
Rice Science ›› 2025, Vol. 32 ›› Issue (1): 67-80.DOI: 10.1016/j.rsci.2024.12.002
• Research Papers • Previous Articles Next Articles
Surangkana Chimthai1, Sulaiman Cheabu2, Wanchana Aesomnuk3, Siriphat Ruengphayak3, Siwaret Arikit1,3, Apichart Vanavichit3, Chanate Malumpong1( )
)
Received:2024-07-29
Accepted:2024-11-11
Online:2025-01-28
Published:2025-02-20
Contact:
Chanate Malumpong
Surangkana Chimthai, Sulaiman Cheabu, Wanchana Aesomnuk, Siriphat Ruengphayak, Siwaret Arikit, Apichart Vanavichit, Chanate Malumpong. Breeding for Heat Tolerant Aromatic Rice Varieties and Identification of Novel QTL Regions Associated with Heat Tolerance During Reproductive Phase by QTL-Seq[J]. Rice Science, 2025, 32(1): 67-80.
Add to citation manager EndNote|Ris|BibTeX
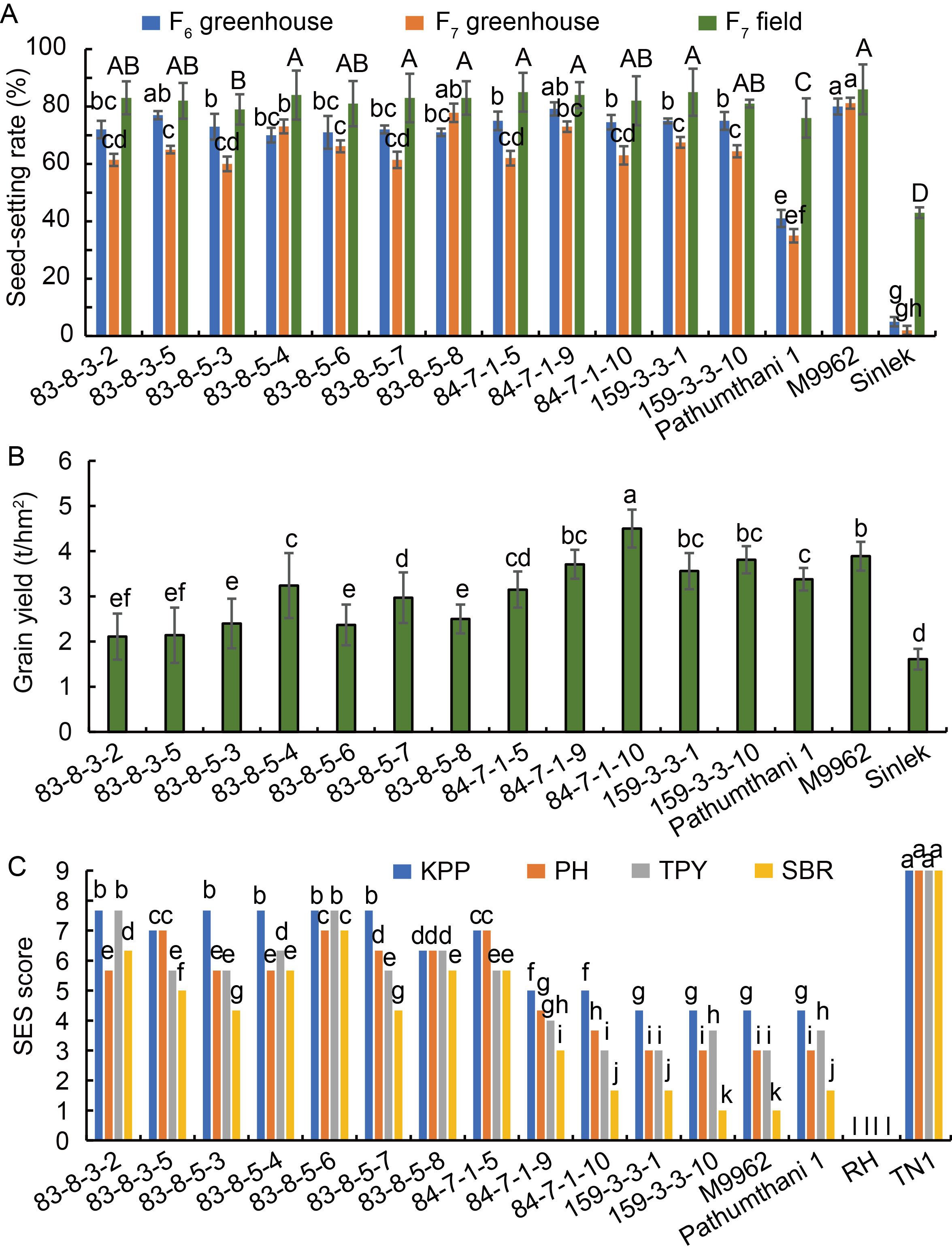
Fig. 1. Seed-setting rate, grain yield, and brown plant- hopper resistance of candidate lines in F6 and F7 generations. A, Seed-setting rates of 12 candidate lines in F6 and F7 generations. B, Grain yields of 12 candidate lines in F7 generation under field conditions. C, Scores of brown planthopper (BPH) resistance in F7 generation under controlled greenhouse. Kamphaeng Phet (KPP), Pak Hai (PH), Ta Phaya (TPY), and Sing Buri (SBR) are four brown planthopper biotypes. SES, Standard evaluation system. Sinlex, RH (Ratuhinati), and TN1 were used as heat susceptible, BPH resistance, and BPH susceptible checks, respectively. Different letters above the bars indicate significant differences at the 0.05 level using least significant difference.
