
Rice Science ›› 2024, Vol. 31 ›› Issue (6): 688-699.DOI: 10.1016/j.rsci.2024.06.002
• Research Papers • Previous Articles Next Articles
Xue Chao1,3,#, Zhao Xinru1,2,#, Chen Xu1, Cai Xingjing1, Hu Yingying2, Li Xiya2, Zhou Yong1,2, Gong Zhiyun1,2( )
)
Received:2024-03-03
Accepted:2024-06-03
Online:2024-11-28
Published:2024-12-10
Contact:
Gong Zhiyun (zygong@yzu.edu.cn)
About author:#These authors contributed equally to this work
Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance[J]. Rice Science, 2024, 31(6): 688-699.
Add to citation manager EndNote|Ris|BibTeX
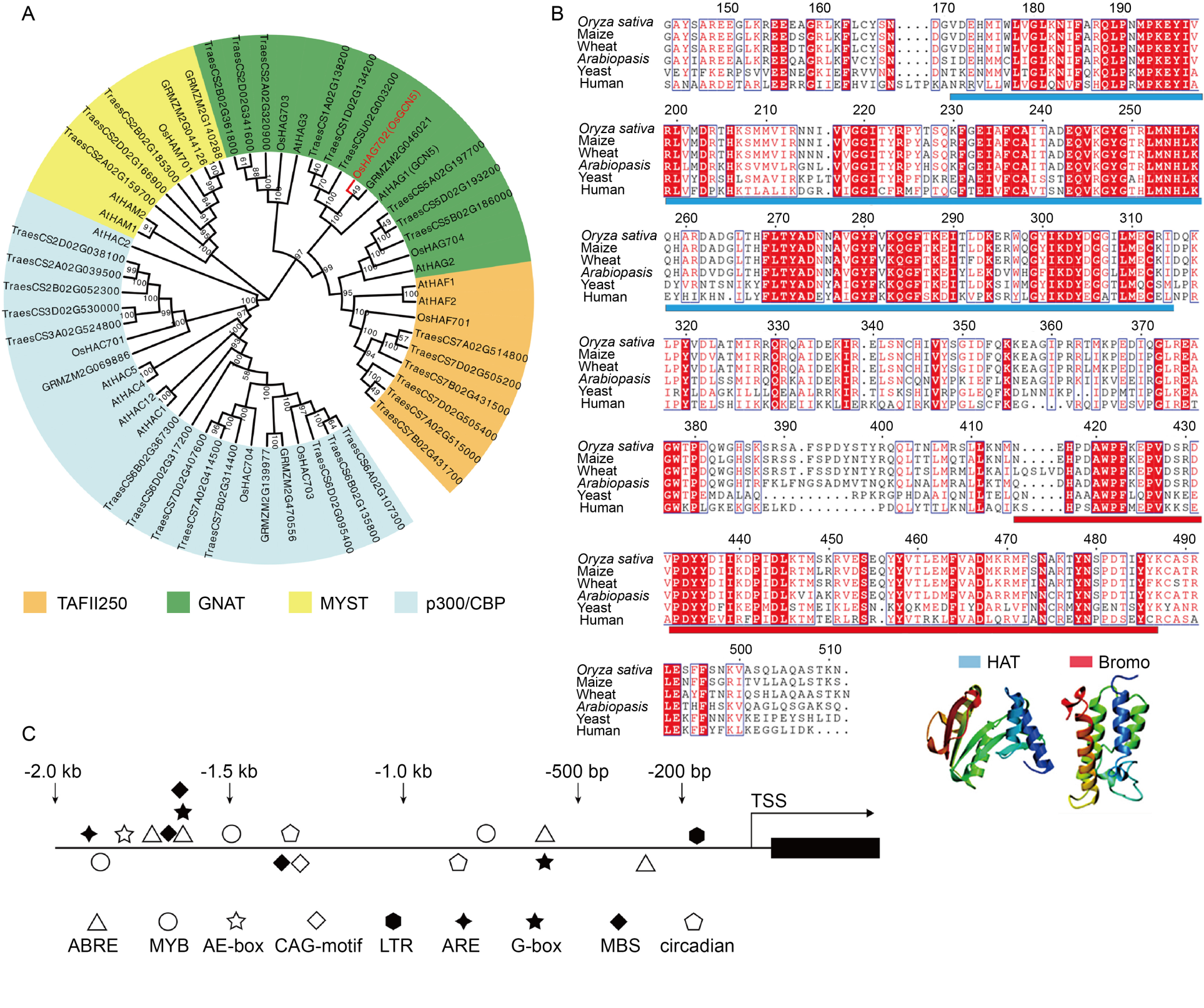
Fig. 1. Phylogenetic tree and cis-acting element of OsGCN5. A, Phylogenetic tree analysis of histone acetyltransferase (HAT) genes in Arabidopsis thaliana (At), Oryza sativa (Os), Triticum aestivum (Traes), and Zea mays (GRMZM). Plant HATs are classified into four families: TATA-binding protein associated factor (TAFII250) family, general control non-repressible 5-related N acetyltransferase (GNAT) family, MOZ, Ybf2/Sas3, Sas2, and Tip60 (MYST) family, and p300/cAMP responsive element-binding protein (p300/CBP) family.B, Alignment of amino acids of GCN5 from Oryza sativa and its homologs from other species, highlighting conserved domains and regions of variability. C, cis-Acting element analysis of OsGCN5 promoter sequence (2 000 bp upstream of the transcription start site, TSS). The analysis reveals the presence of various regulatory elements, including abscisic acid responsive elements (ABRE), transcriptional activator MYB recognition elements (MYB), light response module elements (AE-box, CAG-motif, and G-box), low temperature response element (LTR), cis-regulatory elements of anaerobic induction (ARE), elements involved in drought induction by binding MYB (MBS), and cis-regulatory elements involved in circadian rhythm control (circadian).
