
Rice Science ›› 2025, Vol. 32 ›› Issue (2): 203-216.DOI: 10.1016/j.rsci.2025.02.001
• Research Papers • Previous Articles Next Articles
Chen Ya1,2, Liu Zhiquan1, Yang Linyin1, Wu Fujie1, Cao Zijian1, Shi Huanbin1, Qiu Jiehua1, Kou Yanjun1( )
)
Received:2024-09-18
Accepted:2025-01-23
Online:2025-03-28
Published:2025-04-14
Contact:
Kou Yanjun (kouyanjun@caas.cn)
Chen Ya, Liu Zhiquan, Yang Linyin, Wu Fujie, Cao Zijian, Shi Huanbin, Qiu Jiehua, Kou Yanjun. OsCERK1 Interacts with OsHPP08 to Regulate Copper Uptake and Blast Resistance in Rice[J]. Rice Science, 2025, 32(2): 203-216.
Add to citation manager EndNote|Ris|BibTeX
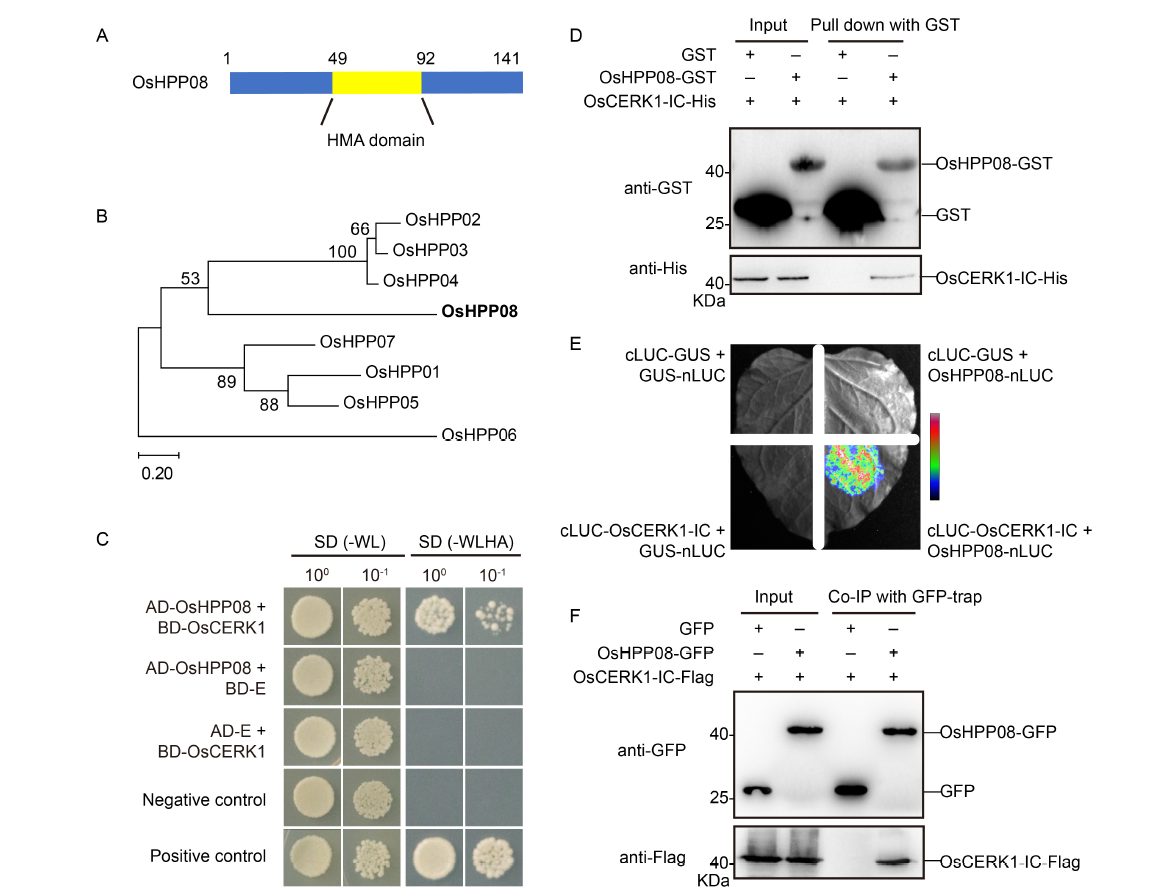
Fig. 1. OsHPP08 interacts with OsCERK1 both in vitro and in vivo. A, OsHPP08 structure with a single heavy metal-associated (HMA) domain is highlighted in yellow. B, Phylogenetic tree of OsHPPs in rice. Clustal W is used to compare the full-length protein sequences, and MEGA7.0 is used to construct the phylogenetic tree using the neighbor-joining method. C, OsHPP08 interacts with OsCERK1 in yeast. The positive and negative controls are PGADT7-T+PGBKT7-53 and PGADT7-T+PGBKT7-lam, respectively. SD (-WL) is a selective medium lacking leucine and tryptophan; SD (-WLHA) is a selective medium lacking leucine, tryptophan, adenine, and histidine; AD-E, The empty vector of PGADT7; BD-E, The empty vector of PGBKT7.D, OsHPP08 interacts with the intracellular domain of OsCERK1 (OsCERK1-IC) in the pull-down assay. GST/OsCERK1-His is used as the control, and OsHPP08-GST/OsCERK1-IC-His is used as the experimental group. GST, Glutathione S-transferase.E, Interaction of OsHPP08 with OsCERK1 in N.icotiana benthamiana using the firefly luciferase complementation assay. The indicated plasmid is transferred into Agrobacterium GV3101 and injected into N. benthamiana. After 48 h, the luminescence of the leaves is observed using a chemiluminescence imager. F, OsHPP08 interacts with the intracellular domain of OsCERK1 in the co-immunoprecipitation (Co-IP) assay. The Agrobacterium containing GFP/OsCERK1-Flag construct is co-injected into N. benthamiana as the control, and OsHPP08-GFP/OsCERK1-IC-Flag is co-injected as the experimental group. After 48 h, the protein of N. benthamiana leaves is extracted and incubated with GFP agarose beads at 4 ºC for 6 h to perform the Co-IP assay.