| Line/ Variety | Aroma (badh2) | Amylose content (wxb) | Gelatinization temperature (SSIIa) | Brown planthopper | |
|---|---|---|---|---|---|
| TPS | Bph3 | ||||
| 83-8-3-2 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-3-5 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-3 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-4 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-6 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-7 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-8 | -/- | -/- | +/+ | +/+ | +/+ |
| 84-7-1-5 | -/- | -/- | +/+ | +/+ | +/+ |
| 84-7-1-9 | +/+ | +/+ | -/+ | +/+ | +/+ |
| 84-7-1-10 | +/+ | +/+ | -/- | +/+ | +/+ |
| 159-3-3-1 | -/- | +/+ | -/- | +/+ | +/+ |
| 159-3-3-10 | -/- | +/+ | -/- | +/+ | +/+ |
| PTT1 | +/+ | +/+ | +/+ | +/+ | +/+ |
| M9962 | -/- | -/- | -/- | +/+ | +/+ |
Table 1. Single nucleotide polymorphism/insertion and deletion (SNP/InDel) marker information on 12 breeding lines identified in F7 generation and their parents.
| Line/ Variety | Aroma (badh2) | Amylose content (wxb) | Gelatinization temperature (SSIIa) | Brown planthopper | |
|---|---|---|---|---|---|
| TPS | Bph3 | ||||
| 83-8-3-2 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-3-5 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-3 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-4 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-6 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-7 | -/- | -/- | +/+ | +/+ | +/+ |
| 83-8-5-8 | -/- | -/- | +/+ | +/+ | +/+ |
| 84-7-1-5 | -/- | -/- | +/+ | +/+ | +/+ |
| 84-7-1-9 | +/+ | +/+ | -/+ | +/+ | +/+ |
| 84-7-1-10 | +/+ | +/+ | -/- | +/+ | +/+ |
| 159-3-3-1 | -/- | +/+ | -/- | +/+ | +/+ |
| 159-3-3-10 | -/- | +/+ | -/- | +/+ | +/+ |
| PTT1 | +/+ | +/+ | +/+ | +/+ | +/+ |
| M9962 | -/- | -/- | -/- | +/+ | +/+ |
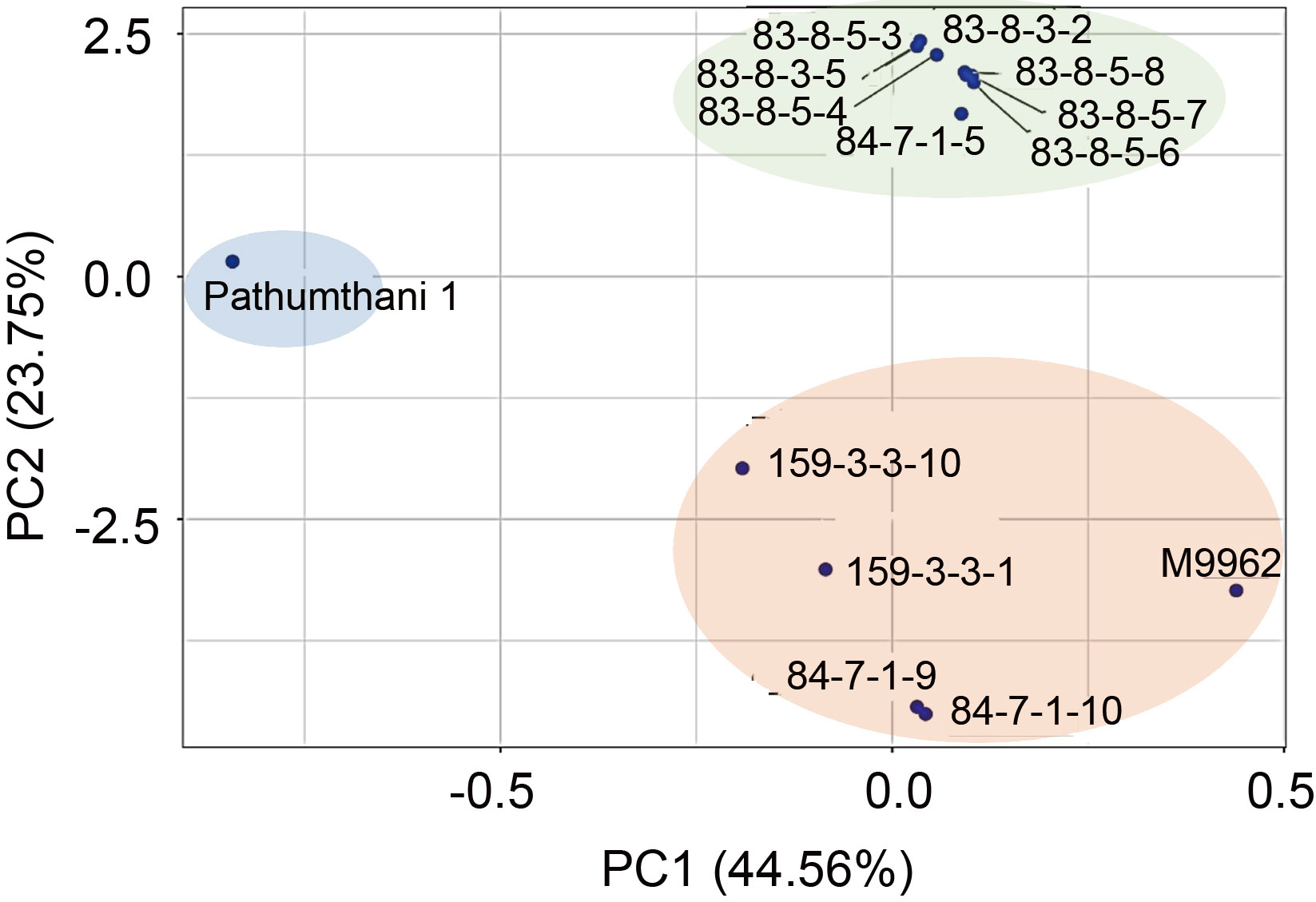
Fig. 2. Principal component analysis (PCA) of 12 candidate lines and their parents to show genetic relationships obtained from whole-genome sequences.