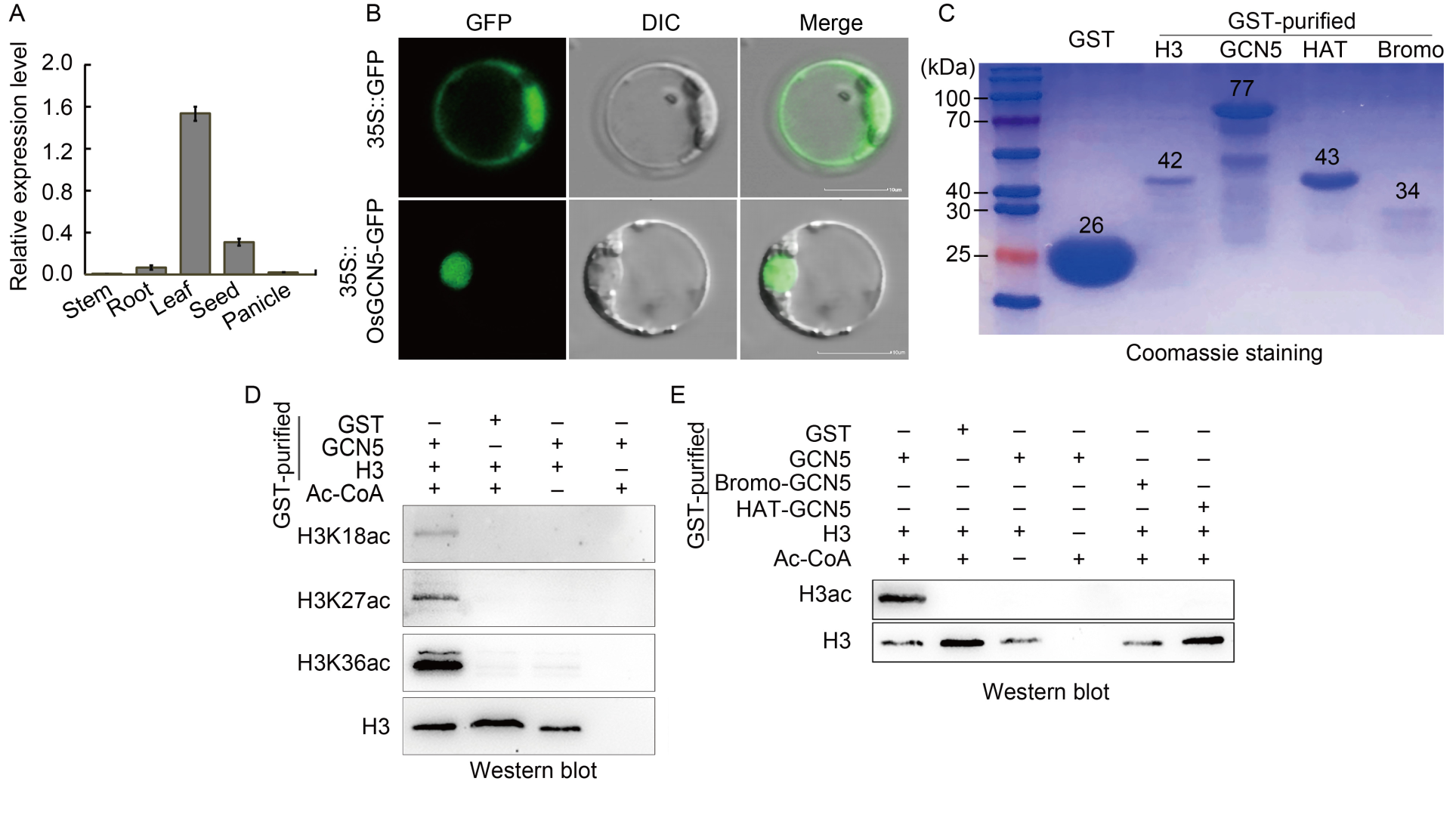
Fig. 2. Expression pattern of OsGCN5 in rice and subcellular localization and histone acetyltransferase activity of OsGCN5. A, Relative expression levels of OsGCN5, quantified by qRT-PCR. OsUBQ was used as an internal reference gene. Data are Mean ± SD (n = 3). B, Subcellular localization of OsGCN5-GFP (Green fluorescent protein) fusion protein in rice protoplasts. DIC, Differential interference contrast. Scale bars, 10 μm. C, SDS-PAGE (Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis. D and E, Western blotting results of histone acetylation assay performed in vitro. GST protein was used as a negative control. H3 antibody was used to detect histone H3 purified with a GST tag. Acetyl coenzyme A (Ac-CoA) was added to the reaction system in vitro as a donor of acetyl groups. Antibodies specific to histone acetylation at H3K18, H3K27, and H3K36 were used to assess the catalytic activity in vitro.
