
Rice Science ›› 2025, Vol. 32 ›› Issue (3): 353-366.DOI: 10.1016/j.rsci.2025.01.008
• Research Papers • Previous Articles Next Articles
Lai Changkai1,2,3, Hu Shikai2, Jiao Guiai2, Wang Ling2, Shao Gaoneng2, Zhao Fengli2, Xie Lihong2, Wei Xiangjin2, Lü Yusong2, Sheng Zhonghua2, Tang Shaoqing2( ), Hu Peisong1,2(
), Hu Peisong1,2( )
)
Received:2024-12-11
Accepted:2025-01-23
Online:2025-05-28
Published:2025-06-16
Contact:
Hu Peisong (Lai Changkai, Hu Shikai, Jiao Guiai, Wang Ling, Shao Gaoneng, Zhao Fengli, Xie Lihong, Wei Xiangjin, Lü Yusong, Sheng Zhonghua, Tang Shaoqing, Hu Peisong. Enhancing Folate Content in Japonica Rice Through Co-expression of OsADCS and OsGTPCHI Indica Alleles[J]. Rice Science, 2025, 32(3): 353-366.
Add to citation manager EndNote|Ris|BibTeX
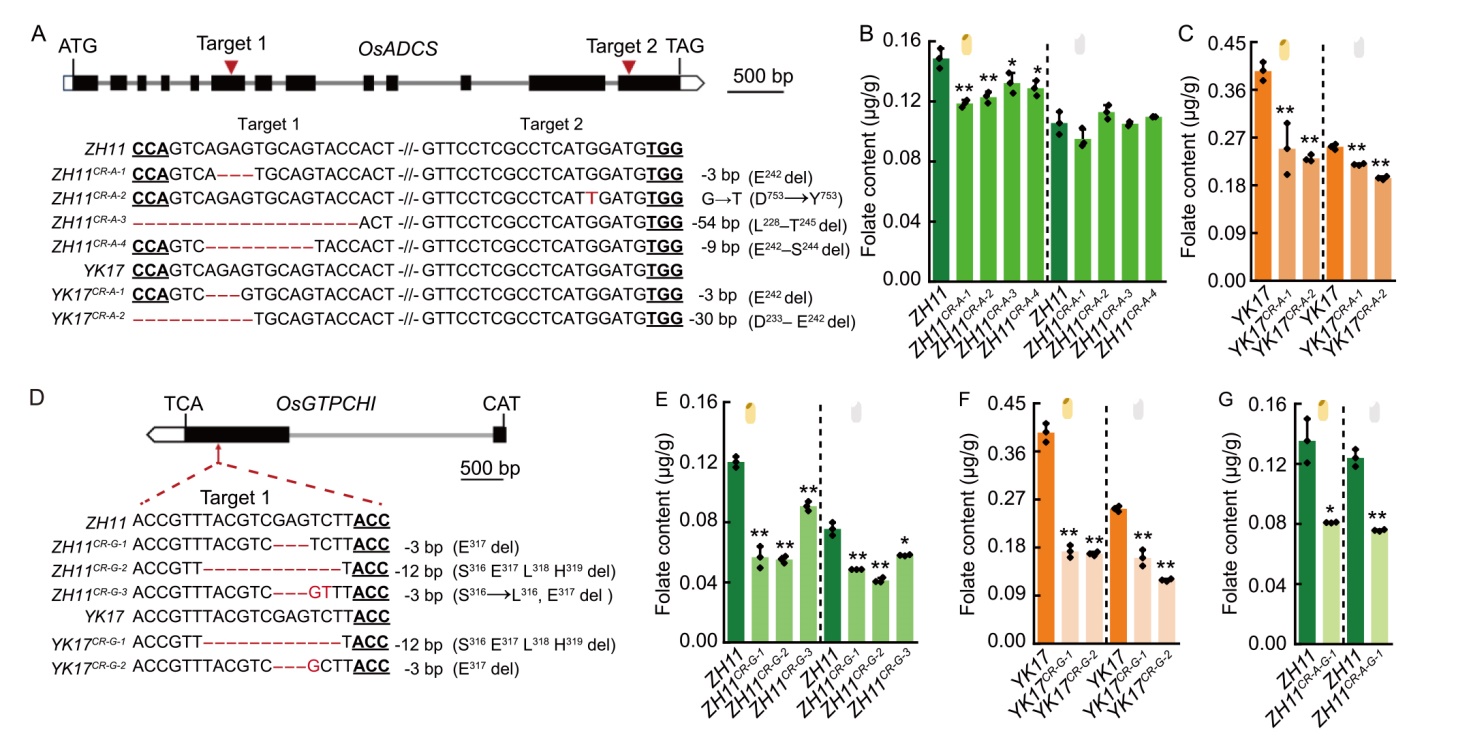
Fig. 1. OsADCS and OsGTPCHI knockout mutants decrease folate content in rice. A and D, Sequence alignment of target sites in OsADCS (A) and OsGTPCHI (D) knockout mutants. Altered nucleotide bases are highlighted in red, with corresponding mutation types indicated on the right. Exons and introns are represented by black boxes and lines, respectively.B, C, and E‒G, Folate content comparisons between: Zhonghua 11 (ZH11) and ZH11 OsADCS knockout lines (ZH11CR-A-1 to ZH11CR-A-4, B); Zhongjiazao 17 (YK17) and YK17 OsADCS knockout lines (YK17CR-A-1 and YK17CR-A-2, C); ZH11 and ZH11 OsGTPCHI knockout lines (ZH11CR-G-1 to ZH11CR-G-3, E); YK17 and YK17 OsGTPCHI knockout lines (YK17CR-G-1 and YK17CR-G-2, F); ZH11 and ZH11 double-knockout OsADCS/OsGTPCHI line (ZH11CR-A-G-1, G). Brown rice (left) and polished rice (right) are shown. Data are presented as Mean ± SD of three biological replicates, with statistical significance denoted by asterisks (*, P < 0.05; **, P < 0.01).