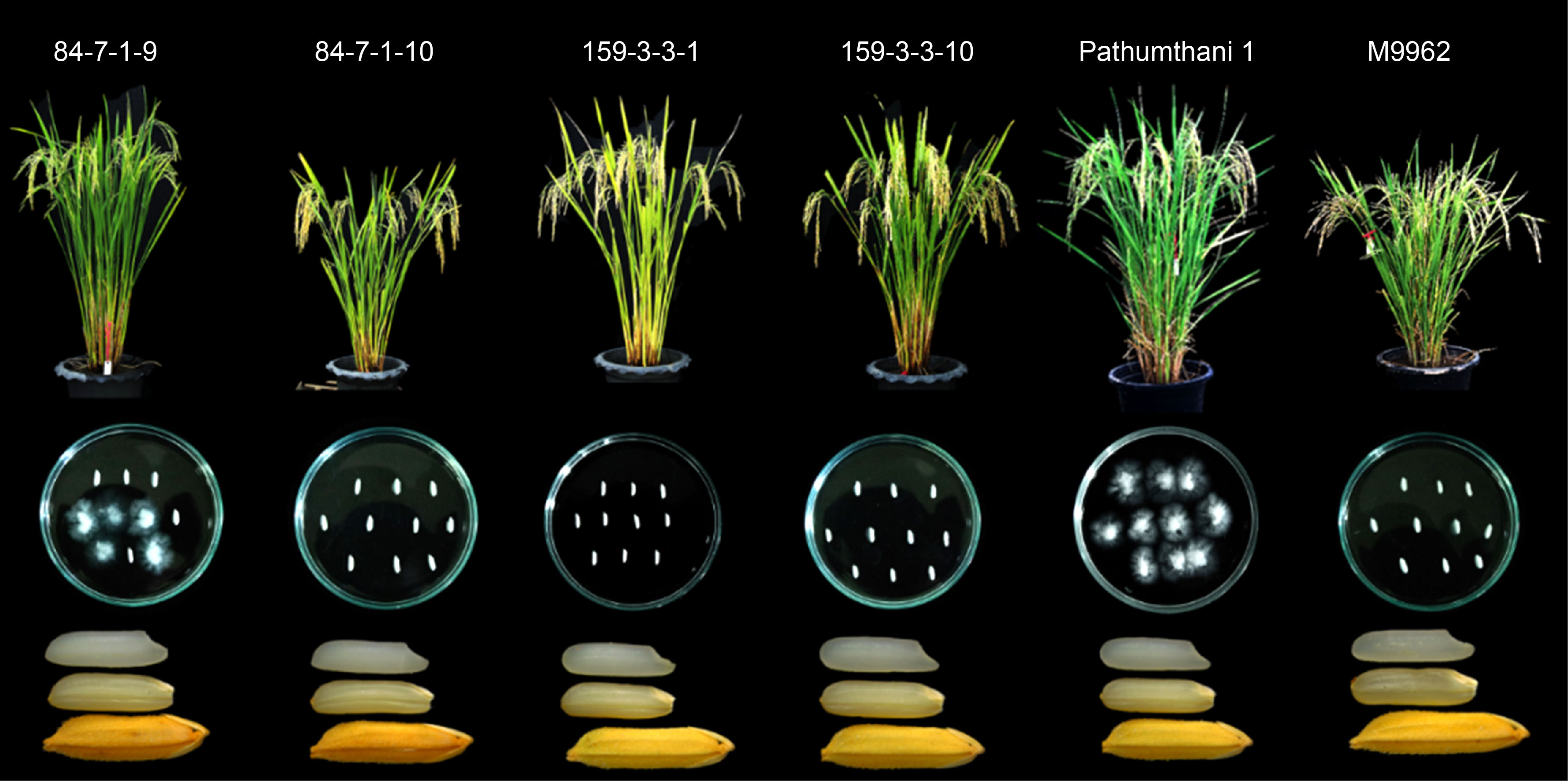
Fig. 3. Plant type, alkaline test, milled grain, brown grain, and paddy grain of four promising lines in F7 generation compared with their parents (Pathumthani 1 and M9962).