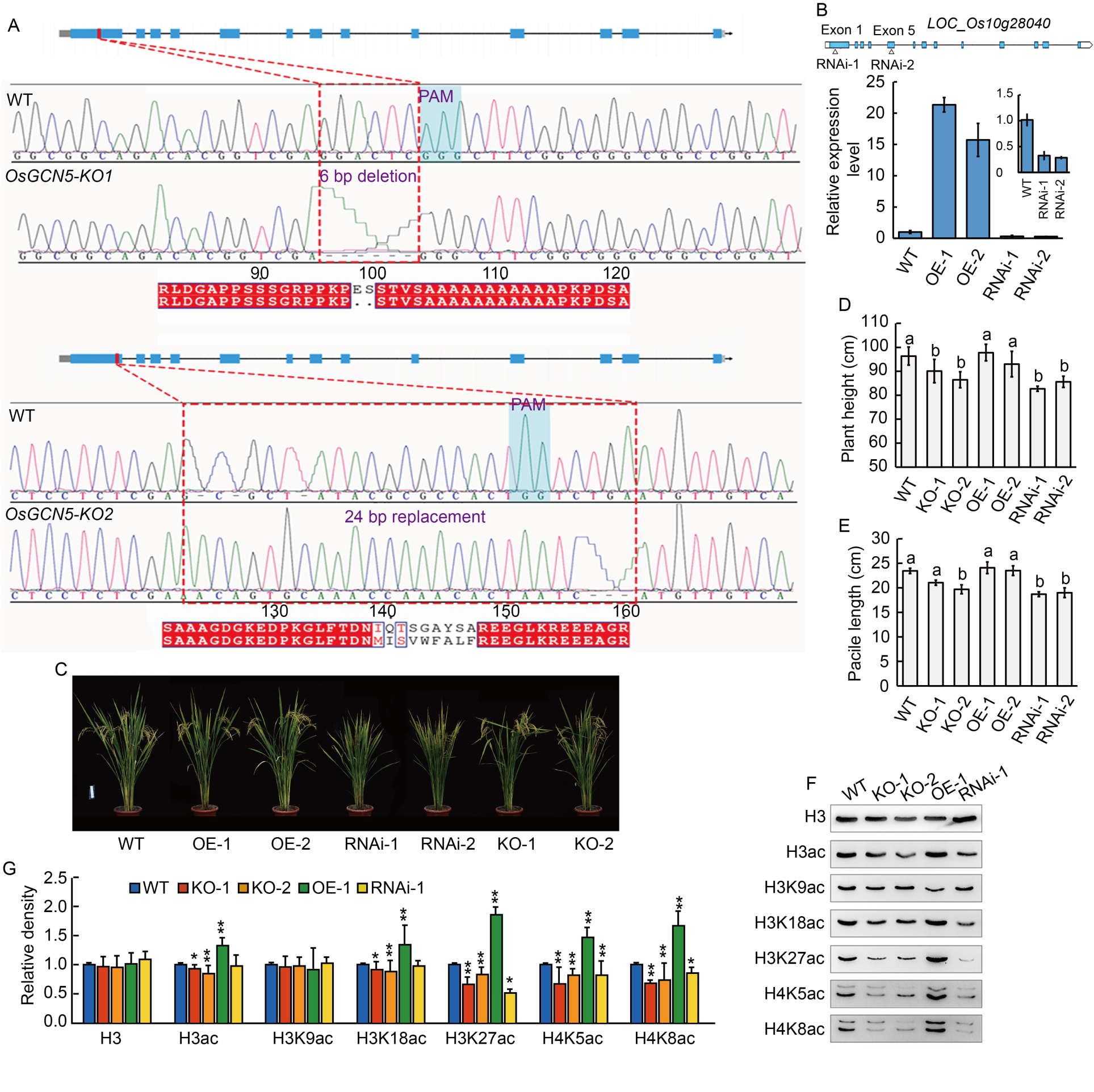
Fig. 3. Characterization of OsGCN5 genetic transformed plants. A, Sequencing results of OsGCN5 knockout lines (KO-1 and KO-2). PAM, Protospacer adjacent motif; WT, Wild type, Nipponbare. B, Expression levels of OsGCN5 in 14-day-old seedling leaves of wild type (WT, Nipponbare), OsGCN5 RNA interference lines (RNAi-1 and RNAi-2), and over-expression lines (OE-1 and OE-2). Data are Mean ± SD (n = 3). RNAi-1 and RNAi-2 lines were generated by interference of sequences on Exon 1 and Exon 5, respectively. UBQ gene was selected as an internal reference. C, Plant phenotypes of WT, OsGCN5 RNAi lines (RNAi-1 and RNAi-2), over-expression lines (OE-1 and OE-2), and knockout lines (KO-1 and KO-2). Scale bar, 10 cm. D and E, Plant height (D) and panicle length (E) of WT, OsGCN5 over-expression lines (OE-1 and OE-2), RNAi lines (RNAi-1 and RNAi-2), and knockout lines (KO-1 and KO-2). Data are Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences at P < 0.05 (one-way analysis of variance followed by Tukey’s multiple-comparison test).F and G, Detection of histone acetylation levels in WT, OsGCN5 knockout lines (KO-1 and KO-2), over-expression line (OE-1), and RNAi line (RNAi-1) by Western blot (F), and analysis of band density by grayscale value (G). H3 was used as a loading control. Data are Mean ± SD (n = 3). * and **, P < 0.05 and P < 0.01 (t-test), respectively.
