
Rice Science ›› 2025, Vol. 32 ›› Issue (2): 193-202.DOI: 10.1016/j.rsci.2024.09.002
• Reviews • Previous Articles Next Articles
Wang Jingqing#, Wang Yaliang#( ), Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping(
), Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping( )
)
Received:2024-08-07
Accepted:2024-09-27
Online:2025-03-28
Published:2025-04-14
Contact:
Wang Yaliang (wangyaliang@caas.cn); Zhang Yuping (cnrrizyp@163.com)
About author: These authors contributed equally to this work
Wang Jingqing, Wang Yaliang, Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping. Progress on Physiological Mechanisms of Rice Spikelet Degeneration at Different Panicle Positions Caused by Abiotic Stress[J]. Rice Science, 2025, 32(2): 193-202.
Add to citation manager EndNote|Ris|BibTeX
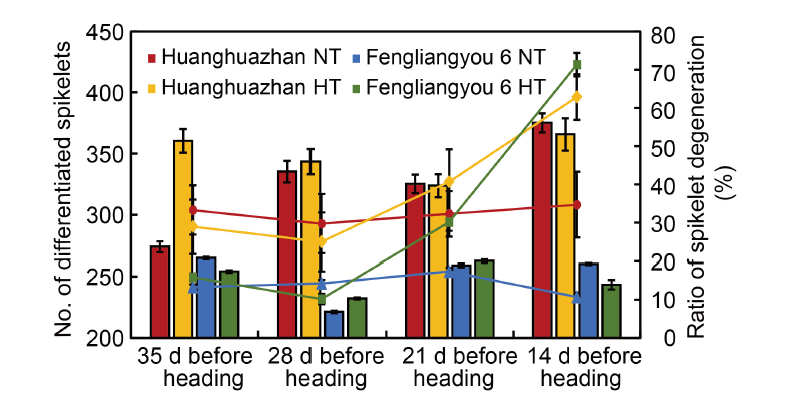
Fig. 2. Number of differentiated spikelets and spikelet degeneration at different panicle initiation periods under high temperatures compared with normal temperatures. Data are from the authors’ pot experiment conducted in 2014 with two rice varieties Huanghuazhan and Fengliangyou 6. The experiment included a high-temperature treatment with a peak temperature of 40 ºC (HT) and a normal-temperature treatment with a peak temperature of 32 ºC as the control (NT). The experiment was conducted for 7 d at 35, 28, 21, and 14 d before panicle heading, respectively. Data are Mean ± SD (n = 3).
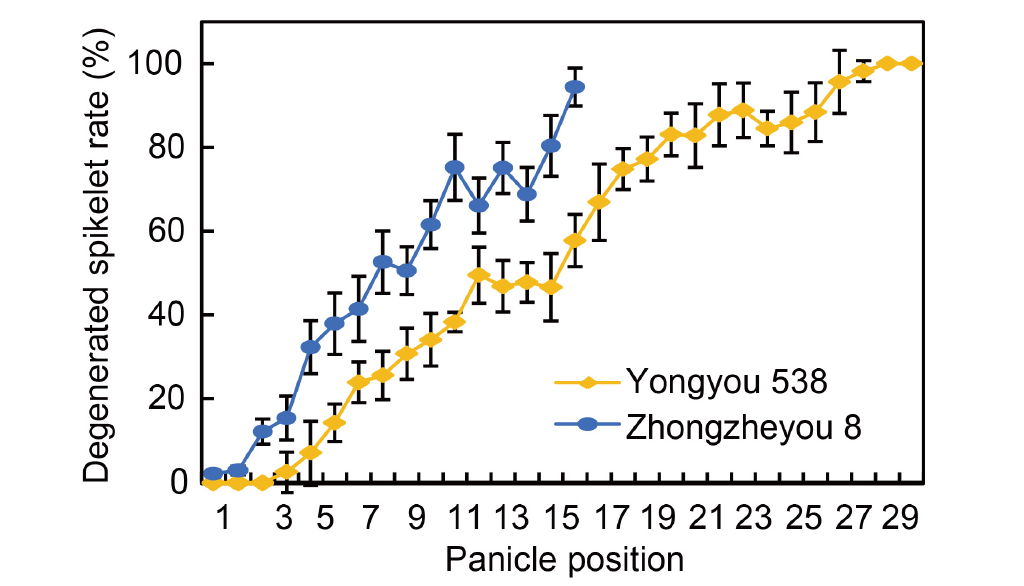
Fig. 3. Frequency of spikelet degeneration and its variability within panicle positions. The data are from an experiment conducted in 2023. The panicle positions were determined by counting the primary branches from the top to the base and were designated as positions 1 through 30. The panicle was divided into three parts, the upper, middle, and basal parts. The upper part was defined as including the top 1/3 of the branches, the middle part as including the middle 1/3 of the branches, and the basal part as including the bottom 1/3 of branches. A spikelet that appeared white and withered on a panicle was defined as degenerated. Data are Mean ± SD (n = 3).