| Line/ Variety | Milled grain width (mm) | Milled grain length (mm) | Chalky grain rate (%) | Alkaline score | Amylose content (%) | 2AP content (mg/kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | Field | GH | Field | GH | Field | GH | Field | GH | Field | GH | Field | ||||||
| 84-7-1-9 | 2.04 a | 2.15 a | 6.89 b | 7.10 b | 12.7 b | 10.5 b | 5 a | 5 a | 16.63 b | 16.89 b | 1.81 a | 3.71 a | |||||
| 84-7-1-10 | 1.95 b | 2.10 b | 7.11 a | 7.18 b | 13.3 b | 10.8 b | 2 b | 2 b | 15.83 b | 16.00 b | 1.52 b | 3.39 b | |||||
| 159-3-3-1 | 1.95 b | 2.10 b | 6.64 c | 6.85 c | 8.7 c | 8.0 c | 2 b | 2 b | 16.63 b | 16.93 b | 0.00 c | 0.00 c | |||||
| 159-3-3-10 | 1.93 b | 2.10 b | 6.62 c | 6.73 c | 11.5 b | 10.7 b | 2 b | 2 b | 16.67 b | 16.85 b | 0.00 c | 0.00 c | |||||
| Pathumthani 1 | 1.92 b | 2.00 c | 7.30 a | 7.38 a | 8.2 c | 7.0 c | 5 a | 5 a | 16.00 b | 16.50 b | 1.78 a | 3.55 b | |||||
| M9962 | 2.01 a | 2.08 a | 6.54 c | 6.50 d | 29.6 a | 20.2 a | 1 b | 1 b | 25.73 a | 25.80 a | 0.00 c | 0.00 c | |||||
| F test (P < 0.05) | * | * | * | * | * | * | * | * | * | * | * | * | |||||
| CV (%) | 1.16 | 2.56 | 1.39 | 3.21 | 8.31 | 10.58 | 0.50 | 0.42 | 10.5 | 11.2 | 9.42 | 7.25 | |||||
Table 2. Grain size, alkalinity, chalkiness, amylose content, and 2-acetyl-1-pyrroline (2AP) content of four promising heat-tolerant lines (F7 generation) compared with those of their parents under controlled greenhouse and field conditions.
| Line/ Variety | Milled grain width (mm) | Milled grain length (mm) | Chalky grain rate (%) | Alkaline score | Amylose content (%) | 2AP content (mg/kg) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GH | Field | GH | Field | GH | Field | GH | Field | GH | Field | GH | Field | ||||||
| 84-7-1-9 | 2.04 a | 2.15 a | 6.89 b | 7.10 b | 12.7 b | 10.5 b | 5 a | 5 a | 16.63 b | 16.89 b | 1.81 a | 3.71 a | |||||
| 84-7-1-10 | 1.95 b | 2.10 b | 7.11 a | 7.18 b | 13.3 b | 10.8 b | 2 b | 2 b | 15.83 b | 16.00 b | 1.52 b | 3.39 b | |||||
| 159-3-3-1 | 1.95 b | 2.10 b | 6.64 c | 6.85 c | 8.7 c | 8.0 c | 2 b | 2 b | 16.63 b | 16.93 b | 0.00 c | 0.00 c | |||||
| 159-3-3-10 | 1.93 b | 2.10 b | 6.62 c | 6.73 c | 11.5 b | 10.7 b | 2 b | 2 b | 16.67 b | 16.85 b | 0.00 c | 0.00 c | |||||
| Pathumthani 1 | 1.92 b | 2.00 c | 7.30 a | 7.38 a | 8.2 c | 7.0 c | 5 a | 5 a | 16.00 b | 16.50 b | 1.78 a | 3.55 b | |||||
| M9962 | 2.01 a | 2.08 a | 6.54 c | 6.50 d | 29.6 a | 20.2 a | 1 b | 1 b | 25.73 a | 25.80 a | 0.00 c | 0.00 c | |||||