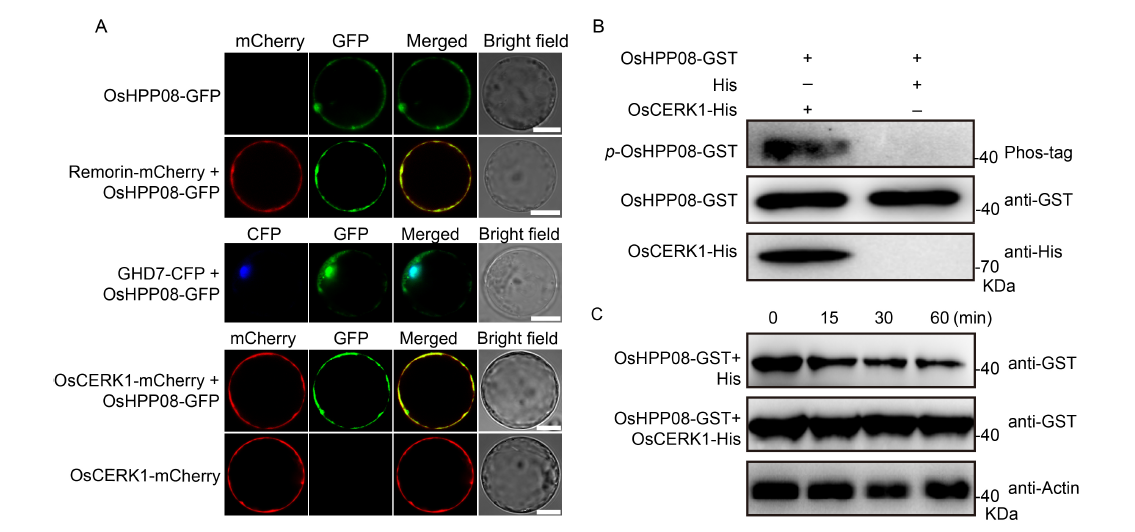
Fig. 2. OsCERK1 phosphorylates and stabilizes OsHPP08. A, OsHPP08 co-localizes with OsCERK1. The C-terminus of OsCERK1 is fused with mCherry, and the C-terminus of OsHPP08 is fused with GFP. OsCERK1-mCherry is transformed into rice protoplasts with OsHPP08-GFP. The Remorin-mCherry is used as a membrane marker, and the GHD7-CFP is served as a nucleus marker. Fluorescent observation reveals that OsHPP08 localizes to the membrane and the nucleus in rice, and co-localizes with OsCERK1 at the membrane. GFP, Green fluorescent protein; CFP, Cyan fluorescent protein. Scale bars, 10 μm. B, OsHPP08 is phosphorylated by OsCERK1. The recombinant plasmid OsHPP08-GST is co-expressed with OsCERK1-His and its empty vector pET-28a (His) in Escherichia coli BL21, respectively. Equal amounts of the GST purified recombinant proteins are detected by immunoblotting using indicated antibodies. p-OsHPP08-GST, Phosphorylated OsHPP08-GST; Phos-tag, Biotinylated Phos-tag zinc BTL111 complex. GST, Glutathione S-transferase.C, Degradation assay of OsHPP08-GST in the absence or presence of OsCERK1-His.