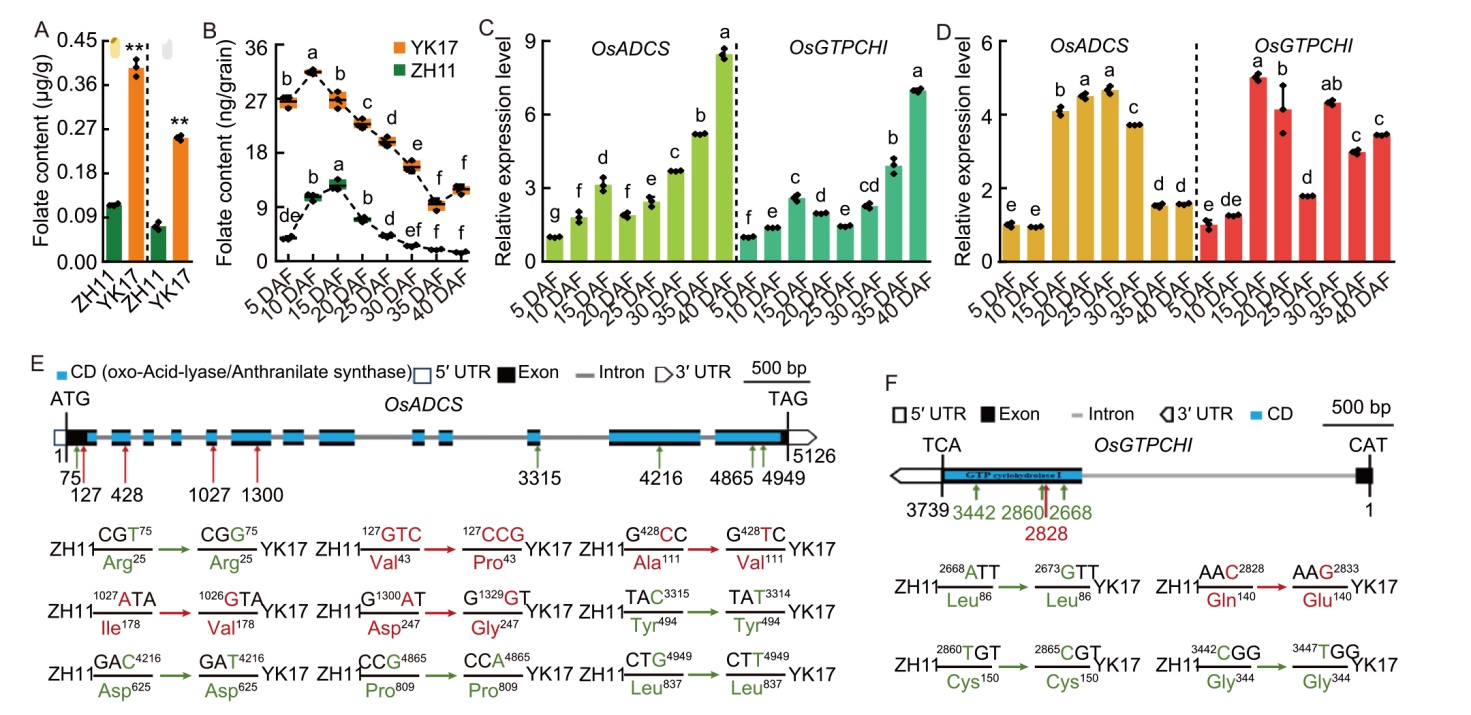
Fig. 2. Folate accumulation and expression patterns of OsADCS and OsGTPCHI genes. A, Comparison of folate contents in grains between Zhonghua 11 (ZH11) and Zhongjiazao 17 (YK17). Brown rice (left) and polished rice (right) are shown.B, Folate contents in single grains of ZH11 and YK17 during seed development stage. DAF, Days after flowering.C and D, Relative transcript levels of OsADCS and OsGTPCHI genes in developing grains of ZH11 (C) and YK17 (D) at 5‒40 DAF, quantified by qRT-PCR with Ubiquitin (LOC_Os03g13170) as an internal control.E and F, Single nucleotide polymorphism (SNP) alignment of coding sequences between ZH11 and YK17 in OsADCS (E) and OsGTPCHI (F). Synonymous and nonsynonymous mutation SNPs are marked in green and red, respectively. UTR, Untranslated region; CD, Conserved domain.Data in A‒D are Mean ± SD (n = 3), with statistical significance denoted by asterisks (**, P < 0.01). Different lowercase letters above bars (B‒D) indicate significant differences at P < 0.05 by a one-way analysis of variance with Tukey’s test.