| F test (P < 0.05) | * | * | * | * | * | * | * | * | * | * | * | * | |||||
| CV (%) | 1.16 | 2.56 | 1.39 | 3.21 | 8.31 | 10.58 | 0.50 | 0.42 | 10.5 | 11.2 | 9.42 | 7.25 | |||||
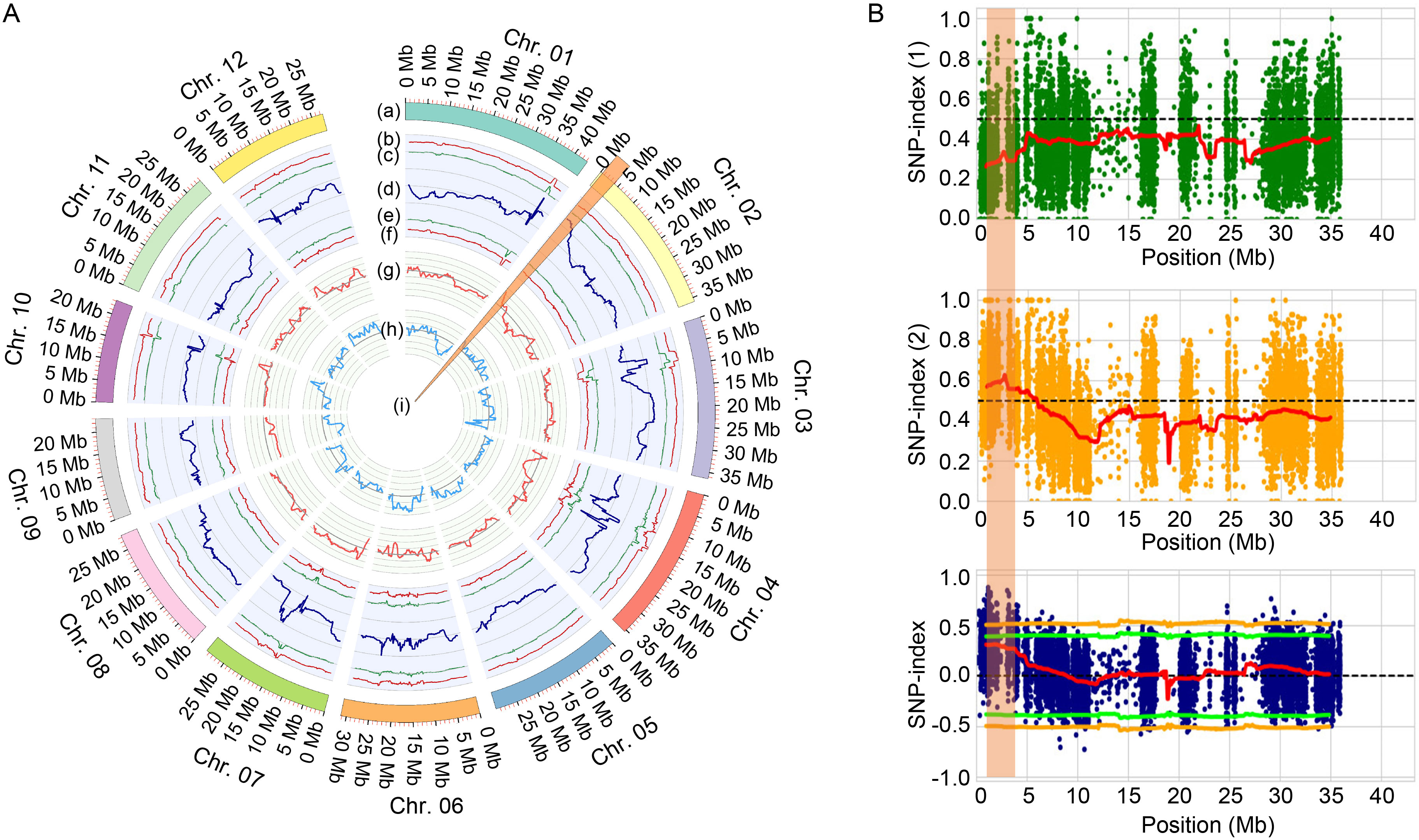
Fig. 4. QTL-seq analysis revealed a putative QTL region on chromosome 2. A, Sliding window plots of the single nucleotide polymorphism (SNP) index for two bulks (HT-bulk and HS-bulk) and a comparison of the ∆(SNP index) between them. (a) Pseuedomolecules of the Nipponbare reference genome (IRGSP 1.0). (b) Upper probability values at the 99% confidence level (P < 0.01). (c) Upper probability values at the 95% confidence level (P < 0.05). (d) Sliding window plots of ∆(SNP index). (e) Lower probability values at the 95% confidence level (P < 0.05). (f) Lower probability values at the 99% confidence level (P < 0.01). (g) Sliding window plots of the average SNP index values in the HT-bulk. (h) Sliding window plots of average SNP index values in the HS-bulk. (i) Candidate genomic regions containing QTLs for the seed-setting rate. B, SNP index and candidate genes on chromosome 2. Plots of the SNP index of SNPs in the HT-bulk (top; green dots) and HS-bulk (middle; orange dots), and plots of the Δ(SNP index) (blue dots) for the two bulks (HT-bulk and HS-bulk). Sliding window plots of the average SNP index, with a 500-kb window size and 10-kb steps, are presented as red lines. The pairs of orange and green lines in the Δ(SNP index) plots represent the 95% and 99% confidence intervals, respectively. The orange shading highlights the detected QTL region.
| QTL | Chromosome | Start (bp) | End (bp) | Interval (bp) | Δ(SNP-index) | HT-bulk SNP-index | HS-bulk SNP-index |
|---|---|---|---|---|---|---|---|
| qSF2.1 | 2 | 311 051 | 3 929 422 | 3 618 371 | 0.94 | 0.94 | 0.25 |
Table 3. QTL detected for the seed-setting rate in rice on chromosome 2.
| QTL | Chromosome | Start (bp) | End (bp) | Interval (bp) | Δ(SNP-index) | HT-bulk SNP-index | HS-bulk SNP-index |
|---|---|---|---|---|---|---|---|
| qSF2.1 | 2 | 311 051 | 3 929 422 | 3 618 371 | 0.94 | 0.94 | 0.25 |

Fig. 5. Candidate genes on chromosome 2 and synonymous single nucleotide polymorphisms/insertions and deletions (SNPs/InDels) among F7 candidate lines, M9962, PTT1, HT-bulk, and HS-bulk. A, Candidate genes within the QTL regions. B, Genes containing synonymous SNPs/InDels among the candidate lines, heat-tolerant parent (M9962) and HT-bulk are highlighted in yellow. HT, Heat tolerant; HS, Heat sensitive; PTT1, Pathumthani 1; PSL2, Phitsanulok.