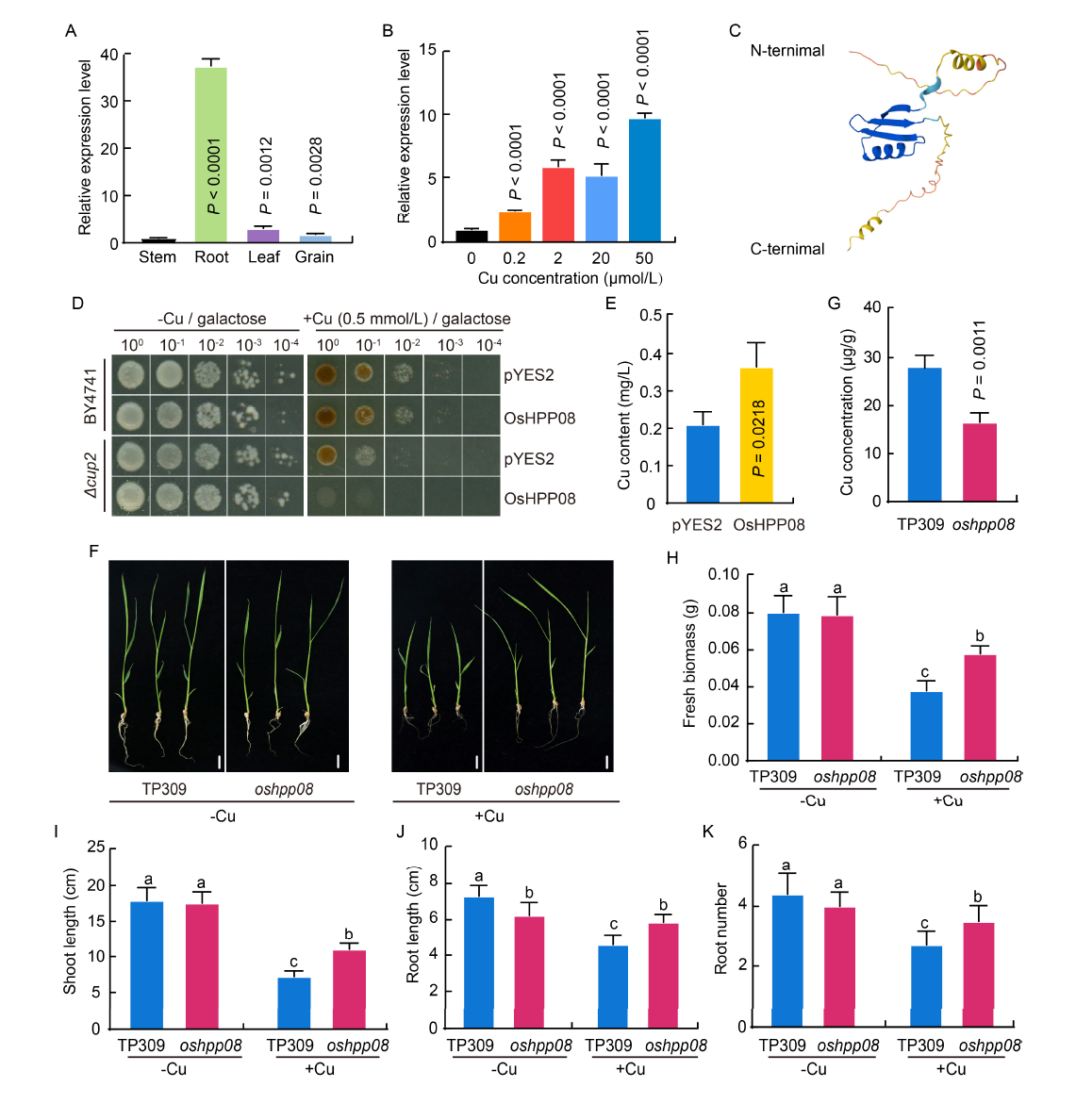
Fig. 3. OsHPP08 positively regulates copper (Cu) uptake in rice. A, Expression levels of OsHPP08 in different tissues of rice. The expression levels of OsHPP08 in the stem, root, leaf, and grain at the four-leaf stage are determined using qRT-PCR analysis. The Ubiquitin gene (LOC_Os03g13170) is served as an internal control.B, Expression levels of OsHPP08 under different Cu concentrations. TP309 is treated with nutrient solution containing 0, 0.2, 2, 20, and 50 μmol/L Cu ions for 12 h, respectively. The Ubiquitin gene is used as an internal control.C, Protein structure of OsHPP08 analyzed with AlphaFold. OsHPP08 contains a classic heavy metal-associated (HMA) domain structure, which may serve as a Cu metalloprotein. D, OsHPP08 has Cu uptake ability in yeast. Yeast monoclonal is diluted with ddH2O, further diluted 10, 102, 103, and 104 times, respectively. Subsequently, the diluted yeast is spot-coated on SD-Ura (galactose) medium, containing 0 or 0.5 mmol/L CuSO4 at 30 ºC for 3-7 d. E, Cu content in yeast. The yeast monoclonal of pYES2-Δcup2 and OsHPP08-pYES2-Δcup2 is cultured in SD-Ura medium (galactose) containing 0.05 mmol/L Cu ions for 2-3 d, and the Cu concentration in yeast is measured.F, Growth of the oshpp08 mutant and its wild type (TP309) before (-Cu) and after (+Cu) treatment with 5 mg/L Cu for 14 d. Scale bars, 1 cm.G, Cu concentration in the oshpp08 mutant and its wild type (TP309) with 5 mg/L Cu treatment. H-K, Fresh biomass (H), shoot length (I), root length (J), and root number (K) of the oshpp08 mutant and its wild-type before (-Cu) and after (+Cu) treatment with 5 mg/L Cu for 14 d.In A, B, E, and G, data are Mean ± SD (n = 3). Student’s t-test is used to analyze the data and generate P values. In H-K, data are Mean ± SD (n = 10). Different lowercase letters above columns indicate statistical differences at P < 0.05 by the Duncan test.