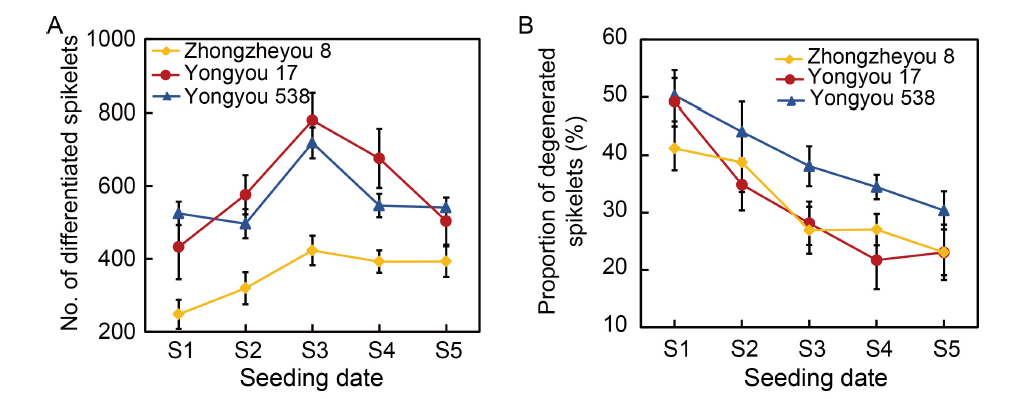
Fig. 4. Characteristics of spikelet differentiation and degeneration in various rice varieties. The data are from a seeding experiment with the application of 180 kg/hm2 nitrogen in 2020. Seeding dates were as follows: S1, 1 April; S2, 21 April; S3, 11 May; S4, 31 May; and S5, 20 June. Rice panicles of uniform size were sampled at the heading stage, and all existing and degenerated primary and secondary spikelets were counted. The number of differentiated spikelets was determined as the total number of developed and degenerated spikelets. The ratio of degenerated spikelets was calculated by dividing the number of all degenerated spikelets (including both primary and secondary spikelets) at each panicle position by the total number of differentiated spikelets. Data are Mean ± SD (n = 3).
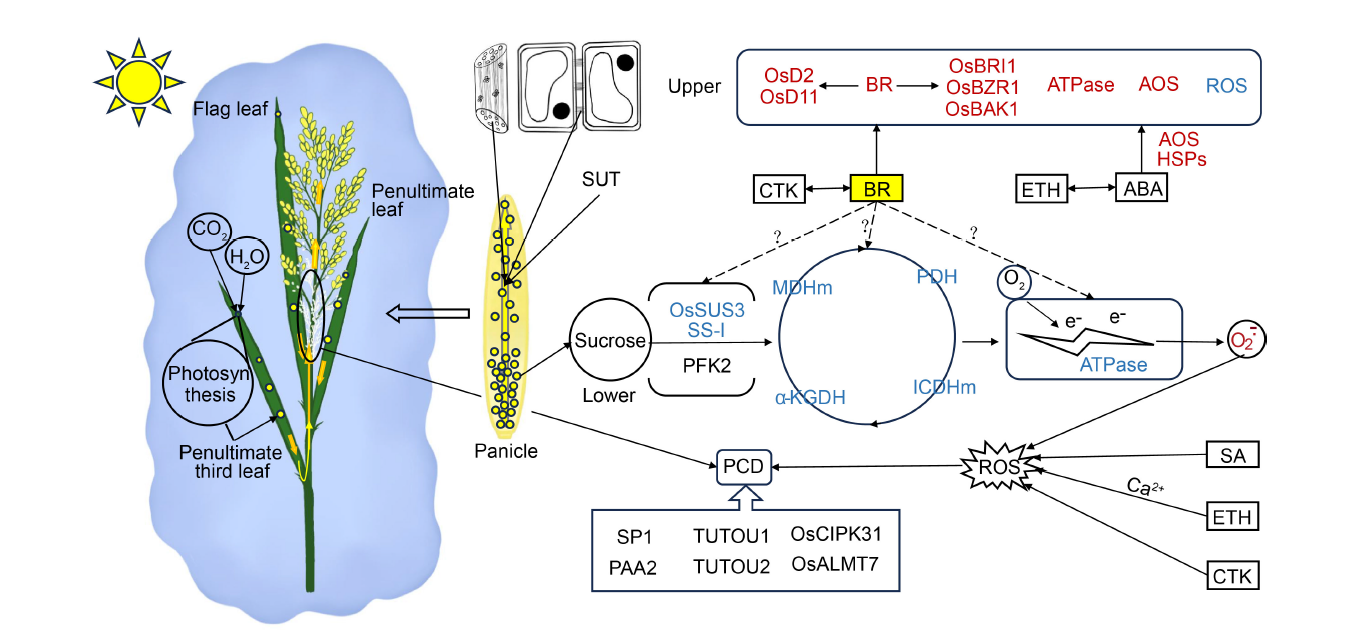
Fig. 5. Physiological pathways underlying positional differences in spikelet degeneration within panicles. Photosynthetic products from the top three leaves of rice plants are transported from the base of the apex of the panicle via the panicle stem. Sucrose transport, facilitated by vascular tissues and plasmodesmata, is regulated by the sucrose transporter SUT. Apical dominance in sucrose utilization may contribute to the growth of the upper part of the spikelet, although the exact relationship is not well understood and may be influenced by BR. The content of BR is higher in the upper part of the spikelet compared with the lower part, and this higher BR content in the upper part of the spikelet may enhance the activity of enzymes involved in sucrose degradation. Similarly, the levels of ATPase and ROS are higher in the upper part of the spikelet, which can promote spikelet growth and development, whereas they are lower in the basal part of the spikelet. Alternatively, a lower frequency of spikelet degeneration may be attributed to programmed cell death, a process regulated by both genes and ROS accumulation. Red font indicates high or increased levels, blue font indicates low or decreased levels, and ‘?’ denotes mechanisms that require further investigation. AOS, Antioxidant system; ROS, Reactive oxygen species; CTK, Cytokinin; BR, Brassinolide; ETH, Ethylene; ABA, Abscisic acid; SA, Salicylic acid; HSPs, Heat shock proteins; PCD, Programmed cell death; PDH, Pyruvate dehydrogenase; ICDHm, Isocitrate dehydrogenase; α-KGDH, Alpha-ketoglutarate dehydrogenase; MDHm, Malate dehydrogenase.
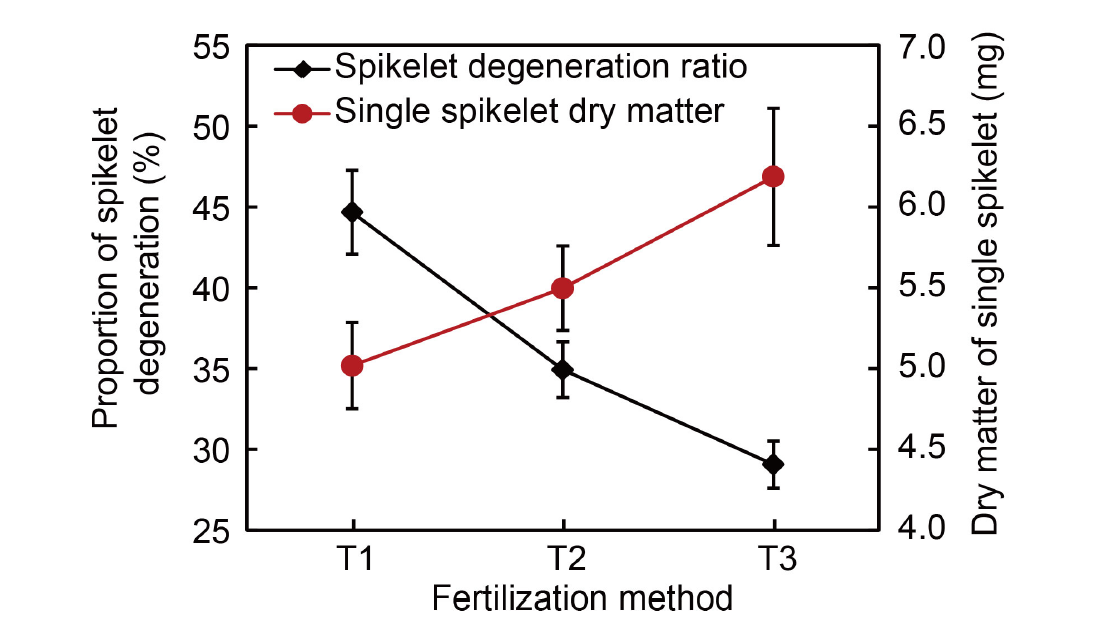
Fig. 6. Effects of different fertilization methods on dry matter accumulation in single spikelets and spikelet degeneration. Data are from authors’ experiment conducted in 2023. The experiment involved three fertilization methods: T1, Application of 100% flowering- promoting fertilizer at 30‒35 d before heading; T2, Application of 50% flowering-promoting fertilizer at 30‒35 d before heading and 50% flowering-preserving fertilizer at 15‒20 d before heading; T3, Application of 100% flowering-preserving fertilizer at 15‒20 d before heading. The total nitrogen application rate was 270 kg/hm2. Data are Mean ± SD (n = 3).