| Gene name | Functional domain and trait ontology | Pathumthani 1 allele | M9962 allele | HT-bulk allele | HS-bulk allele | F7 allele |
|---|---|---|---|---|---|---|
| Os02g0115900 | Endoplasmic reticulum-localized chaperone HSP70 | ATG/ATG | A/A | A/A | ATG/ATG | A/A |
| Os02g0120800 | GTPase, salt tolerance, negative regulation of disease resistance, pollen germination | AGG/AGG | A/A | A/A | AGG/A | A/A |
| Os02g0121000 | Glutamyl-tRNA synthetase, responsible for thermosensitive chlorophyll-deficient phenotype in cde1(t) mutant | G/G | A/A | A/A | G/A | A/A |
| Os02g0121300 | Cyclophilin-like domain, auxin signal transduction, heat tolerance | T/T | C/C | C/C | T/T | C/C |
| Os02g0122400 | Similar to plastid division protein | A/A | G/G | G/G | G/A | G/G |
| Os02g0126400 | Calcium-dependent protein kinase, positive regulator of salt and drought stress responses | A/A | C/C | C/C | A/A | C/C |
| Os02g0128400 | Heat shock protein DnaJ domain protein B3 (HSP40), N-terminal domain-containing protein | TA/TA | T/T | T/T | T/TA | T/T |
| Os02g0141300 | Arabinokinase-like protein, pollen development | C/C | T/T | T/T | C/C | T/T |
| Os02g0149800 | Stress-responsive NAC1-regulated protein phosphatase, drought and oxidative stress tolerance | CTTTT/CTTTT | CTT/CTT | CTT/CTT | CTTTT/CTTTT | CTT/CTT |
Table 4. Candidate genes that may be related to heat tolerance during the reproductive stage in qSF2.1 region.
| Gene name | Functional domain and trait ontology | Pathumthani 1 allele | M9962 allele | HT-bulk allele | HS-bulk allele | F7 allele |
|---|---|---|---|---|---|---|
| Os02g0115900 | Endoplasmic reticulum-localized chaperone HSP70 | ATG/ATG | A/A | A/A | ATG/ATG | A/A |
| Os02g0120800 | GTPase, salt tolerance, negative regulation of disease resistance, pollen germination | AGG/AGG | A/A | A/A | AGG/A | A/A |
| Os02g0121000 | Glutamyl-tRNA synthetase, responsible for thermosensitive chlorophyll-deficient phenotype in cde1(t) mutant | G/G | A/A | A/A | G/A | A/A |
| Os02g0121300 | Cyclophilin-like domain, auxin signal transduction, heat tolerance | T/T | C/C | C/C | T/T | C/C |
| Os02g0122400 | Similar to plastid division protein | A/A | G/G | G/G | G/A | G/G |
| Os02g0126400 | Calcium-dependent protein kinase, positive regulator of salt and drought stress responses | A/A | C/C | C/C | A/A | C/C |
| Os02g0128400 | Heat shock protein DnaJ domain protein B3 (HSP40), N-terminal domain-containing protein | TA/TA | T/T | T/T | T/TA | T/T |
| Os02g0141300 | Arabinokinase-like protein, pollen development | C/C | T/T | T/T | C/C | T/T |
| Os02g0149800 | Stress-responsive NAC1-regulated protein phosphatase, drought and oxidative stress tolerance | CTTTT/CTTTT | CTT/CTT | CTT/CTT | CTTTT/CTTTT | CTT/CTT |
| [1] | Abdul Rahman S M, Ellis R H. 2019. Seed quality in rice is most sensitive to drought and high temperature in early seed development. Seed Sci Res, 29(4): 238-249. |
| [2] | American Association of Cereal Chemists. 2000. Approved methods of the American Association of Cereal Chemists. 10th edn. St. Paul, MN, USA: America Association of Cereal Chemists, John Wiley & Sons Inc. |
| [3] | Amnuaylojaroen T, Limsakul A, Kirtsaeng S, et al. 2022. Effect of the near-future climate change under RCP8.5 on the heat stress and associated work performance in Thailand. Atmosphere, 13(2): 325. |
| [4] | Arshad M S, Farooq M, Asch F, et al. 2017. Thermal stress impacts reproductive development and grain yield in rice. Plant Physiol Biochem, 115: 57-72. |
| [5] | Baker J T, Allen Jr L H, Boote K J. 1992. Response of rice to carbon dioxide and temperature. Agric For Meteor, 60(3/4): 153-166. |
| [6] | Buttery R G, Juliano B O, Ling L C. 1983. Identification of rice aroma compound 2-acetyl-1-pyrroline in pandan leaves. Chem Ind, 478: 478. |
| [7] | Cao Z B, Xie H W, Nie Y Y, et al. 2015. Mapping a QTL (qHTH5) for heat tolerance at the heading stage on rice chromosome 5 and its genetic effect analysis. Chin J Rice Sci, 29(2): 119-125. (in Chinese with English abstract) |
| [8] | Cheabu S, Moung-Ngam P, Arikit S, et al. 2018. Effects of heat stress at vegetative and reproductive stages on spikelet fertility. Rice Sci, 25(4): 218-226. |
| [9] | Cheabu S, Panichawong N, Rattanametta P, et al. 2019. Screening for spikelet fertility and validation of heat tolerance in a large rice mutant population. Rice Sci, 26(4): 229-238. |