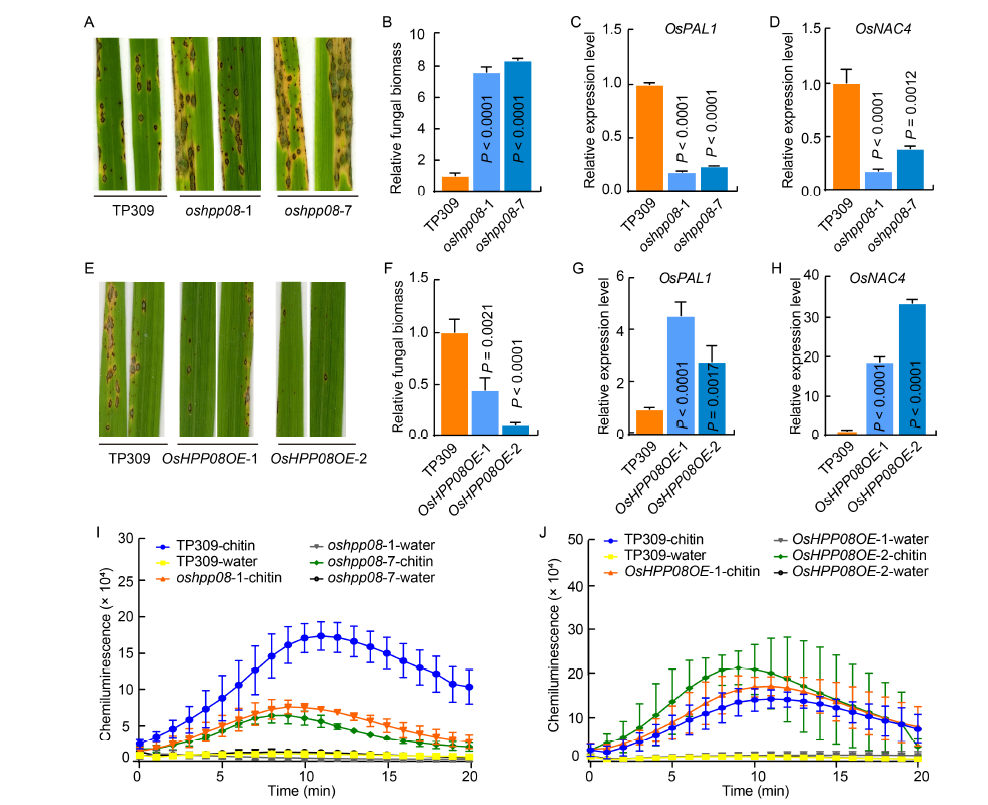
Fig. 4. OsHPP08 positively regulates rice basal resistance to Magnaporthe oryzae. A, Disease symptoms of OsHPP08 mutants and their wild type (TP309) following spray inoculation with M. oryzae. B, Relative fungal biomass in the representative leaves of oshpp08 mutants and TP309 after spray inoculation with M. oryzae. The relative fungal biomass is based on the DNA concentrations of M. oryzae Pot2 against the rice genomic Ubiquitin DNA level using quantitative PCR analysis.C and D, Expression levels of pathogenesis-related genes OsPAL1 (C) and OsNAC4 (D) after inoculation with M. oryzae. The Ubiquitin gene is served as an internal control.E, Disease symptoms of OsHPP08OE plants inoculated with M. oryzae. F, Relative fungal biomass of the representative leaves of OsHPP08OE plants. G and H, Expression levels of pathogenesis-related genes OsPAL1 (G) and OsNAC4 (H) of OsHPP08OE plants after inoculation with M. oryzae. I and J, Reactive oxygen species (ROS) accumulation dynamics in the leaves of the oshpp08 mutants (I) and OsHPP08OE (J) plants. Data are Mean ± SD (n = 3). Different lowercase letters above columns indicate statistical differences at P < 0.05 by the Duncan test.