| [1] | Aoki N, Hirose T, Scofield G N, et al. 2003. The sucrose transporter gene family in rice. Plant Cell Physiol, 44(3): 223-232. |
| [2] | Bai J T, Zhu X D, Wang Q, et al. 2015. Rice TUTOU1 encodes a suppressor of cAMP receptor-like protein that is important for actin organization and panicle development. Plant Physiol, 169(2): 1179-1191. |
| [3] | Meier U. 2001. Growth Stages of Mono and Dicotyledonous Plants. Bonn, Germany: Federal Biological Research Centre for Agriculture and Forestry: 18-22. |
| [4] | Bolouri-Moghaddam M R, Le Roy K, Xiang L, et al. 2010. Sugar signalling and antioxidant network connections in plant cells. FEBS J, 277(9): 2022-2037. |
| [5] | Boyer J S, Westgate M E. 2004. Grain yields with limited water. J Exp Bot, 55: 2385-2394. |
| [6] | Cao D, Chabikwa T, Barbier F, et al. 2023. Auxin-independent effects of apical dominance induce changes in phytohormones correlated with bud outgrowth. Plant Physiol, 192(2): 1420-1434. |
| [7] | Chen L Q, Qu X Q, Hou B H, et al. 2012. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science, 335: 207-211. |
| [8] | Chen Y H, Chen H Z, Xiang J, et al. 2021. Rice spikelet formation inhibition caused by decreased sugar utilization under high temperature is associated with brassinolide decomposition. Environ Exp Bot, 190: 104585. |
| [9] | Chu G, Chen S, Xu C M, et al. 2023. Ethylene and polyamines interact in rice spikelet degeneration in response to water stress during meiosis. Plant Growth Regul, 101(3): 617-628. |
| [10] | Ding C Q, Wang Y, Chang Z Y, et al. 2016. Comparative proteomic analysis reveals nitrogen fertilizer increases spikelet number per panicle in rice by repressing protein degradation and 14-3-3 proteins. J Plant Growth Regul, 35(3): 744-754. |
| [11] | Fu R, Shang B, Zhang G Y, et al. 2021. Differential effects of ozone pollution on photosynthesis and growth of rice during two growth stages. J Agro-Environ Sci, 40(10): 2066-2075. (in Chinese with English abstract) |
| [12] | Gill S S, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem, 48(12): 909-930. |
| [13] | Guo C H, Zhu X F, Sun S, et al. 2023. Analysis of high temperature hazard in different climate regions of China. J Trop Meteor, 39(1): 66-77. (in Chinese with English abstract) |
| [14] | Heng Y Q, Wu C Y, Long Y, et al. 2018. OsALMT7 maintains panicle size and grain yield in rice by mediating malate transport. Plant Cell, 30(4): 889-906. |
| [15] | Itoh J I, Nonomura K I, Ikeda K, et al. 2005. Rice plant development: From zygote to spikelet. Plant Cell Physiol, 46(1): 23-47. |