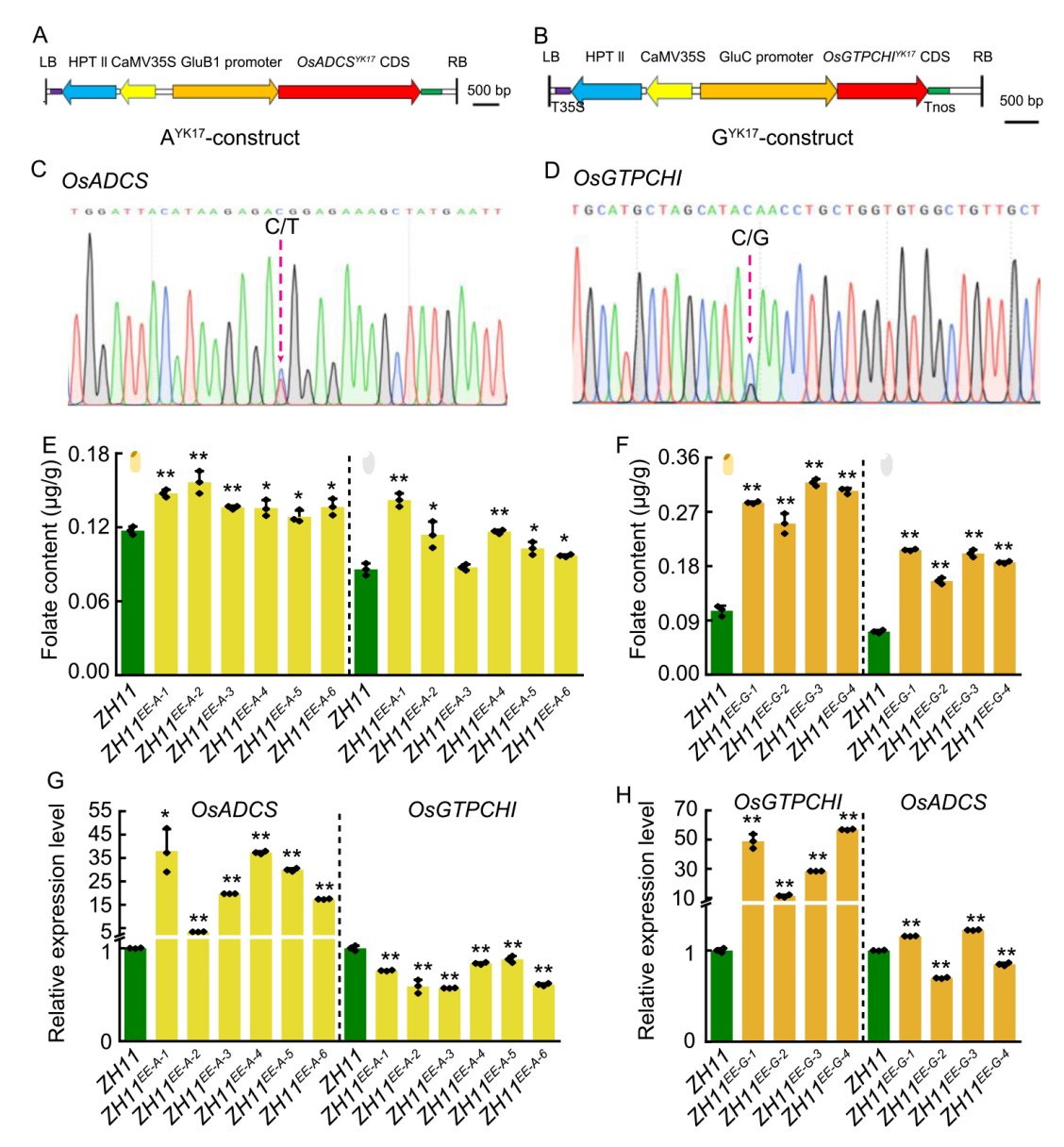
Fig. 3. Endosperm-specific expression of OsADCS and OsGTPCHI in rice. A and B, Schematic diagrams of T-DNA regions in plant transformation vectors for AYK17-construct (A) and GYK17-construct (B). LB/RB, Left/right T-DNA borders; T35S, 35S transcriptional terminator; Tnos, Nopaline synthase terminator; CaMV35S, Cauliflower mosaic virus 35S promoter; HPTII, Hygromycin phosphotransferase II. AYK17 and GYK17 indicate endosperm-specific expression vectors with indica (Zhongjiazao 17, YK17) alleles of OsADCS and OsGTPCHI, respectively.C, Single nucleotide polymorphism (SNP) detection at position 4 216 bp in OsADCS from GluB1 promoter-derived expression lines (ZH11EE-A-1 to ZH11EE-A-6). Blue peak (C) and red peak (T) represent the japonica (Zhonghua 11, ZH11) and indica YK17 alleles, respetively. D, SNP analysis at position 2 828 bp in OsGTPCHI from GluC promoter-derived expression lines (ZH11EE-G-1 to ZH11EE-G-4). Blue (C, ZH11) and black (G, YK17) peaks indicate parental alleles. E and F, Folate content comparisons between: ZH11 and endosperm-specific expression of OsADCS lines (ZH11EE-A-1 to ZH11EE-A-6, E); ZH11 and endosperm-specific expression of OsGTPCHI lines (ZH11EE-G-1 to ZH11EE-G-4, F). Brown rice (left) and polished rice (right) are shown.G and H, Relative expression levels of OsADCS (G) and OsGTPCHI (H) genes in rice grains at 15 d after flowering from endosperm-specific expression lines versus ZH11 control, normalized to Ubiquitin (LOC_ Os03g13170) gene.Data in E‒H are Mean ± SD from three biological replicates, with statistical significance denoted by asterisks (*, P < 0.05; **, P < 0.01).