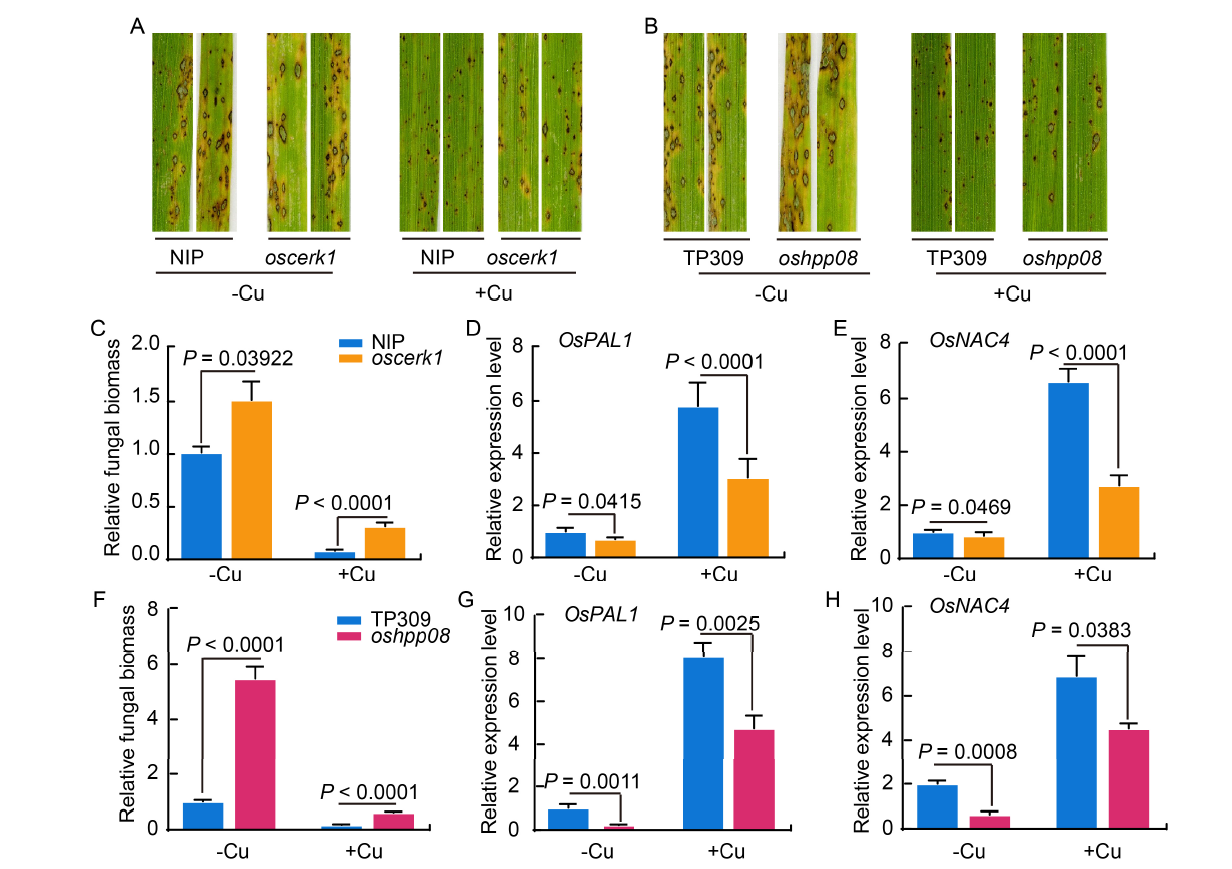
Fig. 5. OsCERK1 and OsHPP08 contribute to Cu-induced resistance to Magnaporthe oryzae in rice. A and B, Disease symptoms of oscerk1 (A) and oshpp08 (B) mutants and their wild type Nipponbare (NIP) plants after spray inoculation with M. oryzae before (-Cu) and after (+Cu) treatment with 5 mg/L Cu for 14 d. C-H, Relative fungal biomass of the representative leaves of oscerk1 mutants (C), oshpp08 mutants (F), and their wild type plants after spray inoculation with M. oryzae before (-Cu) and after (+Cu) treatment with 5 mg/L Cu for 14 d. The expression levels of pathogenesis-related genes OsPAL1 and OsNAC4 in the oscerk1 (D and E) and oshpp08 (G and H) mutants after inoculation with M. oryzae. Data are Mean ± SD (n = 3). Student’s t-test is used to analyze the data and generate P values.
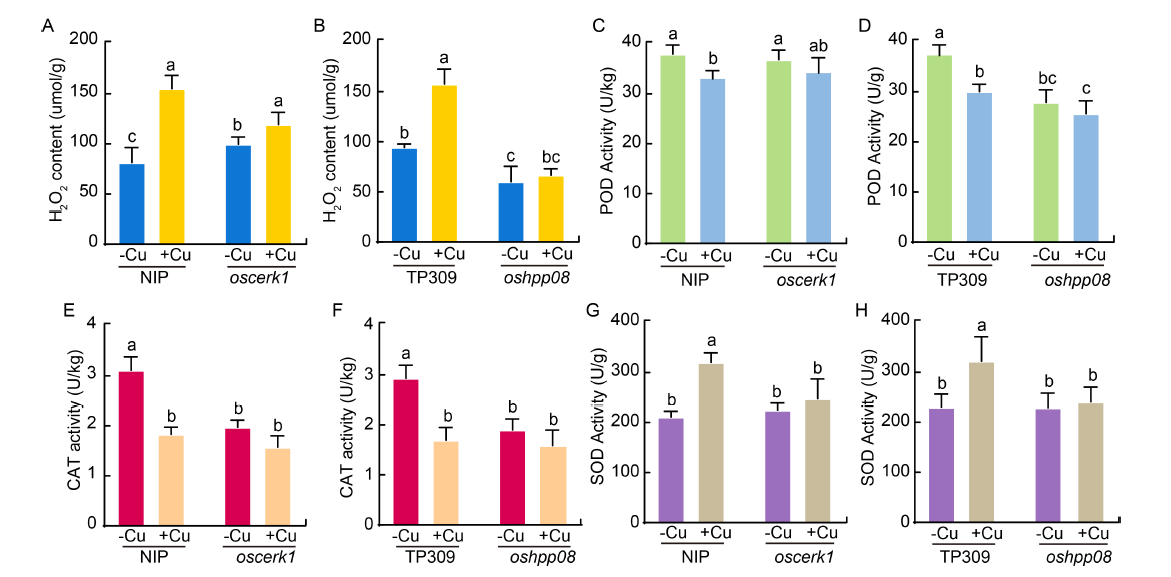
Fig. 6. Activities of antioxidant enzymes in Nipponbare (NIP), oshpp08, and oscerk1 mutants and their wild type seedlings before (-Cu) and after (+Cu) treatment with 5 mg/L copper (Cu) for 14 d. A and B, Content of hydrogen peroxide (H2O2) in oscerk1 and oshpp08 mutants treated with Cu. C and D, Activities of peroxidase (POD) in oscerk1 and oshpp08 mutants treated with Cu. E and F, Activities of catalase (CAT) in the oscerk1 and oshpp08 mutants treated with Cu. G and H, Activities of superoxide dismutase (SOD) in oscerk1 and oshpp08 mutants treated with Cu. Data are Mean ± SD (n = 3). Different lowercase letters above columns indicate statistical differences at P < 0.05 by the Duncan test.