| [16] | Jagadish K S V, Craufurd P, Shi W J, et al. 2014. A phenotypic marker for quantifying heat stress impact during microsporogenesis in rice (Oryza sativa L.). Funct Plant Biol, 41(1): 48-55. |
| [17] | Jiang N, Yu P H, Fu W M, et al. 2020. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ, 43(5): 1273-1287. |
| [18] | Jiang Z R, Chen Q L, Chen L, et al. 2021. Efficiency of sucrose to starch metabolism is related to the initiation of inferior grain filling in large panicle rice. Front Plant Sci, 12: 732867. |
| [19] | Jin Z X, Wang J, Wang S Y, et al. 2020. Analysis of gene expression related to young panicle differentiation of rice varieties with different panicle types. J Northeast Agric Univ, 51(12): 14-23. (in Chinese with English abstract) |
| [20] | Kobata T, Yoshida H, Masiko U, et al. 2013. Spikelet sterility is associated with a lack of assimilate in high-spikelet-number rice. Agron J, 105(6): 1821-1831. |
| [21] | Li G H, Wang H Z, Wang S H, et al. 2010. Effect of nitrogen applied at rice panicle initiation stage on carbon and nitrogen metabolism and spikelets per panicle. J Nanjing Agric Univ, 33(1): 1-5. (in Chinese with English abstract) |
| [22] | Li L F, Sun X T, Ouyang L J, et al. 2018. Influencing factors and genetic research progress of rice spikelet degeneration. J Nucl Agric Sci, 32(2): 291-296. (in Chinese with English abstract) |
| [23] | Li S B, Qian Q, Fu Z M, et al. 2009. Short panicle1 encodes a putative PTR family transporter and determines rice panicle size. Plant J, 58(4): 592-605. |
| [24] | Li W X, Dai L, Wang L H, et al. 2022. Carbon and nitrogen metabolism and yield composition in multi-panicle and large- panicle super rice varieties at young panicle differentiation stage. Acta Agric Bor-Sin, 37(4): 103-112. (in Chinese with English abstract) |
| [25] | Li Z, Mao B G, Heng Y Q, et al. 2014. Fine mapping of the panicle apical abortion PAA2 in rice (Oryza sativa). J Plant Genet Resour, 15(5): 1023-1027. (in Chinese with English abstract) |
| [26] | Li Z T, Wang S Y, Jiang W Y, et al. 2018. Physiological mechanisms of promoting source, sink, and grain filling by 24-epibrassinolide (EBR) applied at panicle initiation stage of rice. Acta Agron Sin, 44(4): 581-590. (in Chinese with English abstract) |
| [27] | Liang Z M, Cao X D, Gao R, et al. 2024. Brassinosteroids alleviates wheat floret degeneration under low nitrogen stress by promoting the distribution of sucrose from stems to spikes. J Integr Agric, (in press) |
| [28] | Liu K, Zhou S Q, Li S Y, et al. 2022. Differences and mechanisms of post-anthesis dry matter accumulation in rice varieties with different yield levels. Crop Environ, 1(4): 262-272. |
| [29] | Liu Y, Xiao W H, Cai W L, et al. 2023. Advances in studies on the roles of plant hormones in grain filling, grain weight and quality of rice. China Rice, 29(3): 9-14/23. (in Chinese with English abstract) |