| [10] | Chen L, Wang Q, Tang M Y, et al. 2021. QTL mapping and identification of candidate genes for heat tolerance at the flowering stage in rice. Front Genet, 11: 621871. |
| [11] | Counce P A, Keisling T C, Mitchell A J. 2000. A uniform, objective, and adaptive system for expressing rice development. Crop Sci, 40(2): 436-443. |
| [12] | Eckhardt U, Grimm B, Hörtensteiner S. 2004. Recent advances in chlorophyll biosynthesis and breakdown in higher plants. Plant Mol Biol, 56(1): 1-14. |
| [13] | Farooq M, Hussain M, Wahid A, et al. 2012. Drought stress in plants: An overview. In: Aroca R. Plant Responses to Drought Stress. Heidelberg, Berlin, Germany: Springer Berlin Heidelberg: 1-33. |
| [14] | He C, Xiao Y L, Yu J H, et al. 2019. Pyramiding Xa21, Bph14, and Bph15 genes into the elite restorer line Yuehui9113 increases resistance to bacterial blight and the brown planthopper in rice. Crop Prot, 115: 31-39. |
| [15] | Heinrichs E A, Medrano F G, Rapusas H R. 1985. Genetic evaluation for insect resistance in rice. Los Banos, the Philippines: International Rice Research Institute. |
| [16] | Hu C M, Jiang J H, Li Y L, et al. 2022. QTL mapping and identification of candidate genes using a genome-wide association study for heat tolerance at anthesis in rice (Oryza sativa L.). Front Genet, 13: 983525. |
| [17] | IRRI. 2013. Standard Evaluation System for Rice. Manila, the Philippines: International Rice Research Institute: 35-36. |
| [18] | Jagadish S V K, Cairns J, Lafitte R, et al. 2010. Genetic analysis of heat tolerance at anthesis in rice. Crop Sci, 50(5): 1633-1641. |
| [19] | Jagadish K S V, Craufurd P, Shi W J, et al. 2013. A phenotypic marker for quantifying heat stress impact during microsporogenesis in rice (Oryza sativa L.). Funct Plant Biol, 41(1): 48-55. |
| [20] | Kamau P K, Sano S, Takami T, et al. 2015. A mutation in GIANT CHLOROPLAST encoding a PARC6 homolog affects spikelet fertility in rice. Plant Cell Physiol, 56(5): 977-991. |
| [21] | Kilasi N L, Singh J, Vallejos C E, et al. 2018. Heat stress tolerance in rice (Oryza sativa L.): Identification of quantitative trait loci and candidate genes for seedling growth under heat stress. Front Plant Sci, 9: 1578. |
| [22] | Kou S Y, Wu Z G, Li H Y, et al. 2023. Heterologous expression of heat-shock protein PpHSP70 improves high temperature and drought tolerance in rice. Plant Stress, 10: 100273. |
| [23] | Krzywinski M, Schein J, Birol I, et al. 2009. Circos: An information aesthetic for comparative genomics. Genome Res, 19(9): 1639-1645. |
| [24] | Kumboonreang N. 2011. Effect of light duration on panicle initiation and advantages of off-season growing KDML105 rice using short day period. Nakhon Ratchasima, Tailand: Suranaree University of Technology. |
| [25] | Lang N T, Ha P T T, Tru P C, et al. 2015. Breeding for heat tolerance rice based on marker-assisted backcrosing in Vietnam. Plant Breed Biotechnol, 3(3): 274-281. |
| [26] | Langkulsen U, Rwodzi D. 2019. Extreme weather and climate events and occupational health in Thailand. In: Akhtar R. Extreme Weather Events and Human Health. Cham: Springer International Publishing: 209-225. |
| [27] | Langmead B, Salzberg S L. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods, 9(4): 357-359. |
| [28] | LGC Group. 2016. SNPline genotyping automation. [2024-01-05]. https://www.lgcgroup.com/products/genotypinginstruments/snpline/#.XFCv91xKhaQ. |
| [29] | Li J Z, Qian X G, Sha B D. 2009. Heat shock protein 40: Structural studies and their functional implications. Protein Pept Lett, 16(6): 606-612. |
| [30] | Li M M, Li X, Yu L Q, et al. 2018. Identification of QTLs associated with heat tolerance at the heading and flowering stage in rice (Oryza sativa L.). Euphytica, 214(4): 70. |
| [31] | Liao J L, Zhang H Y, Shao X L, et al. 2011. Identification for heat tolerance in backcross recombinant lines and screening of backcross introgression lines with heat tolerance at milky stage in rice. Rice Sci, 18(4): 279-286. |
| [32] | Liu D H, Zhang J L, Cao J H, et al. 2010. The reduction of amylose content in rice grain and decrease of Wx gene expression during endosperm development in response to drought stress. J Food Agric Environ, 8(3): 873-878. |