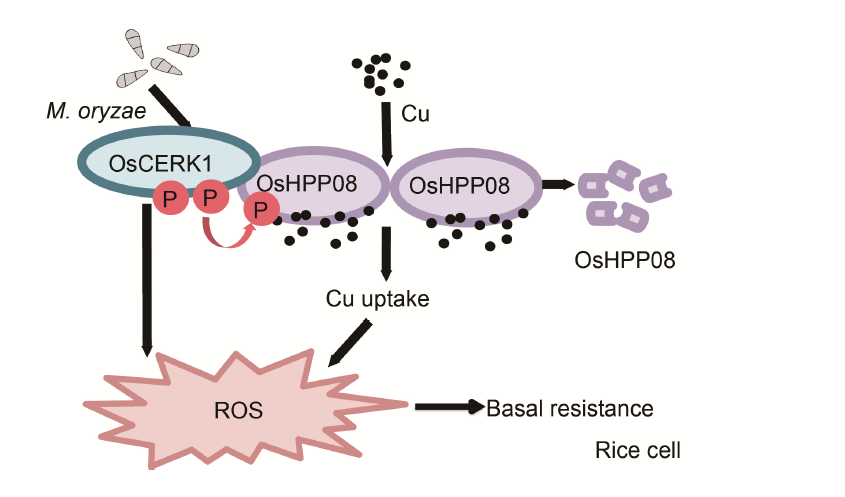
Fig. 7. A proposed model illustrating that OsCERK1-OsHPP08 regulates copper (Cu) uptake to confer resistance against blast disease in rice. OsCERK1 likely phosphorylates and stabilizes OsHPP08 to positively regulate Cu uptake. The reactive oxygen species (ROS) accumulation triggered by Cu in the cell enhances the basal resistance against Magnaporthe oryzae in rice.
| [1] | Ao Y, Li Z Q, Feng D R, et al. 2014. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J, 80(6): 1072-1084. |
| [2] | Bi G Z, Hu M, Fu L, et al. 2022. The cytosolic thiol peroxidase PRXIIB is an intracellular sensor for H2O2 that regulates plant immunity through a redox relay. Nat Plants, 8(10): 1160-1175. |
| [3] | Cao H W, Zhao Y N, Liu X S, et al. 2022. A metal chaperone OsHIPP16 detoxifies cadmium by repressing its accumulation in rice crops. Environ Pollut, 311: 120058. |
| [4] | Chen G Q, Xiong S. 2021. OsHIPP24 is a copper metallochaperone which affects rice growth. J Plant Biol, 64(2): 145-153. |
| [5] | Chen Y, Liu Z Q, Meng S, et al. 2022. OsCERK1 contributes to cupric oxide nanoparticles induced phytotoxicity and basal resistance against blast by regulating the anti-oxidant system in rice. J Fungi, 9(1): 36. |
| [6] | Choudhary R C, Kumaraswamy R V, Kumari S, et al. 2017. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci Rep, 7(1): 9754. |
| [7] | Chowdhury A R, Kumar R, Mahanty A, et al. 2024. Inhibitory role of copper and silver nanocomposite on important bacterial and fungal pathogens in rice (Oryza sativa). Sci Rep, 14(1): 1779. |
| [8] | Colangelo E P, Guerinot M L. 2006. Put the metal to the petal: Metal uptake and transport throughout plants. Curr Opin Plant Biol, 9(3): 322-330. |
| [9] | de Abreu-Neto J B, Turchetto-Zolet A C, de Oliveira L F V, et al. 2013. Heavy metal-associated isoprenylated plant protein (HIPP): Characterization of a family of proteins exclusive to plants. FEBS J, 280(7): 1604-1616. |
| [10] | Dykema P E, Sipes P R, Marie A, et al. 1999. A new class of proteins capable of binding transition metals. Plant Mol Biol, 41(1): 139-150. |
| [11] | Espinoza C, Liang Y, Stacey G. 2017. Chitin receptor CERK 1 links salt stress and chitin-triggered innate immunity in Arabidopsis. Plant J, 89(5): 984-995. |
| [12] | Feng S J, Liu X S, Cao H W, et al. 2021. Identification of a rice metallochaperone for cadmium tolerance by an epigenetic mechanism and potential use for clean up in wetland. Environ Pollut, 288: 117837. |
| [13] | Hayafune M, Berisio R, Marchetti R, et al. 2014. Chitin-induced activation of immune signaling by the rice receptor CEBiP relies on a unique sandwich-type dimerization. Proc Natl Acad Sci USA, 111(3): E404-E413. |
| [14] | Kennedy P H, Alborzian Deh Sheikh A, Balakar M, et al. 2024. Post-translational modification-centric base editor screens to assess phosphorylation site functionality in high throughput. Nat Methods, 21(6): 1033-1043. |
| [15] | Khan I U, Rono J K, Zhang B Q, et al. 2019. Identification of novel rice (Oryza sativa) HPP and HIPP genes tolerant to heavy metal toxicity. Ecotoxicol Environ Saf, 175: 8-18. |
| [16] | Khan I U, Rono J K, Liu X S, et al. 2020. Functional characterization of a new metallochaperone for reducing cadmium concentration in rice crop. J Clean Prod, 272: 123152. |
| [17] | Li Z Y, Wei X J, Tong X H, et al. 2022. The OsNAC23-Tre6P- SnRK1a feed-forward loop regulates sugar homeostasis and grain yield in rice. Mol Plant, 15(4): 706-722. |
| [18] | Liu B, Li J F, Ao Y, et al. 2012. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell, 24(8): 3406-3419. |
| [19] | Liu Z Q, Qiu J H, Shen Z N, et al. 2023. The E3 ubiquitin ligase OsRGLG5 targeted by the Magnaporthe oryzae effector AvrPi9 confers basal resistance against rice blast. Plant Commun, 4(5): 100626. |