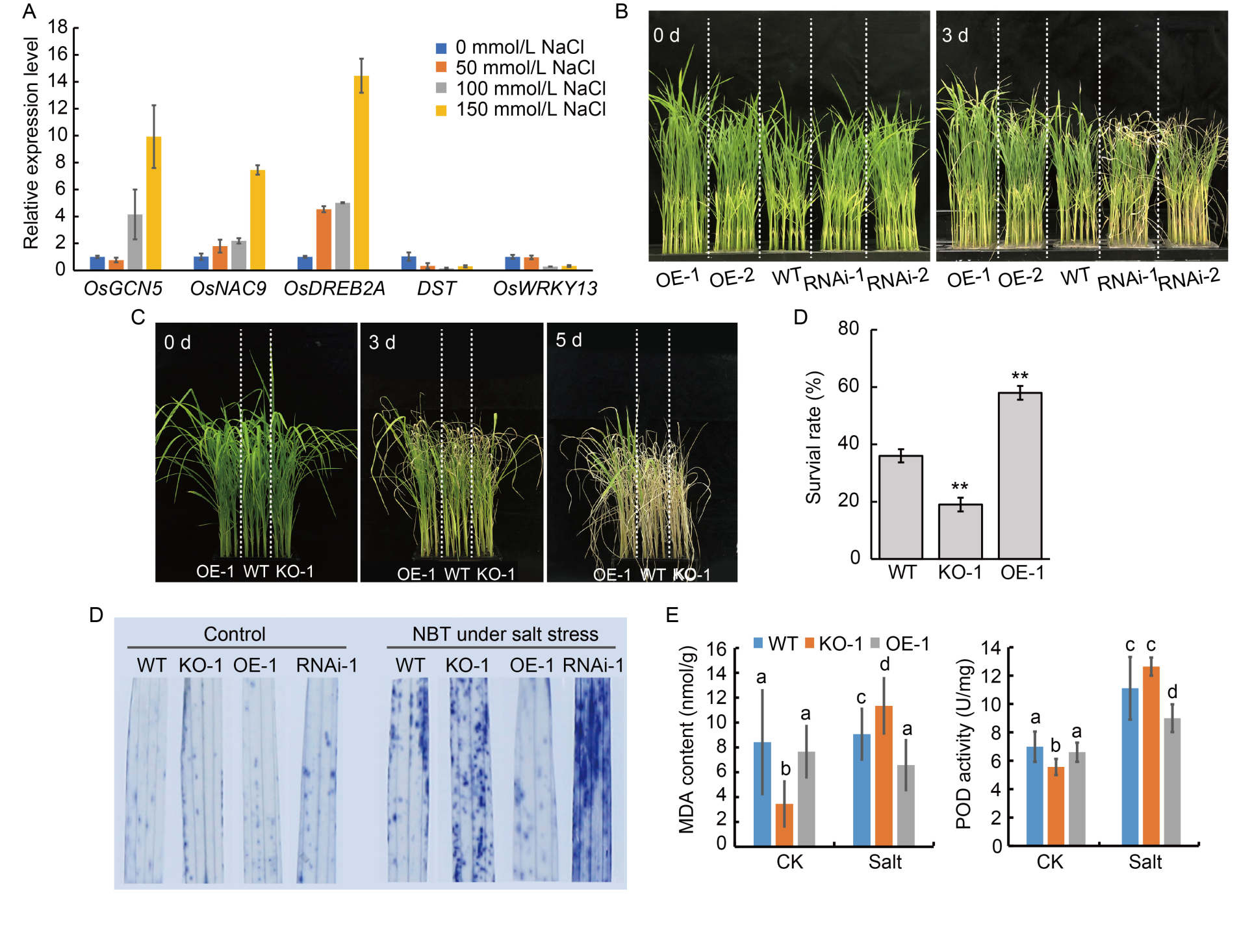
Fig. 4. Phenotypes and physiological changes of OsGCN5 transgenic lines under salt stress. A, Relative expression levels of OsGCN5 and some salt-responsive genes from rice seedlings (grown for 2 weeks) under normal conditions (0 mmol/L) and different NaCl concentrations (50, 100, and 150 mmol/L) for 24 h, quantified by qRT-PCR. UBQ gene was selected as an internal reference. B, Seedling (grown for 2 weeks) phenotypes of over-expression lines (OE-1 and OE-2), RNA interference lines (RNAi-1 and RNAi-2), and wild type (WT) under salt untreated (0 d) and 150 mmol/L NaCl treatments for 3 d. C and D, Seedling (grown for 2 weeks) (C) and survival rate (D) of over-expression line (OE-1), knockout line (KO-1), and wild type (WT) under salt untreated (0 d) and 150 mmol/L NaCl treatments for 5 d and recovery for 5 d. Data are Mean ± SD (n = 3). **, P < 0.01 (t-test). E, Reactive oxygen species accumulation in OsGCN5 over-expression line (OE-1), RNAi line (RNAi-1), knockout line (KO-1), and WT after 150 mmol/L NaCl stress detected by NBT (nitrotetrazolium blue chloride) staining. The color depth represents the amount of reactive oxygen species accumulation. F, Determination of malondialdehyde (MDA) content and peroxidase (POD) activity in WT and OsGCN5 transgenic lines before (CK) and after 150 mmol/L NaCl stress. Data are Mean ± SD (n = 3). Different lowercase letters above the bars indicate significant differences at P < 0.05 (One-way analysis of variance followed by Tukey’s multiple-comparison test).