| [30] | Long Q, Qiu S C, Man J M, et al. 2023. OsAAI1 increases rice yield and drought tolerance dependent on ABA-mediated regulatory and ROS scavenging pathway. Rice, 16(1): 35. |
| [31] | Mason M G, Ross J J, Babst B A, et al. 2014. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc Natl Acad Sci USA, 111(16): 6092-6097. |
| [32] | Matsushima S, Manaka T. 1957. Analysis of developmental factors determining yield and yield prediction in lowland rice. Proc Crop Sci Soc Jpn, 25(4): 203-206. |
| [33] | Miao N Y, Tang S, Chen W Z, et al. 2017. Research of nitrogen granular fertilizer alleviating high temperature stress at rice grain filling stage and its physiological mechanism. J Nanjing Agric Univ, 40(1): 1-10. (in Chinese with English abstract) |
| [34] | Nakamura A, Nakajima N, Goda H, et al. 2006. Arabidopsis Aux/IAA genes are involved in brassinosteroid-mediated growth responses in a manner dependent on organ type. Plant J, 45(2): 193-205. |
| [35] | Onukwufor J O, Berry B J, Wojtovich A P. 2019. Physiologic implications of reactive oxygen species production by mitochondrial complex I reverse electron transport. Antioxidants, 8(8): 285. |
| [36] | Panigrahi S, Kariali E, Dash S K, et al. 2023. Ethylene sensitivity underscores the yield advantage of high-grain numbers in cylinder-shaped rice panicles. Environ Exp Bot, 214: 105466. |
| [37] | Peng Y B, Hou F X, Bai Q, et al. 2018. Rice calcineurin B-like protein-interacting protein kinase 31 (OsCIPK31) is involved in the development of panicle apical spikelets. Front Plant Sci, 9: 1661. |
| [38] | Rezaul I M, Baohua F, Tingting C, et al. 2019. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol Plant, 165(3): 644-663. |
| [39] | Sun W, Lu C J, Wen L Y, et al. 2024. Low sucrose availability reduces basal spikelet fertility by inducing abscisic acid and jasmonic acid synthesis in wheat. J Exp Bot, 75(7): 1967-1981. |
| [40] | Takehara K, Murata K, Yamaguchi T, et al. 2018. Thermo-responsive allele of sucrose synthase 3 (Sus3) provides high-temperature tolerance during the ripening stage in rice (Oryza sativa L.). Breed Sci, 68(3): 336-342. |
| [41] | Tian J, Cheng Y Q, Kong X Y, et al. 2017. Induction of reactive oxygen species and the potential role of NADPH oxidase in hyperhydricity of garlic plantlets in vitro. Protoplasma, 254(1): 379-388. |
| [42] | Torres M A, Jones J D G, Dangl J L. 2006. Reactive oxygen species signaling in response to pathogens. Plant Physiol, 141(2): 373-378. |
| [43] | van Breusegem F, Vranová E, Dat J F, et al. 2001. The role of active oxygen species in plant signal transduction. Plant Sci, 161(3): 405-414. |
| [44] | Vescovi M, Riefler M, Gessuti M, et al. 2012. Programmed cell death induced by high levels of cytokinin in Arabidopsis cultured cells is mediated by the cytokinin receptor CRE1/ AHK4. J Exp Bot, 63(7): 2825-2832. |