| [20] | Maidment J H R, Franceschetti M, Maqbool A, et al. 2021. Multiple variants of the fungal effector AVR-Pik bind the HMA domain of the rice protein OsHIPP19, providing a foundation to engineer plant defense. J Biol Chem, 296: 100371. |
| [21] | Park C H, Chen S B, Shirsekar G, et al. 2012. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell, 24(11): 4748-4762. |
| [22] | Priya M, Venkatesan R, Deepa S, et al. 2023. Green synthesis, characterization, antibacterial, and antifungal activity of copper oxide nanoparticles derived from Morinda citrifolia leaf extract. Sci Rep, 13(1): 18838. |
| [23] | Qiu J H, Chen Y, Liu Z Q, et al. 2023. The application of zinc oxide nanoparticles: An effective strategy to protect rice from rice blast and abiotic stresses. Environ Pollut, 331: 121925. |
| [24] | Raffaele S, Bayer E, Lafarge D, et al. 2009. Remorin, a solanaceae protein resident in membrane rafts and plasmodesmata, impairs Potato virus X movement. Plant Cell, 21(5): 1541-1555. |
| [25] | Rono J K, Sun D, Yang Z M. 2022. Metallochaperones: A critical regulator of metal homeostasis and beyond. Gene, 822: 146352. |
| [26] | Shi H B, Meng S, Qiu J H, et al. 2021. MoWhi2 regulates appressorium formation and pathogenicity via the MoTor signalling pathway in Magnaporthe oryzae. Mol Plant Pathol, 22(8): 969-983. |
| [27] | Shi Y, Jiang N, Wang M T, et al. 2023. OsHIPP17 is involved in regulating the tolerance of rice to copper stress. Front Plant Sci, 14: 1183445. |
| [28] | Song H D, Lin B R, Huang Q L, et al. 2021. The Meloidogyne graminicola effector MgMO289 targets a novel copper metallochaperone to suppress immunity in rice. J Exp Bot, 72(15): 5638-5655. |
| [29] | Tehseen M, Cairns N, Sherson S, et al. 2010. Metallochaperone- like genes in Arabidopsis thaliana. Metallomics, 2(8): 556-564. |
| [30] | Tsuda K, Katagiri F. 2010. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Curr Opin Plant Biol, 13(4): 459-465. |
| [31] | Wang C, Wang G, Zhang C, et al. 2017. OsCERK1-mediated chitin perception and immune signaling requires receptor-like cytoplasmic kinase 185 to activate an MAPK cascade in rice. Mol Plant, 10(4): 619-633. |
| [32] | Wang J W, Li Y, Zhang Y X, et al. 2013. Molecular cloning and characterization of a Brassica juncea yellow stripe-like gene, BjYSL7, whose overexpression increases heavy metal tolerance of tobacco. Plant Cell Rep, 32(5): 651-662. |
| [33] | Wintz H, Fox T, Wu Y Y, et al. 2003. Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem, 278(48): 47644-47653. |
| [34] | Xiong S, Kong X H, Chen G Q, et al. 2023. Metallochaperone OsHIPP9 is involved in the retention of cadmium and copper in rice. Plant Cell Environ, 46(6): 1946-1961. |
| [35] | Xu L, Wang J Z, Xiao Y, et al. 2022. Structural insight into chitin perception by chitin elicitor receptor kinase 1 of Oryza sativa. J Integr Plant Biol, 65(1): 235-248. |
| [36] | Yamaguchi K, Imai K, Akamatsu A, et al. 2012. SWAP70 functions as a Rac/Rop guanine nucleotide-exchange factor in rice. Plant J, 70(3): 389-397. |
| [37] | Yang C, Liu R, Pang J H, et al. 2021. Poaceae-specific cell wall- derived oligosaccharides activate plant immunity via OsCERK1 during Magnaporthe oryzae infection in rice. Nat Commun, 12(1): 2178. |
| [38] | Yeon J, Park A R, Nguyen H T T, et al. 2022. Inhibition of oomycetes by the mixture of maleic acid and copper sulfate. Plant Dis, 106(3): 960-965. |
| [39] | Yuan M, Chu Z H, Li X H, et al. 2010. The bacterial pathogen Xanthomonas oryzae overcomes rice defenses by regulating host copper redistribution. Plant Cell, 22(9): 3164-3176. |
| [40] | Zhang B Q, Liu X S, Feng S J, et al. 2020. Developing a cadmium resistant rice genotype with OsHIPP29 locus for limiting cadmium accumulation in the paddy crop. Chemosphere, 247: 125958. |