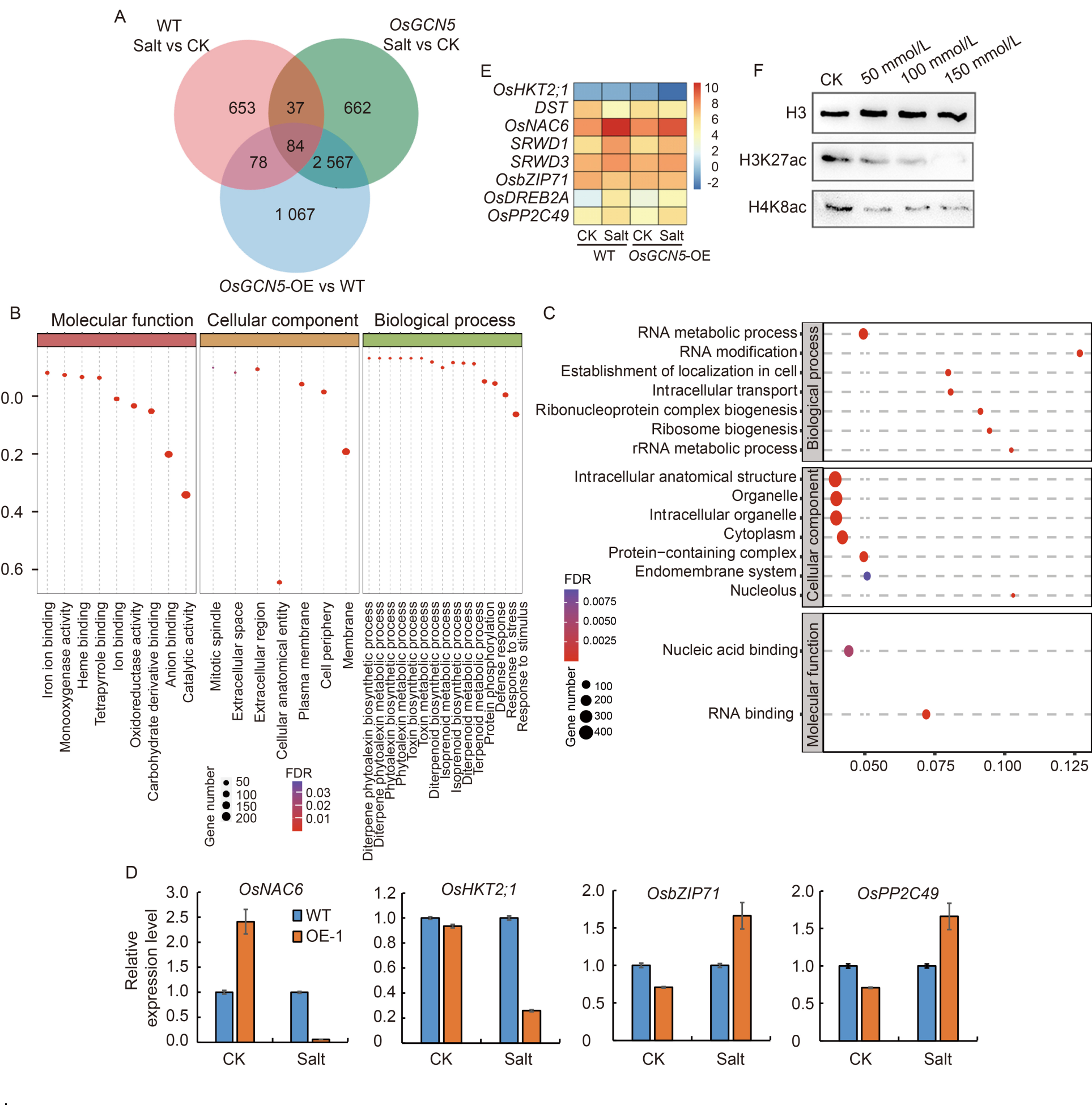
Fig. 5. Transcriptome sequencing analysis and qRT-PCR verification. A, Venn diagram of the number of up-regulated genes. B, Gene Ontology (GO) analysis of the 2 651 co-upregulated genes between over-expressed lines and WT under 150 mmol/L NaCl treatment for 24 h. C, GO enrichment analysis of 662 unique up-regulated genes in OE lines under salt stress (150 mmol/L NaCl treatment for 24 h). D, qRT-PCR verification of some differentially expressed genes in WT and OE-1 rice seedlings (grown for 2 weeks) under normal conditions (0 mmol/L) and different NaCl concentrations (50, 100, and 150 mmol/L) for 24 h. The rice ubiquitin gene (UBQ) was selected as an internal reference gene. Data are Mean ± SD (n = 3). E, Heat maps of differentially expressed genes associated with salt stress response. F, Immunoblot analysis of the histone acetylation levels under salt stress. H3 was used as a loading control. CK, Control check; Salt, 150 mmol/L NaCl treatment for 24 h; WT, Wild type; OE, Over-expressed lines; FDR, False discovery rate.