| [45] | Wang C G, Zhang P Y, He Y, et al. 2023. Exogenous spraying of IAA improved the efficiency of microspore embryogenesis in Wucai (Brassica campestris L.) by affecting the balance of endogenous hormones, energy metabolism, and cell wall degradation. BMC Genomics, 24(1): 380. |
| [46] | Wang Y F, Lei B, Deng H B, et al. 2023. Exogenous abscisic acid affects the heat tolerance of rice seedlings by influencing the accumulation of ROS. Antioxidants, 12(7): 1404. |
| [47] | Wang Y L, Zhang Y P, Zeng Y H, et al. 2015. Effect of high temperature stress on rice spikelet differentiation and degeneration during panicle initiation stage. Chin J Agrometeorol, 36(6): 724-731. (in Chinese with English abstract) |
| [48] | Wang Y L, Zhang Y P, Zhu D F, et al. 2016. Effect of heat stress on spikelet degeneration and grain filling at panicle initiation period of rice. Acta Agron Sin, 42(9): 1402-1410. (in Chinese with English abstract) |
| [49] | Wang Y L, Zhang Y P, Xiang J, et al. 2017. Response of indica rice spikelet differentiation and degeneration to air temperature and solar radiation of different sowing dates. J Appl Ecol, 28(11): 3571-3580. (in Chinese with English abstract) |
| [50] | Wang Y L, Zhang Y K, Shi Q H, et al. 2020. Decrement of sugar consumption in rice young panicle under high temperature aggravates spikelet number reduction. Rice Sci, 27(1): 44-55. |
| [51] | Wang Z Q, Zhang W Y, Yang J C. 2018. Physiological mechanism underlying spikelet degeneration in rice. J Integr Agric, 17(7): 1475-1481. |
| [52] | Wen T G, Wang W Z, Yang W F, et al. 2019. Effects of exogenous plant growth regulator treatments on rice spikelet differentiation and degeneration during panicle initiation stage. Jiangsu J Agric Sci, 35(3): 514-522. (in Chinese with English abstract) |
| [53] | Wu C, Cui K H, Wang W C, et al. 2017. Heat-induced cytokinin transportation and degradation are associated with reduced panicle cytokinin expression and fewer spikelets per panicle in rice. Front Plant Sci, 8: 371. |
| [54] | Xu W B, Miao Y M, Kong J, et al. 2024. ROS signaling and its involvement in abiotic stress with emphasis on heat stress-driven anther sterility in plants. Crop Environ, 3(2): 65-74. |
| [55] | Yang K F, Yang L X, Wang Y X, et al. 2009. Effects of increasing surface ozone concentration on spikelet formation of hybrid rice cultivars. J Appl Ecol, 20(3): 609-614. (in Chinese with English abstract) |
| [56] | Yao J Y, Yu J X, Wang Z Q, et al. 2021. Response of endogenous brassinosteroids to nitrogen rates and its regulatory effect on spikelet degeneration in rice. Acta Agron Sin, 47(5): 894-903. (in Chinese with English abstract) |
| [57] | Yao Y L, Yamamoto Y, Wang Y L, et al. 2000. Heterosis in numbers of differentiated, degenerated, and surviving spikelets and their relations to the dry matter production in F1 hybrids of rice. Soil Sci Plant Nutr, 46(4): 951-962. |