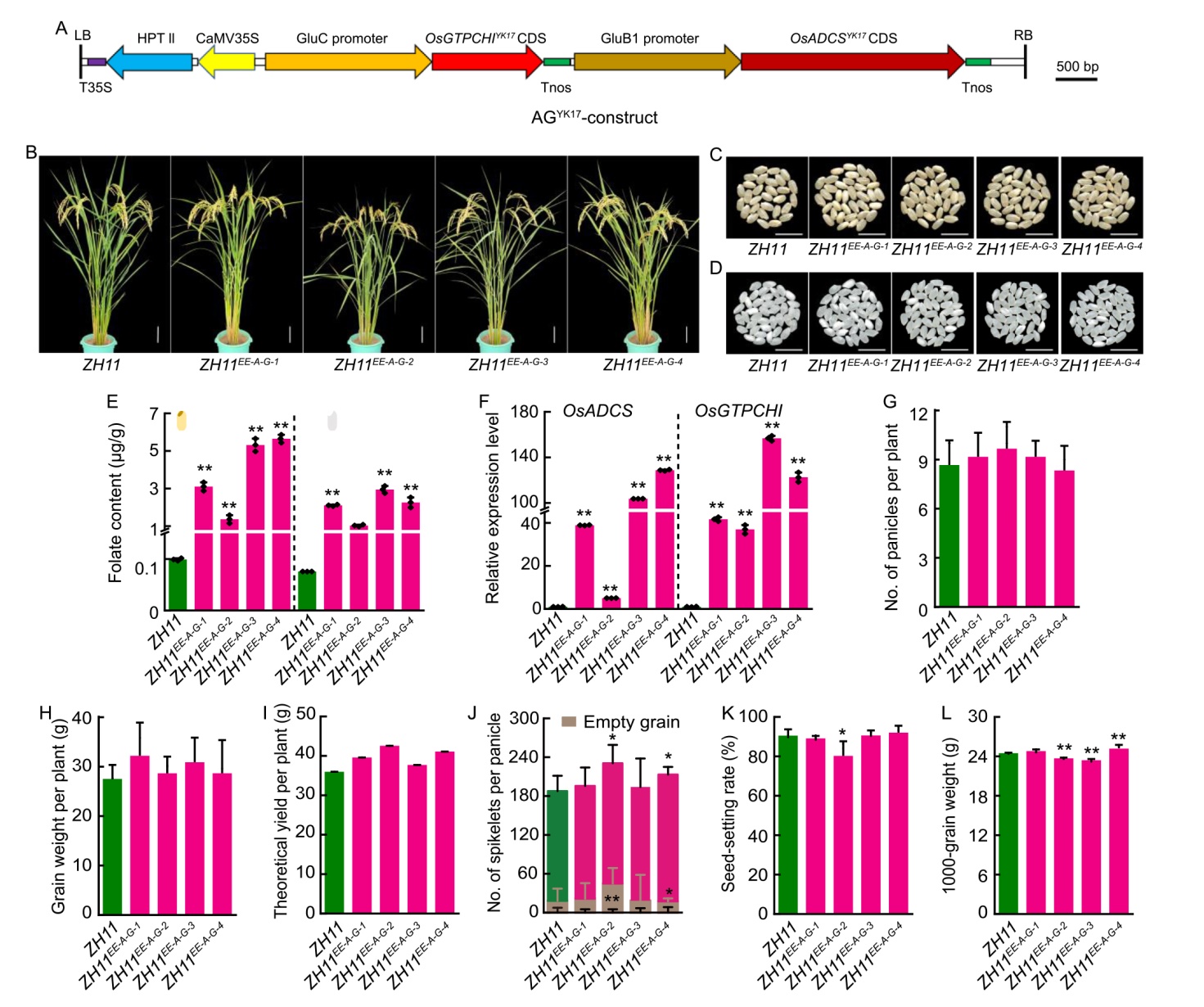
Fig. 4. Co-expression of OsADCS and OsGTPCHI enhanced folate accumulation. A, Structure schematic diagram of AGYK17-construct in plant transformation vector. LB/RB, Left/right T-DNA borders; T35S, 35S transcriptional terminator; Tnos, Nopaline synthase terminator; CaMV35S, Cauliflower mosaic virus 35S promoter; HPTII, Hygromycin phosphotransferase II. AGYK17 contains both OsADCS and OsGTPCHI alleles from indica variety Zhongjiazao 17 (YK17) for endosperm-specific co-expression.B, Plant morphology of Zhonghua 11 (ZH11) and ZH11AG+-lines (ZH11EE-A-G-1 to ZH11EE-A-G-4) at the late grain-filling stage. Scale bars, 10 cm.C and D, Grain morphology of brown rice (C) and polished rice(D) in ZH11 and ZH11AG+-lines. Scale bars, 10 mm.E, Comparison of folate contents between ZH11 and ZH11AG+-lines. Brown rice (left) and polished rice (right) are shown.F, Relative expression levels of OsADCS and OsGTPCHI genes in developing grains (15 d after flowering) from ZH11 and ZH11AG+-lines, normalized to Ubiquitin (LOC_Os03g13170). G‒L, Panicle number per plant (G), grain weight per plant (H), theoretical yield per plant (I), spikelet number per panicle (J), seed-setting rate (K), and 1000-grain weight (L) from ZH11 and ZH11AG+-lines.In B‒L, ZH11AG+ indicates endosperm-specific co-expression of OsADCS and OsGTPCHI in the background of japonica rice variety ZH11. Data in E‒K are presented as Mean ± SD from three (E, F, and L) and six (G‒K) biological replicates. Asterisks denote statistical significance by two-tailed t-test (*, P < 0.05; **, P < 0.01), compared with ZH11.