| [1] | An D, Chen J G, Gao Y Q, Li X, Chao Z F, Chen Z R, Li Q Q, Han M L, Wang Y L, Wang Y F, Chao D Y. 2017. AtHKT1 drives adaptation of Arabidopsis thaliana to salinity by reducing floral sodium content. PLoS Genet, 13(10): e1007086. |
| [2] | Ardie S W, Xie L N, Takahashi R, Liu S K, Takano T. 2009. Cloning of a high-affinity K+ transporter gene PutHKT2;1 from Puccinellia tenuiflora and its functional comparison with OsHKT2;1 from rice in yeast and Arabidopsis. J Exp Bot, 60(12): 3491-3502. |
| [3] | Grant P A, Duggan L, Côté J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, Berger S L, Workman J L. 1997. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: Characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev, 11(13): 1640-1650. |
| [4] | Hawar A, Xiong S Q, Yang Z, Sun B. 2022. Histone acetyltransferase SlGCN5 regulates shoot meristem and flower development in Solanum lycopersicum. Front Plant Sci, 12: 805879. |
| [5] | Horie T, Costa A, Kim T H, Han M J, Horie R, Leung H Y, Miyao A, Hirochika H, An G, Schroeder J I. 2007. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J, 26(12): 3003-3014. |
| [6] | Hu J Z, Cai J, Park S J, Lee K, Li Y X, Chen Y, Yun J Y, Xu T, Kang H. 2021. N6-Methyladenosine mRNA methylation is important for salt stress tolerance in Arabidopsis. Plant J, 106(6): 1759-1775. |
| [7] | Hu Z R, Song N, Zheng M, Liu X Y, Liu Z S, Xing J W, Ma J H, Guo W W, Yao Y Y, Peng H R, Xin M M, Zhou D X, Ni Z F, Sun Q X. 2015. Histone acetyltransferase GCN5 is essential for heat stress-responsive gene activation and thermotolerance in Arabidopsis. Plant J, 84(6): 1178-1191. |
| [8] | Jha U C, Chaturvedi S K, Bohra A, Basu P S, Khan M S, Barh D. 2014. Abiotic stresses, constraints and improvement strategies in chickpea. Plant Breed, 133(2): 163-178. |
| [9] | Kim S, Piquerez S J M, Ramirez-Prado J S, Mastorakis E, Veluchamy A, Latrasse D, Manza-Mianza D, Brik-Chaouche R, Huang Y, Rodriguez- Granados N Y, Concia L, Blein T, Citerne S, Bendahmane A, Bergounioux C, Crespi M, Mahfouz M M, Raynaud C, Hirt H, Ntoukakis V, Benhamed M. 2020. GCN5 modulates salicylic acid homeostasis by regulating H3K14ac levels at the 5′ and 3′ ends of its target genes. Nucleic Acids Res, 48(11): 5953-5966. |
| [10] | Kobayashi N I, Yamaji N, Yamamoto H, Okubo K, Ueno H, Costa A, Tanoi K, Matsumura H, Fujii-Kashino M, Horiuchi T, Nayef M A, Shabala S, An G, Ma J F, Horie T. 2017. OsHKT1;5 mediates Na+ exclusion in the vasculature to protect leaf blades and reproductive tissues from salt toxicity in rice. Plant J, 91(4): 657-670. |
| [11] | Kotak J, Saisana M, Gegas V, Pechlivani N, Kaldis A, Papoutsoglou P, Makris A, Burns J, Kendig A L, Sheikh M, Kuschner C E, Whitney G, Caiola H, Doonan J H, Vlachonasios K E, McCain E R, Hark A T. 2018. The histone acetyltransferase GCN5 and the transcriptional coactivator ADA2b affect leaf development and trichome morphogenesis in Arabidopsis. Planta, 248(3): 613-628. |
| [12] | Kubo T, Yoshimura A, Kurata N. 2022. Loss of OsEAF6, a subunit of the histone acetyltransferase complex, causes hybrid breakdown in intersubspecific rice crosses. Front Plant Sci, 13: 866404. |
| [13] | Kumar V, Thakur J K, Prasad M. 2021. Histone acetylation dynamics regulating plant development and stress responses. Cell Mol Life Sci, 78(10): 4467-4486. |
| [14] | Li H, Yan S H, Zhao L, Tan J J, Zhang Q, Gao F, Wang P, Hou H L, Li L J. 2014. Histone acetylation associated up-regulation of the cell wall related genes is involved in salt stress induced maize root swelling. BMC Plant Biol, 14: 105. |
| [15] | Li S, Lin Y C J, Wang P Y, Zhang B F, Li M, Chen S, Shi R, Tunlaya-Anukit S, Liu X Y, Wang Z F, Dai X F, Yu J, Zhou C G, Liu B G, Wang J P, Chiang V L, Li W. 2019. transcription factor influences histone acetylation to regulate drought responses and tolerance in Populus trichocarpa. Plant Cell, 31(3): 663-686. |