| [58] | Zhang B, Zheng J C, Huang S, et al. 2008. Temperature differences of air-rice plant under different irrigated water depths at spiking stage. J Appl Ecol, 19(1): 87-92. (in Chinese with English abstract) |
| [59] | Zhang C X, Feng B H, Chen T T, et al. 2017. Sugars, antioxidant enzymes and IAA mediate salicylic acid to prevent rice spikelet degeneration caused by heat stress. Plant Growth Regul, 83(2): 313-323. |
| [60] | Zhang C X, Feng B H, Chen T T, et al. 2018. Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ Exp Bot, 155: 718-733. |
| [61] | Zhang G Y, Hu Y X, Pan X Y, et al. 2023. Effects of increased ozone on rice panicle morphology. iScience, 26(4): 106471. |
| [62] | Zhang W D, Dong M H, Li Y, et al. 2023. Effects of nitrogen application rate on accumulation and distribution of non-structural carbohydrates and spikelets formation in rice. J Yangzhou Univ: Agric Life Sci, 44(1): 29-39/48. (in Chinese with English abstract) |
| [63] | Zhang W Y, Chen Y J, Wang Z Q, et al. 2017. Polyamines and ethylene in rice young panicles in response to soil drought during panicle differentiation. Plant Growth Regul, 82(3): 491-503. |
| [64] | Zhang W Y, Zhu K Y, Wang Z Q, et al. 2019. Brassinosteroids function in spikelet differentiation and degeneration in rice. J Integr Plant Biol, 61(8): 943-963. |
| [65] | Zhang W Y, Fu L D, Men C B, et al. 2020. Response of brassinosteroids to nitrogen rates and their regulation on rice spikelet degeneration during meiosis. Food Energy Secur, 9(3): e201. |
| [66] | Zhang W Y, Huang H H, Zhou Y J, et al. 2023. Brassinosteroids mediate moderate soil-drying to alleviate spikelet degeneration under high temperature during meiosis of rice. Plant Cell Environ, 46(4): 1340-1362. |
| [67] | Zhang W Y, Wu M Y, Zhong X H, et al. 2024. Involvement of brassinosteroids and abscisic acid in spikelet degeneration in rice under soil drying during meiosis. J Exp Bot, 75(5): 1580-1600. |
| [68] | Zhao Q, Zhou L J, Liu J C, et al. 2018a. Involvement of CAT in the detoxification of HT-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep, 37(5): 741-757. |
| [69] | Zhao Q, Zhou L J, Liu J C, et al. 2018b. Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem, 122: 90-101. |
| [70] | Zheng Y, Fu D B, Yang Z N. 2023. OsDPE2 regulates rice panicle morphogenesis by modulating the content of starch. Rice, 16(1): 5. |
| [71] | Zhou C Y, Li G H, Xu K, et al. 2021. Advances in translocation mechanism and cultivation regulation of nonstructural carbohydrate in rice stem and sheath. Chin Bull Life Sci, 33(1): 111-120. (in Chinese with English abstract) |
| [72] | Zhu Z C, Luo S, Lei B, et al. 2022. Locus TUTOU2 determines the panicle apical abortion phenotype of rice (Oryza sativa L.) in tutou2 mutant. J Integr Agric, 21(3): 621-630. |