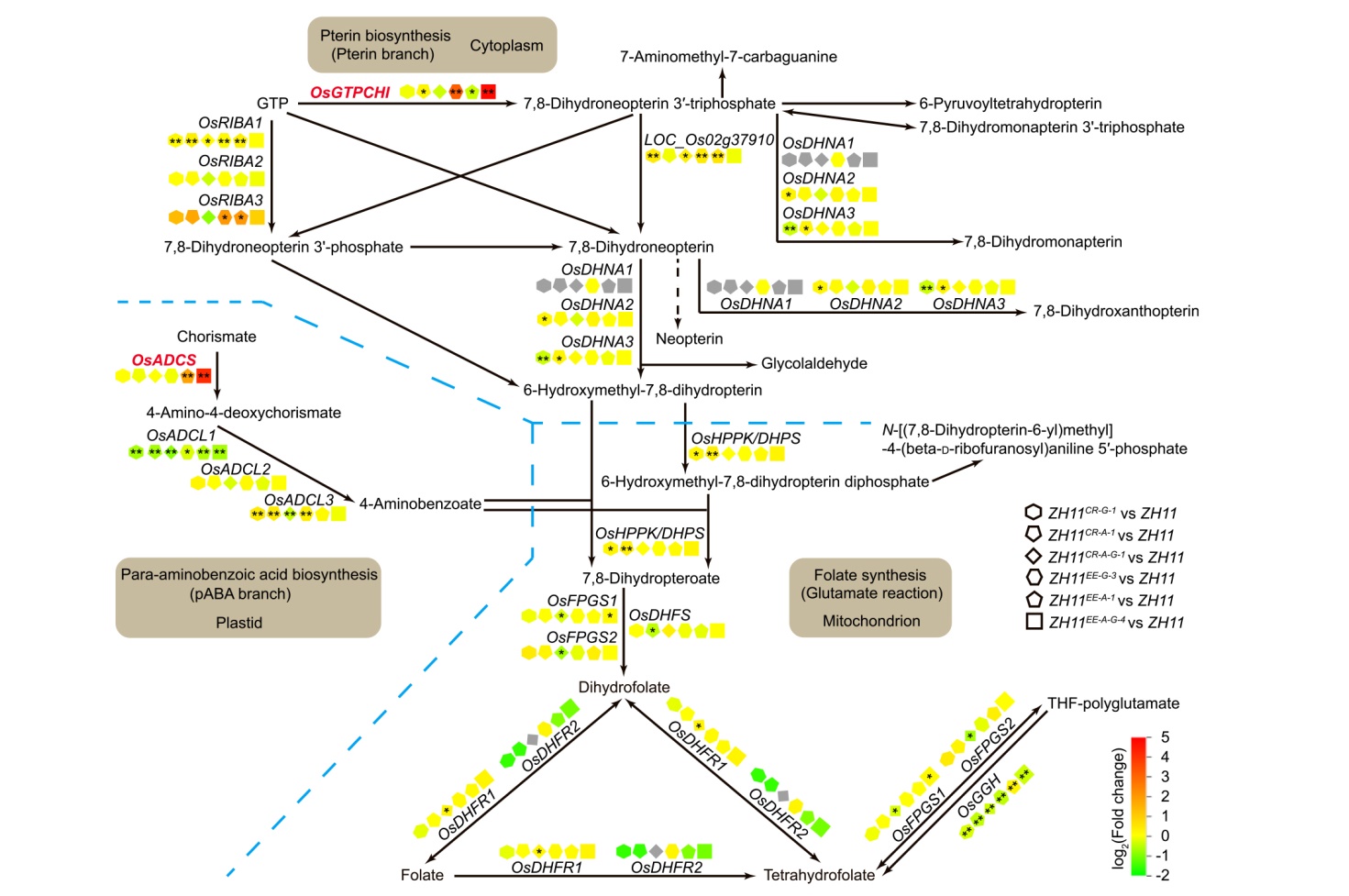
Fig. 5. Biosynthesis pathway and expression patterns of folate synthesis genes in rice grains at 25 d after flowering, according to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway database. ADCS, Aminodeoxychorismate synthase; ADCL, Aminodeoxychorismate lyase; GTP, Guanosine triphosphate; GTPCHI, GTP cyclohydrolase I; RIBA, Riboflavin biosynthesis protein; DHNA, Dihydroneopterinaldolase; HPPK/DHPS, Hydroxymethyldihydropterin pyrophosphokinase/dihydropteroate synthase; FPGS, Folylpolyglutamate synthase; DHFS, Dihydrofolate synthetase; DHFR, Dihydrofolate reductase; GGH, γ-Glutamyl hydrolase; THF, Tetrahydrofolate. Bule dashed lines represent different cell compartmentation, black arrows indicate folate synthesis direction. Asterisks denote statistical significance by hypergeometric distribution test (*, P < 0.05; **, P < 0.01).
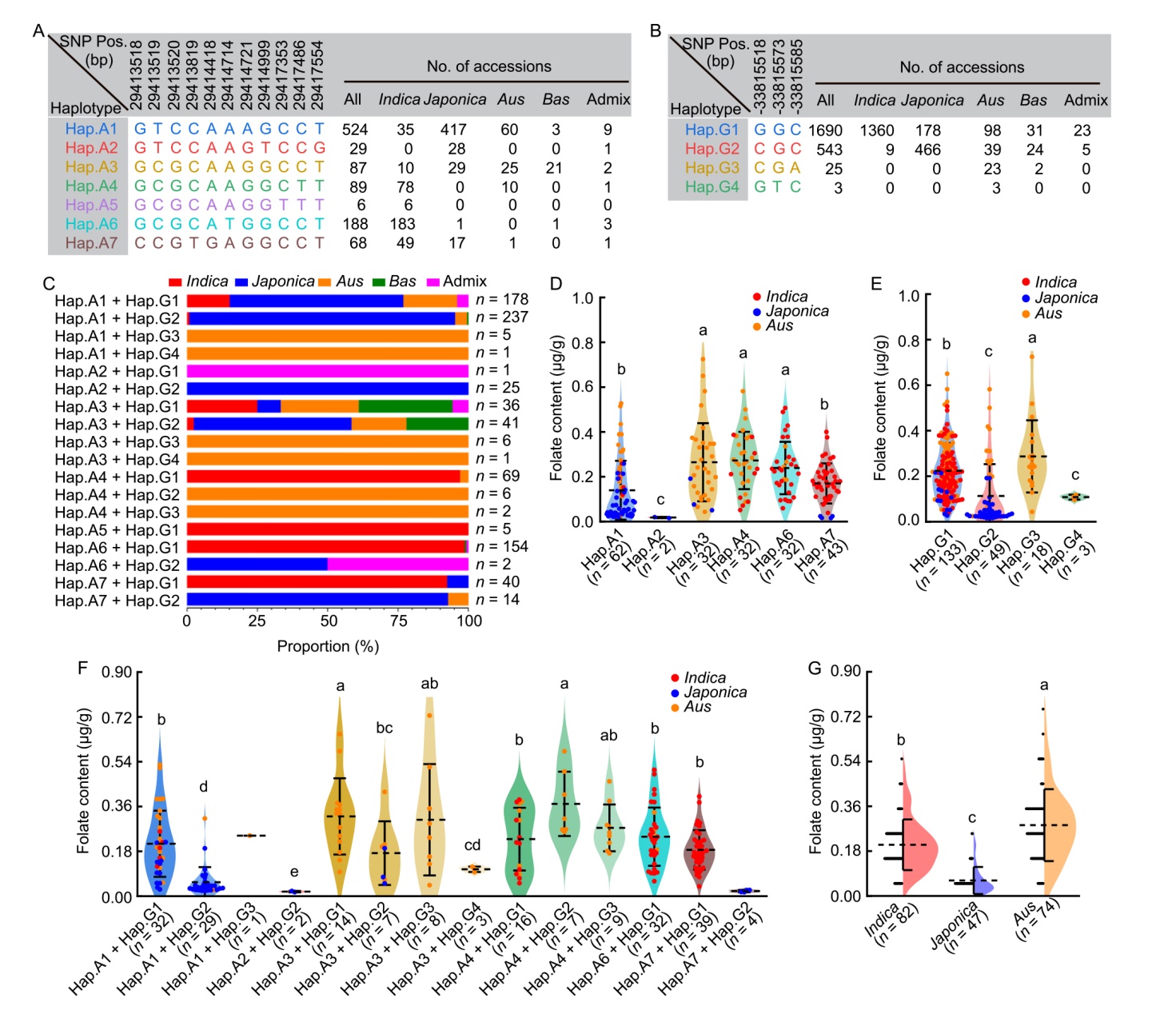
Fig. 6. Natural variation in OsADCS and OsGTPCHI is associated with differences on rice folate content. A, Haplotype analysis of OsADCS (LOC_Os06g48620) in 991 accessions from Rice SNP-Seek Database using 11 nonsynonymous single nucleotide polymorphisms (SNPs) in coding sequence (CDS).B, Haplotype analysis of OsGTPCHI (LOC_Os04g56710) in 2 261 accessions form Rice SNP-Seek Database using 3 nonsynonymous SNPs in CDS.C, Haplotype combination proportion of OsADCS and OsGTPCHI in 823 accessions from Rice SNP-Seek Database.D‒F, Folate content distribution in brown rice among 203 collected germplasms in OsADCS haplotypes (D), OsGTPCHI haplotypes (E), and haplotype combinations (F).G, Comparison of folate content of brown rice between indica rice, japonica rice, and Aus rice, in 203 collected germplasms. Short horizontal lines represent sample size for each group.Data in D‒G are Mean ± SD. Different lowercase letters above bars indicate statistically significant differences (P < 0.05, two-tailed t-tests).
| [1] | Aiyswaraya K S, Saraswathi R, Ramchander S, et al. 2020. Review on: Folate in crop plants. Int J Curr Microbiol Appl Sci, 9(3): 2831-2836. |
| [2] | Akhtar T A, Orsomando G, Mehrshahi P, et al. 2010. A central role for gamma-glutamyl hydrolases in plant folate homeostasis. Plant J, 64(2): 256-266. |
| [3] | Anukul N, Ramos R A, Mehrshahi P, et al. 2010. Folate polyglutamylation is required for rice seed development. Rice, 3: 181-193. |
| [4] | Basset G, Quinlivan E P, Ziemak M J, et al. 2002. Folate synthesis in plants: The first step of the pterin branch is mediated by a unique bimodular GTP cyclohydrolase I. Proc Natl Acad Sci USA, 99(19): 12489-12494. |
| [5] | Basset G J C, Quinlivan E P, Ravanel S, et al. 2004a. Folate synthesis in plants: The p-aminobenzoate branch is initiated by a bifunctional PabA-PabB protein that is targeted to plastids. Proc Natl Acad Sci USA, 101(6): 1496-1501. |
| [6] | Basset G J C, Ravanel S, Quinlivan E P, et al. 2004b. Folate synthesis in plants: The last step of the p-aminobenzoate branch is catalyzed by a plastidial aminodeoxychorismate lyase. Plant J, 40(4): 453-461. |
| [7] | Bhullar N K, Gruissem W. 2013. Nutritional enhancement of rice for human health: The contribution of biotechnology. Biotechnol Adv, 31(1): 50-57. |
| [8] | Blancquaert D, Storozhenko S, Loizeau K, et al. 2010. Folates and folic acid: From fundamental research toward sustainable health. Crit Rev Plant Sci, 29(1): 14-35. |