| [16] | Li Z, Zhang H, Cai C J, Lin Z, Zhen Z, Chu J, Guo K. 2022. Histone acetyltransferase GCN5-mediated lysine acetylation modulates salt stress aadaption of Trichoderma. Appl Microbiol Biotechnol, 106(8): 3033-3049. |
| [17] | Liao B, Xiang Y H, Li Y, Yang K Y, Shan J X, Ye W W, Dong N Q, Kan Y, Yang Y B, Zhao H Y, Yu H X, Lu Z Q, Zhao Y, Zhao Q, Guo D L, Guo S Q, Lei J J, Mu X R, Cao Y J, Han B, Lin H X. 2024. Dysfunction of duplicated pair rice histone acetyltransferases causes segregation distortion and an interspecific reproductive barrier. Nat Commun, 15(1): 996. |
| [18] | Lin C J, Yang S Y, Hsu L H, Yu S J, Chen Y L. 2023. The Gcn5- Ada2-Ada3 histone acetyltransferase module has divergent roles in pathogenesis of Candida glabrata. Med Mycol, 61( 2): myad004. |
| [19] | Oomen R J F J, Benito B, Sentenac H, Rodríguez-Navarro A, Talón M, Véry A A, Domingo C. 2012. HKT2;2/1, a K⁺-permeable transporter identified in a salt-tolerant rice cultivar through surveys of natural genetic polymorphism. Plant J, 71(5): 750-762. |
| [20] | Poulios S, Tsilimigka F, Mallioura A, Pappas D, Seira E, Vlachonasios K. 2022. Histone acetyltransferase GCN5 affects auxin transport during root growth by modulating histone acetylation and gene expression of PINs. Plants, 11(24): 3572. |
| [21] | Roodt D. 2021. Worth its salt: A histone acetyltransferase gene enhances salt tolerance in bread wheat. Plant Physiol, 186(4): 1752-1753. |
| [22] | Servet C, Conde e S N, Zhou D X. 2010. Histone acetyltransferase AtGCN5/HAG1 is a versatile regulator of developmental and inducible gene expression in Arabidopsis. Molecular Plant. 3(4): 670-677. |
| [23] | Suzuki K, Yamaji N, Costa A, Okuma E, Kobayashi N I, Kashiwagi T, Katsuhara M, Wang C, Tanoi K, Murata Y, Schroeder J I, Ma J F, Horie T. 2016. OsHKT1;4-mediated Na+ transport in stems contributes to Na+ exclusion from leaf blades of rice at the reproductive growth stage upon salt stress. BMC Plant Biol, 16: 22. |
| [24] | Wang H, Jiao X, Kong X, Hamera S, Wu Y, Chen X, Fang R, Yan Y. 2016. A signaling cascade from miR444 to RDR1 in rice antiviral RNA silencing pathway. Plant Physiol, 170(4): 2365-2377. |
| [25] | Wang L, Dent S Y R. 2014. Functions of SAGA in development and disease. Epigenomics, 6(3): 329-339. |
| [26] | Wang R, Jing W, Xiao L Y, Jin Y K, Shen L K, Zhang W H. 2015. The rice high-affinity potassium Transporter1;1 is involved in salt tolerance and regulated by an MYB-type transcription factor. Plant Physiol, 168(3): 1076-1090. |
| [27] | Wang W X, Vinocur B, Altman A. 2003. Plant responses to drought, salinity and extreme temperatures: Towards genetic engineering for stress tolerance. Planta, 218(1): 1-14. |
| [28] | Weake V M, Workman J L. 2012. SAGA function in tissue-specific gene expression. Trends Cell Biol, 22(4): 177-184. |
| [29] | Wei H, Wang X L, He Y Q, Xu H, Wang L. 2021. Clock component OsPRR73 positively regulates rice salt tolerance by modulating OsHKT2;1-mediated sodium homeostasis. EMBO J, 40(3): e105086. |
| [30] | Wu C J, Yuan D Y, Liu Z Z, Xu X, Wei L, Cai X W, Su Y N, Li L, Chen S, He X J. 2023. Conserved and plant-specific histone acetyltransferase complexes cooperate to regulate gene transcription and plant development. Nat Plants, 9(3): 442-459. |
| [31] | Xue C, Liu S, Chen C, Zhu J., Yang X B, Zhou Y, Guo R, Liu X Y, Gong Z Y. 2018. Global proteome analysis links lysine acetylation to diverse functions in Oryza Sativa. Proteomics, 18(1): doi: 10.1002/pmic.201700036. |
| [32] | Yang Y Q, Guo Y. 2018. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol, 217(2): 523-539. |
| [33] | Zhang M X, Zhao R R, Wang H T, Ren S L, Shi L Y, Huang S Z, Wei Z Q, Guo B Y, Jin J Y, Zhong Y, Chen M J, Jiang W Z, Wu T, Du X L. 2023. OsWRKY28 positively regulates salinity tolerance by directly activating OsDREB1B expression in rice. Plant Cell Rep, 42(2): 223-234. |
| [34] | Zhao S, Zhang X R, Li H T. 2018. Beyond histone acetylation-writing and erasing histone acylations. Curr Opin Struct Biol, 53: 169-177. |