| [41] | Zhang C, He J M, Dai H L, et al. 2021. Discriminating symbiosis and immunity signals by receptor competition in rice. Proc Natl Acad Sci USA, 118(16): e2023738118. |
| [42] | Zhang X, Liu Y, Yuan G X, et al. 2024. The synthetic NLR RGA5HMA5 requires multiple interfaces within and outside the integrated domain for effector recognition. Nat Commun, 15(1): 1104. |
| [43] | Zhang Y Y, Chen K, Zhao F J, et al. 2018. OsATX1 interacts with heavy metal P1B-type ATPases and affects copper transport and distribution. Plant Physiol, 178(1): 329-344. |
| [44] | Zhao Y N, Wang M Q, Li C, et al. 2022. The metallochaperone OsHIPP56 gene is required for cadmium detoxification in rice crops. Environ Exp Bot, 193: 104680. |
| [45] | Zheng T H, Sun J, Zhou S R, et al. 2019. Post-transcriptional regulation of Ghd7 protein stability by phytochrome and OsGI in photoperiodic control of flowering in rice. New Phytol, 224(1): 306-320. |
| [46] | Zschiesche W, Barth O, Daniel K, et al. 2015. The zinc-binding nuclear protein HIPP 3 acts as an upstream regulator of the salicylate-dependent plant immunity pathway and of flowering time in Arabidopsis thaliana. New Phytol, 207(4): 1084-1096. |
| [1] | Wang Jingqing, Wang Yaliang, Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping. Progress on Physiological Mechanisms of Rice Spikelet Degeneration at Different Panicle Positions Caused by Abiotic Stress [J]. Rice Science, 2025, 32(2): 193-202. |
| [2] | Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight [J]. Rice Science, 2025, 32(1): 118-130. |
| [3] | Wang Mingyue, Zhao Weibo, Feng Xiaoya, Chen Yi, Li Junhao, Fu Jinmei, Yan Yingchun, Chu Zhaohui, Huang Wenchao. Disruption of Energy Metabolism and Reactive Oxygen Species Homeostasis in Honglian Type-Cytoplasmic Male Sterility (HL-CMS) Rice Pollen [J]. Rice Science, 2025, 32(1): 81-93. |
| [4] | Yu Shicong, Luo Ruxian, Zheng Shuqin, Ning Jing, Shi Yuanzhu, Guo Daiming, Jia Liangmeng, Wang Sen, Xiao Guizong, Guo Pengwang, Li Yang, Ma Xiaoding. CHOLINE TRANSPORTER-RELATED 4 (CTR4) Is Involved in Drought and Saline Tolerance in Rice [J]. Rice Science, 2025, 32(1): 52-66. |
| [5] | Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance [J]. Rice Science, 2024, 31(6): 688-699. |
| [6] | Li Wei, Zhang Mengchen, Yang Yaolong, Weng Lin, Hu Peisong, Wei Xinghua. Molecular Evolution of Rice Blast Resistance Gene bsr-d1 [J]. Rice Science, 2024, 31(6): 700-711. |
| [7] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [8] | Wu Lijuan, Han Cong, Wang Huimei, He Yuchang, Lin Hai, Wang Lei, Chen Chen, E Zhiguo. OsbZIP53 Negatively Regulates Immunity Response by Involving in Reactive Oxygen Species and Salicylic Acid Metabolism in Rice [J]. Rice Science, 2024, 31(2): 190-202. |
| [9] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [10] | Xia Xiaodong, Zhang Xiaobo, Wang Zhonghao, Cheng Benyi, Sun Huifeng, Xu Xia, Gong Junyi, Yang Shihua, Wu Jianli, Shi Yongfeng, Xu Rugen. Mapping and Functional Analysis of LE Gene in a Lethal Etiolated Rice Mutant at Seedling Stage [J]. Rice Science, 2023, 30(6): 567-576. |
| [11] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| [12] | Md. Dhin Islam, Adam H. Price, Paul D. Hallett. Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth [J]. Rice Science, 2023, 30(5): 459-472. |
| [13] | Jiang Changjie, Liang Zhengwei, Xie Xianzhi. Priming for Saline-Alkaline Tolerance in Rice: Current Knowledge and Future Challenges [J]. Rice Science, 2023, 30(5): 417-425. |
| [14] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [15] | Fabiano T. P. K. Távora, Anne Cécile Meunier, Aurore Vernet, Murielle Portefaix, Joëlle Milazzo, Henri Adreit, Didier Tharreau, Octávio L. Franco, Angela Mehta. CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae [J]. Rice Science, 2022, 29(6): 535-544. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||