| [9] | Blancquaert D, van Daele J, Storozhenko S, et al. 2013a. Rice folate enhancement through metabolic engineering has an impact on rice seed metabolism, but does not affect the expression of the endogenous folate biosynthesis genes. Plant Mol Biol, 83(4/5): 329-349. |
| [10] | Blancquaert D, Storozhenko S, van Daele J, et al. 2013b. Enhancing pterin and para-aminobenzoate content is not sufficient to successfully biofortify potato tubers and Arabidopsis thaliana plants with folate. J Exp Bot, 64(12): 3899-3909. |
| [11] | Blancquaert D, van Daele J, Strobbe S, et al. 2015. Improving folate (vitamin B9) stability in biofortified rice through metabolic engineering. Nat Biotechnol, 33(10): 1076-1078. |
| [12] | Boggio S B, Palatnik J F, Heldt H W, et al. 2000. Changes in amino acid composition and nitrogen metabolizing enzymes in ripening fruits of Lycopersicon esculentum Mill. Plant Sci, 159(1): 125-133. |
| [13] | Bouis H E, Saltzman A. 2017. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob Food Sec, 12: 49-58. |
| [14] | Cole P D, Kamen B A, Gorlick R, et al. 2001. Effects of over-expression of gamma-glutamyl hydrolase on methotrexate metabolism and resistance. Cancer Res, 61(11): 4599-4604. |
| [15] | de la Garza R D, Quinlivan E P, Klaus S M J, et al. 2004. Folate biofortification in tomatoes by engineering the pteridine branch of folate synthesis. Proc Natl Acad Sci USA, 101(38): 13720-13725. |
| [16] | de la Garza R I D,Gregory 3rd J F, Hanson A D. 2007. Folate biofortification of tomato fruit. Proc Natl Acad Sci USA, 104(10): 4218-4222. |
| [17] | Dong W, Cheng Z J, Wang X L, et al. 2011. Determination of folate content in rice germplasm (Oryza sativa L.) using tri-enzyme extraction and microbiological assays. Int J Food Sci Nutr, 62(5): 537-543. |
| [18] | Dong W, Cheng Z J, Lei C L, et al. 2014. Overexpression of folate biosynthesis genes in rice (Oryza sativa L.) and evaluation of their impact on seed folate content. Plant Foods Hum Nutr, 69(4): 379-385. |
| [19] | Fabio L C M, Lucas T M R, Sandra H U T, et al. 2018. Rice (Oryza sativa) breeding strategies for grain biofortification. Afr J Biotechnol, 17(14): 466-477. |
| [20] | Gillies S A, McIntosh S R, Henry R J. 2008. A Cereal Crop with Enhanced Folate: Rice Transgenic for Wheat HPPK/DHPS. ComBio, Canberra, ACT, September 21-25, 2008. |
| [21] | Gorelova V, Ambach L, Rébeillé F, et al. 2017. Folates in plants: Research advances and progress in crop biofortification. Front Chem, 5: 21. |
| [22] | Hossain T, Rosenberg I, Selhub J, et al. 2004. Enhancement of folates in plants through metabolic engineering. Proc Natl Acad Sci USA, 101(14): 5158-5163. |
| [23] | Hui S Z, Li H J, Mawia A M, et al. 2022. Production of aromatic three-line hybrid rice using novel alleles of BADH2. Plant Biotechnol J, 20(1): 59-74. |
| [24] | Khan A, Pudhuvai B, Shrestha A, et al. 2024. CRISPR-mediated iron and folate biofortification in crops: Advances and perspectives. Biotechnol Genet Eng Rev, 40(4): 4138-4168. |
| [25] | Kiekens F, Blancquaert D, Devisscher L, et al. 2015. Folates from metabolically engineered rice: A long-term study in rats. Mol Nutr Food Res, 59(3): 490-500. |
| [26] | Kim D, Langmead B, Salzberg S L. 2015. HISAT: A fast spliced aligner with low memory requirements. Nat Methods, 12(4): 357-360. |
| [27] | Lian T, Wang X X, Li S, et al. 2022. Comparative transcriptome analysis reveals mechanisms of folate accumulation in maize grains. Int J Mol Sci, 23(3): 1708. |