| [1] | Chen Ya, Liu Zhiquan, Yang Linyin, Wu Fujie, Cao Zijian, Shi Huanbin, Qiu Jiehua, Kou Yanjun. OsCERK1 Interacts with OsHPP08 to Regulate Copper Uptake and Blast Resistance in Rice [J]. Rice Science, 2025, 32(2): 203-216. |
| [2] | Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight [J]. Rice Science, 2025, 32(1): 118-130. |
| [3] | Yu Shicong, Luo Ruxian, Zheng Shuqin, Ning Jing, Shi Yuanzhu, Guo Daiming, Jia Liangmeng, Wang Sen, Xiao Guizong, Guo Pengwang, Li Yang, Ma Xiaoding. CHOLINE TRANSPORTER-RELATED 4 (CTR4) Is Involved in Drought and Saline Tolerance in Rice [J]. Rice Science, 2025, 32(1): 52-66. |
| [4] | Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance [J]. Rice Science, 2024, 31(6): 688-699. |
| [5] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [6] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [7] | Xia Xiaodong, Zhang Xiaobo, Wang Zhonghao, Cheng Benyi, Sun Huifeng, Xu Xia, Gong Junyi, Yang Shihua, Wu Jianli, Shi Yongfeng, Xu Rugen. Mapping and Functional Analysis of LE Gene in a Lethal Etiolated Rice Mutant at Seedling Stage [J]. Rice Science, 2023, 30(6): 567-576. |
| [8] | Md. Dhin Islam, Adam H. Price, Paul D. Hallett. Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth [J]. Rice Science, 2023, 30(5): 459-472. |
| [9] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [10] | Ratan Kumar Ganapati, Shahzad Amir Naveed, Sundus Zafar, Wang Wensheng, Xu Jianlong. Saline-Alkali Tolerance in Rice: Physiological Response, Molecular Mechanism, and QTL Identification and Application to Breeding [J]. Rice Science, 2022, 29(5): 412-434. |
| [11] | Yousef Alhaj Hamoud, Hiba Shaghaleh, Wang Ruke, Willy Franz Gouertoumbo, Amar Ali Adam hamad, Mohamed Salah Sheteiwy, Wang Zhenchang, Guo Xiangping. Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation [J]. Rice Science, 2022, 29(5): 473-488. |
| [12] | Chen Wei, Cai Yicong, Shakeel Ahmad, Wang Yakun, An Ruihu, Tang Shengjia, Guo Naihui, Wei Xiangjin, Tang Shaoqing, Shao Gaoneng, Jiao Guiai, Xie Lihong, Hu Shikai, Sheng Zhonghua, Hu Peisong. NRL3 Interacts with OsK4 to Regulate Heading Date in Rice [J]. Rice Science, 2022, 29(3): 237-246. |
| [13] | Vijayaraghavareddy Preethi, Xinyou Yin, C. Struik Paul, Makarla Udayakumar, Sreeman Sheshshayee. Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases [J]. Rice Science, 2020, 27(4): 345-354. |
| [14] | Shuting Yuan, Chunjue Xu, Wei Yan, Zhenyi Chang, Xingwang Deng, Zhufeng Chen, Jianxin Wu, Xiaoyan Tang. Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis [J]. Rice Science, 2020, 27(4): 289-301. |
| [15] | Hussain Kashif, Yingxing Zhang, Anley Workie, Riaz Aamir, Abbas Adil, Hasanuzzaman Rani Md., Hong Wang, Xihong Shen, Liyong Cao, Shihua Cheng. Association Mapping of Quantitative Trait Loci for Grain Size in Introgression Line Derived from Oryza rufipogon [J]. Rice Science, 2020, 27(3): 246-254. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||