| [79] | Thomine S, Lelièvre F, Debarbieux E, et al. 2003. AtNRAMP3, a multispecific vacuolar metal transporter involved in plant responses to iron deficiency. Plant J, 34(5): 685-695. |
| [80] | Tiwari M, Sharma D, Dwivedi S, et al. 2014. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ, 37(1): 140-152. |
| [81] | Uraguchi S, Kamiya T, Clemens S, et al. 2014. Characterization of OsLCT1, a cadmium transporter from indica rice (Oryza sativa). Physiol Plant, 151(3): 339-347. |
| [82] | Wang C, Chen X, Yao Q, et al. 2019. Overexpression of TtNRAMP6 enhances the accumulation of Cd in Arabidopsis. Gene, 696: 225-232. |
| [83] | Wang N, Cui Y, Liu Y, et al. 2013. Requirement and functional redundancy of Ib subgroup bHLH proteins for iron deficiency responses and uptake in Arabidopsis thaliana. Mol Plant, 6(2): 503-513. |
| [84] | Wang T K, Li Y X, Fu Y F, et al. 2019. Mutation at different sites of metal transporter gene OsNramp5 affects Cd accumulation and related agronomic traits in rice (Oryza sativa L.). Front Plant Sci, 10: 1081. |
| [85] | Wei W, Chai T Y, Zhang Y X, et al. 2009. The Thlaspi caerulescens NRAMP homologue TcNRAMP3 is capable of divalent cation transport. Mol Biotechnol, 41(1): 15-21. |
| [86] | Wu D Z, Yamaji N, Yamane M, et al. 2016. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol, 172(3): 1899-1910. |
| [87] | Wu T H, Li Y K, Sun Y T, et al. 2021. Cloning, expression and bioinformatical analysis of OsNRAMP7 gene in rice. Mol Plant Breed, 19: 2103-2110. (in Chinese with English abstract) |
| [88] | Xia J X, Yamaji N, Kasai T, et al. 2010. Plasma membrane-localized transporter for aluminum in rice. Proc Natl Acad Sci USA, 107: 18381-18385. |
| [89] | Xia J X, Yamaji N, Che J, et al. 2014. Differential expression of Nrat1 is responsible for Al-tolerance QTL on chromosome 2 in rice. J Exp Bot, 65(15): 4297-4304. |
| [90] | Xiong H C, Kobayashi T, Kakei Y, et al. 2012. AhNRAMP1 iron transporter is involved in iron acquisition in peanut. J Exp Bot, 63(12): 4437-4446. |
| [91] | Xu H X, Jin J, DeFelice L J, et al. 2004. A spontaneous, recurrent mutation in divalent metal transporter-1 exposes a calcium entry pathway. PLoS Biol, 2(3): e50. |
| [92] | Xue W J, Wang P P, Tang L, et al. 2021. Citric acid inhibits Cd uptake by improving the preferential transport of Mn and triggering the defense response of amino acids in grains. Ecotoxicol Environ Saf, 211: 111921. |
| [93] | Yamaji N, Sasaki A, Xia J X, et al. 2013. A node-based switch for preferential distribution of manganese in rice. Nat Commun, 4: 2442. |
| [94] | Yang M. 2014. Function analysis of rice NRAMP genes in Mn and Cd transporter. Wuhan, China: Huazhong Agricultural University. (in Chinese with English abstract) |
| [95] | Yang M, Zhang W, Dong H X, et al. 2013. OsNRAMP3 is a vascular bundles-specific manganese transporter that is responsible for manganese distribution in rice. PLoS One, 8(12): e83990. |
| [96] | Yang M, Zhang Y Y, Zhang L J, et al. 2014. OsNRAMP5 contributes to manganese translocation and distribution in rice shoots. J Exp Bot, 65(17): 4849-4861. |
| [97] | Yang W, Chen L, Ma Y M, et al. 2023. OsNRAMP2 facilitates Cd efflux from vacuoles and contributes to the difference in grain Cd accumulation between japonica and indica rice. Crop J, 11(2): 417-426. |
| [98] | Yu E, Wang W G, Yamaji N, et al. 2022. Duplication of a manganese/ cadmium transporter gene reduces cadmium accumulation in rice grain. Nat Food, 3(8): 597-607. |
| [99] | Yu J, Chen J S, Zhang Z P, et al. 2024. Cloning and biological function analysis of Nramps in blueberry. Sci Hortic, 324: 112554. |
| [100] | Yu X F, Yang L, Fan C Y, et al. 2023. Abscisic acid (ABA) alleviates cadmium toxicity by enhancing the adsorption of cadmium to root cell walls and inducing antioxidant defense system of Cosmos bipinnatus Ecotoxicol Environ Saf, 261: 115101. |
| [101] | Yu Y B, Zhang S, Yu Y, et al. 2023. The pivotal role of MYB transcription factors in plant disease resistance. Planta, 258(1): 16. |
| [102] | Yuan J J, Zhao Y N, Yu S H, et al. 2024. The Arabidopsis receptor-like kinase WAKL4 limits cadmium uptake via phosphorylation and degradation of NRAMP1 transporter. Nat Commun, 15: 9537. |
| [103] | Zeng X Y, Bian W, Liu Z W, et al. 2023. Muscle-derived stem cell exosomes with overexpressed miR-214 promote the regeneration and repair of rat sciatic nerve after crush injury to activate the JAK2/STAT3 pathway by targeting PTEN. Front Mol Neurosci, 16: 1146329. |
| [104] | Zhang H M, Sun B L, Wu W, et al. 2024. The MYB transcription factor OsMYBxoc1 regulates resistance to Xoc by directly repressing transcription of the iron transport gene OsNRAMP5 in rice. Plant Commun, 5(6): 100859. |
| [105] | Zhang Q, Chen H F, Xu C, et al. 2019. Heavy metal uptake in rice is regulated by pH-dependent iron plaque formation and the expression of the metal transporter genes. Environ Exp Bot, 162: 392-398. |
| [106] | Zhang W Y, Guan M Y, Chen M X, et al. 2024. Mutation of OsNRAMP5 reduces cadmium xylem and phloem transport in rice plants and its physiological mechanism. Environ Pollut, 341: 122928. |
| [107] | Zhao F J, Chang J D. 2022. A weak allele of OsNRAMP5 for safer rice. J Exp Bot, 73(18): 6009-6012. |
| [108] | Zhou X J, Yang Y N. 2004. Differential expression of rice Nramp genes in response to pathogen infection, defense signal molecules and metal ions. Physiol Mol Plant Pathol, 65(5): 235-243. |
| No related articles found! |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||