| [28] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25(4): 402-408. |
| [29] | Love M I, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2 Genome Biol, 15(12): 550. |
| [30] | Majumder S, Datta K, Datta S K. 2019. Rice biofortification: High iron, zinc, and vitamin-A to fight against ‘hidden hunger’. Agronomy, 9(12): 803. |
| [31] | McIntosh S R, Brushett D, Henry R J. 2008. GTP cyclohydrolase 1 expression and folate accumulation in the developing wheat seed. J Cereal Sci, 48(2): 503-512. |
| [32] | Mohidem N A, Hashim N, Shamsudin R, et al. 2022. Rice for food security: Revisiting its production, diversity, rice milling process and nutrient content. Agriculture, 12(6): 741. |
| [33] | Nar H, Huber R, Auerbach G, et al. 1995. Active site topology and reaction mechanism of GTP cyclohydrolase I. Proc Natl Acad Sci USA, 92(26): 12120-12125. |
| [34] | Paine J A, Shipton C A, Chaggar S, et al. 2005. Improving the nutritional value of Golden Rice through increased pro-vitamin A content. Nat Biotechnol, 23(4): 482-487. |
| [35] | Qian D D, Qiu B, Zhou N, et al. 2020. Hypotensive activity of transgenic rice seed accumulating multiple antihypertensive peptides. J Agric Food Chem, 68(27): 7162-7168. |
| [36] | Saltzman A, Birol E, Bouis H E, et al. 2013. Biofortification: Progress toward a more nourishing future. Glob Food Secur, 2(1): 9-17. |
| [37] | Shan Q J, Liu J H, Li W, et al. 2019. Comprehensive evaluation of biosynthesis, accumulation, regulation of folate and vitamin C in waxy maize (Zea mays L. var. Ceratina) with kernel development. J Cereal Sci, 87: 215-224. |
| [38] | Sharma P, Aggarwal P, Kaur A. 2017. Biofortification: A new approach to eradicate hidden hunger. Food Rev Int, 33(1): 1-21. |
| [39] | Shohag M J I, Wei Y Y, Zhang J, et al. 2020. Genetic and physiological regulation of folate in pak choi (Brassica rapa subsp. Chinensis) germplasm. J Exp Bot, 71(16): 4914-4929. |
| [40] | Sohta Y, Ohta T, Masada M. 1997. Purification and some properties of GTP cyclohydrolase I from spinach leaves. Biosci Biotechnol Biochem, 61(7): 1081-1085. |
| [41] | Storozhenko S, de Brouwer V, Volckaert M, et al. 2007. Folate fortification of rice by metabolic engineering. Nat Biotechnol, 25(11): 1277-1279. |
| [42] | Strobbe S, van der Straeten D. 2017. Folate biofortification in food crops. Curr Opin Biotechnol, 44: 202-211. |
| [43] | van der Straeten D, Bhullar N K, de Steur H, et al. 2020. Multiplying the efficiency and impact of biofortification through metabolic engineering. Nat Commun, 11(1): 5203. |
| [44] | Viswanathan V K, Green J M, Nichols B P. 1995. Kinetic characterization of 4-amino 4-deoxychorismate synthase from Escherichia coli. J Bacteriol, 177(20): 5918-5923. |
| [45] | Wakasa K, Hasegawa H, Nemoto H, et al. 2006. High-level tryptophan accumulation in seeds of transgenic rice and its limited effects on agronomic traits and seed metabolite profile. J Exp Bot, 57(12): 3069-3078. |
| [46] | Waller J C, Akhtar T A, Lara-Núñez A, et al. 2010. Developmental and feedforward control of the expression of folate biosynthesis genes in tomato fruit. Mol Plant, 3(1): 66-77. |
| [47] | Wirth J, Poletti S, Aeschlimann B, et al. 2009. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J, 7(7): 631-644. |
| [48] | Zhao M C, Lin Y J, Chen H. 2020. Improving nutritional quality of rice for human health. Theor Appl Genet, 133(5): 1397-1413. |