| [35] | Zheng M, Liu X B, Lin J C, Liu X Y, Wang Z Y, Xin M M, Yao Y Y, Peng H R, Zhou D X, Ni Z F, Sun Q X, Hu Z R. 2019. Histone acetyltransferase GCN5 contributes to cell wall integrity and salt stress tolerance by altering the expression of cellulose synthesis genes. Plant J, 97(3): 587-602. |
| [36] | Zheng M, Lin J C, Liu X B, Chu W, Li J P, Gao Y J, An K X, Song W J, Xin M M, Yao Y Y, Peng H R, Ni Z F, Sun Q X, Hu Z R. 2021. Histone acetyltransferase TaHAG1 acts as a crucial regulator to strengthen salt tolerance of hexaploid wheat. Plant Physiol, 186(4): 1951-1969. |
| [37] | Zhou S L, Jiang W, Long F, Cheng S F, Yang W J, Zhao Y, Zhou D X. 2017. Rice homeodomain protein WOX11 recruits a histone acetyltransferase complex to establish programs of cell proliferation of crown root meristem. Plant Cell, 29(5): 1088-1104. |
| [38] | Zhu J K. 2016. Abiotic stress signaling and responses in plants. Cell, 167(2): 313-324. |
| [1] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [2] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [3] | Xia Xiaodong, Zhang Xiaobo, Wang Zhonghao, Cheng Benyi, Sun Huifeng, Xu Xia, Gong Junyi, Yang Shihua, Wu Jianli, Shi Yongfeng, Xu Rugen. Mapping and Functional Analysis of LE Gene in a Lethal Etiolated Rice Mutant at Seedling Stage [J]. Rice Science, 2023, 30(6): 567-576. |
| [4] | Md. Dhin Islam, Adam H. Price, Paul D. Hallett. Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth [J]. Rice Science, 2023, 30(5): 459-472. |
| [5] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [6] | Yousef Alhaj Hamoud, Hiba Shaghaleh, Wang Ruke, Willy Franz Gouertoumbo, Amar Ali Adam hamad, Mohamed Salah Sheteiwy, Wang Zhenchang, Guo Xiangping. Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation [J]. Rice Science, 2022, 29(5): 473-488. |
| [7] | Chen Wei, Cai Yicong, Shakeel Ahmad, Wang Yakun, An Ruihu, Tang Shengjia, Guo Naihui, Wei Xiangjin, Tang Shaoqing, Shao Gaoneng, Jiao Guiai, Xie Lihong, Hu Shikai, Sheng Zhonghua, Hu Peisong. NRL3 Interacts with OsK4 to Regulate Heading Date in Rice [J]. Rice Science, 2022, 29(3): 237-246. |
| [8] | Shuting Yuan, Chunjue Xu, Wei Yan, Zhenyi Chang, Xingwang Deng, Zhufeng Chen, Jianxin Wu, Xiaoyan Tang. Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis [J]. Rice Science, 2020, 27(4): 289-301. |
| [9] | Vijayaraghavareddy Preethi, Xinyou Yin, C. Struik Paul, Makarla Udayakumar, Sreeman Sheshshayee. Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases [J]. Rice Science, 2020, 27(4): 345-354. |
| [10] | Hussain Kashif, Yingxing Zhang, Anley Workie, Riaz Aamir, Abbas Adil, Hasanuzzaman Rani Md., Hong Wang, Xihong Shen, Liyong Cao, Shihua Cheng. Association Mapping of Quantitative Trait Loci for Grain Size in Introgression Line Derived from Oryza rufipogon [J]. Rice Science, 2020, 27(3): 246-254. |
| [11] | Jinjun Zhou, Peina Ju, Fang Zhang, Chongke Zheng, Bo Bai, Yaping Li, Haifeng Wang, Fan Chen, Xianzhi Xie. OsSRK1, an Atypical S-Receptor-Like Kinase Positively Regulates Leaf Width and Salt Tolerance in Rice [J]. Rice Science, 2020, 27(2): 133-142. |
| [12] | Kazemi Sheidollah, Reza Eshghizadeh Hamid, Zahedi Morteza. Responses of Four Rice Varieties to Elevated CO2 and Different Salinity Levels [J]. Rice Science, 2018, 25(3): 142-151. |
| [13] | Radhesh Krishnan Subramanian, Muthuramalingam Pandiyan, Pandian Subramani, Banupriya Ramachandradoss, Chithra Gunasekar, Ramesh Manikandan. Sprouted Sorghum Extract Elicits Coleoptile Emergence, Enhances Shoot and Root Acclimatization, and Maintains Genetic Fidelity in indica Rice [J]. Rice Science, 2018, 25(2): 61-72. |
| [14] | Nurdiani Dini, Widyajayantie Dwi, Nugroho Satya. OsSCE1 Encoding SUMO E2-Conjugating Enzyme Involves in Drought Stress Response of Oryza sativa [J]. Rice Science, 2018, 25(2): 73-81. |
| [15] | Fernando Polesi Luís, Bruder Silveira Sarmento Silene, Guidolin Canniatti-Brazaca Solange. Starch Digestibility and Functional Properties of Rice Starch Subjected to Gamma Radiation [J]. Rice Science, 2018, 25(1): 42-51. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||