| [1] | Sara Cannavò, Chiara Paleni, Alma Costarelli, Maria Cristina Valeri, Martina Cerri, Antonietta Saccomanno, Veronica Gregis, Graziella Chini Zittelli, Petre I. Dobrev, Lara Reale, Martin M. Kater, Francesco Paolocci. Assessing Changes in Root Architecture, Developmental Timing, Transcriptional and Hormonal Profiles in Rice Co-Cultivated with Azolla filiculoides [J]. Rice Science, 2025, 32(3): 426-444. |
| [2] | Nie Lixiao, Guo Xiayu, Wang Weiqin, Qi Yucheng, Ai Zhiyong, He Aibin. Regulation of Regeneration Rate to Enhance Ratoon Rice Production [J]. Rice Science, 2025, 32(2): 177-192. |
| [3] | Wang Jingqing, Wang Yaliang, Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping. Progress on Physiological Mechanisms of Rice Spikelet Degeneration at Different Panicle Positions Caused by Abiotic Stress [J]. Rice Science, 2025, 32(2): 193-202. |
| [4] | Chen Ya, Liu Zhiquan, Yang Linyin, Wu Fujie, Cao Zijian, Shi Huanbin, Qiu Jiehua, Kou Yanjun. OsCERK1 Interacts with OsHPP08 to Regulate Copper Uptake and Blast Resistance in Rice [J]. Rice Science, 2025, 32(2): 203-216. |
| [5] | Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight [J]. Rice Science, 2025, 32(1): 118-130. |
| [6] | Yu Shicong, Luo Ruxian, Zheng Shuqin, Ning Jing, Shi Yuanzhu, Guo Daiming, Jia Liangmeng, Wang Sen, Xiao Guizong, Guo Pengwang, Li Yang, Ma Xiaoding. CHOLINE TRANSPORTER-RELATED 4 (CTR4) Is Involved in Drought and Saline Tolerance in Rice [J]. Rice Science, 2025, 32(1): 52-66. |
| [7] | Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance [J]. Rice Science, 2024, 31(6): 688-699. |
| [8] | Sitthikorn Bodeerath, Jeeraporn Veeradittakit, Sansanee Jamjod, Chanakan Prom-U-Thai. Applying Boron Fertilizer at Different Growth Stages Promotes Boron Uptake and Productivity in Rice [J]. Rice Science, 2024, 31(6): 751-760. |
| [9] | Kunhikrishnan Hemalatha Dhanyalakshmi, Reshma Mohan, Sasmita Behera, Uday Chand Jha, Debashis Moharana, Ahalya Behera, Sini Thomas, Preman Rejitha Soumya, Rameswar Prasad Sah, Radha Beena. Next Generation Nutrition: Genomic and Molecular Breeding Innovations for Iron and Zinc Biofortification in Rice [J]. Rice Science, 2024, 31(5): 526-544. |
| [10] | Nitin Sharma, Bhupinder Singh, Subbaiyan Gopala Krishnan, Haritha Bollinedi, Pranab Kumar Mandal, Milan Kumar Lal, Prakash Kumar Jha, P. V. Vara Prasad, Anjali Anand. Higher Grain-Filling Rate in Inferior Spikelets of Tolerant Rice Genotype Offset Grain Yield Loss under Post-Anthesis High Night Temperatures [J]. Rice Science, 2024, 31(5): 572-586. |
| [11] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [12] | Supranee Santanoo, Wichian Sangwongchai, Maysaya Thitisaksakul, Suphatta Phothiset, Paweena Pongdontri, Noppawan Nounjan, Piyada Theerakulpisut. Rice Grains from Slightly Saline Field Exhibited Unchanged Starch Physicochemical Properties but Enhanced Nutritional Values [J]. Rice Science, 2024, 31(3): 343-360. |
| [13] | Hong Weiyuan, Duan Meiyang, Wang Yifei, Chen Yongjian, Mo Zhaowen, Qi Jianying, Pan Shenggang, Tang Xiangru. Enriching Iodine and Regulating Grain Aroma, Appearance Quality, and Yield in Aromatic Rice by Foliar Application of Sodium Iodide [J]. Rice Science, 2024, 31(3): 328-342. |
| [14] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [15] | Ji Dongling, Xiao Wenhui, Sun Zhiwei, Liu Lijun, Gu Junfei, Zhang Hao, Matthew Tom Harrison, Liu Ke, Wang Zhiqin, Wang Weilu. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 598-612. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||