| [33] | Liu H Q, Zeng B H, Zhao J L, et al. 2023. Genetic research progress: Heat tolerance in rice. Int J Mol Sci, 24(8): 7140. |
| [34] | Liu W Z, Fu Y P, Hu G C, et al. 2007. Identification and fine mapping of a thermo-sensitive chlorophyll deficient mutant in rice (Oryza sativa L.). Planta, 226(3): 785-795. |
| [35] | Malumpong C, Cheabu S, Mongkolsiriwatana C, et al. 2019. Spikelet fertility and heat shock transcription factor (Hsf) gene responses to heat stress in tolerant and susceptible rice (Oryza sativa L.) genotypes. J Agric Sci, 157(4): 283-299. |
| [36] | Malumpong C, Buadchee R, Thammasamisorn B, et al. 2020. Backcross breeding for improvement of heat tolerance at reproductive phase in Thai rice (Oryza sativa L.) varieties. J Agric Sci, 158(6): 496-510. |
| [37] | Masutomi Y, Takimoto T, Manabe T, et al. 2023. Breeding targets for heat-tolerant rice varieties in Japan in a warming climate. Mitig Adapt Strateg Glob Change, 28(1): 2. |
| [38] | McKenna A, Hanna M, Banks E, et al. 2010. The genome analysis toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res, 20(9): 1297-1303. |
| [39] | Milne I, Shaw P, Stephen G, et al. 2010. Flapjack: Graphical genotype visualization. Bioinformatics, 26(24): 3133-3134. |
| [40] | Mitsui T, Shiraya T, Kaneko K, et al. 2013. Proteomics of rice grain under high temperature stress. Front Plant Sci, 4: 36. |
| [41] | Nicolas M E, Gleadow R M, Dalling M J. 1985. Effect of post- anthesis drought on cell division and starch accumulation in developing wheat grains. Ann Bot, 55(3): 433-444. |
| [42] | Nubankoh P, Wanchana S, Saensuk C, et al. 2020. QTL-seq reveals genomic regions associated with spikelet fertility in response to a high temperature in rice (Oryza sativa L.). Plant Cell Rep, 39(1): 149-162. |
| [43] | Okpala N E, Potcho M P, An T Y, et al. 2020. Low temperature increased the biosynthesis of 2-AP, cooked rice elongation percentage and amylose content percentage in rice. J Cereal Sci, 93: 102980. |
| [44] | Pitija K, Srisedkha T, Wiwatsamretkun C. 2021. Quantification of rice aroma, 2-acetyl-1-pyrroline (2-AP), using TurboMatrix Headspace Trap coupled with GC/NPD and GC/MS. [2024-01-05]. http:www.https://resources.perkinelmer.com/lab-solutions/resources/docs/APP_013275_01-Quantification-of-Rice-Aroma-Application-Note.pdf. |
| [45] | Prasad P V V, Boote K J, Allen Jr L H, et al. 2006. Species, ecotype and cultivar differences in spikelet fertility and harvest index of rice in response to high temperature stress. Field Crops Res, 95(2/3): 398-411. |
| [46] | Prasanth V V, Basava K R, Babu M S, et al. 2016. Field level evaluation of rice introgression lines for heat tolerance and validation of markers linked to spikelet fertility. Physiol Mol Biol Plants, 22(2): 179-192. |
| [47] | Prodhan Z H, Faruq G, Rashid K A, et al. 2017. Effects of temperature on volatile profile and aroma quality in rice. Int J Agric Biol, 19(5): 1065-1072. |
| [48] | Prodhan Z H, Shu Q Y. 2020. Rice aroma: A natural gift comes with price and the way forward. Rice Sci, 27(2): 86-100. |
| [49] | Sarkar N K, Kundnani P, Grover A. 2013. Functional analysis of Hsp70 superfamily proteins of rice (Oryza sativa). Cell Stress Chaperones, 18(4): 427-437. |
| [50] | Senapeng P, Prahadchai T, Guayjarernpanishk P, et al. 2022. Spatial modeling of extreme temperature in Northeast Thailand. Atmosphere, 13(4): 589. |
| [51] | Shanmugavadivel P S, Amitha Mithra S V, Chandra P, et al. 2017. High resolution mapping of QTLs for heat tolerance in rice using a 5K SNP array. Rice, 10(1): 28. |
| [52] | Singh S P, Singh M K, Kumar S, et al. 2019. Cultivation of aromatic rice: A review. In: Hasanuzzaman M. Agronomic Crops. Singapore: Springer Singapore: 175-198. |
| [53] | Sreethong T, Prom-u-thai C, Rerkasem B, et al. 2018. Variation of milling and grain physical quality of dry season Pathum Thani 1 in Thailand. Chiang Mai Univ J Nat Sci, 17(3): 191-202. |
| [54] | Su P H, Li H M. 2008. Arabidopsis stromal 70-kD heat shock proteins are essential for plant development and important for thermotolerance of germinating seeds. Plant Physiol, 146(3): 1231-1241. |
| [55] | Sukkeo S, Rerkasem B, Jamjod S. 2017. Heat tolerance in Thai rice varieties. ScienceAsia, 43(2): 61-69. |
| [56] | Takagi H, Abe A, Yoshida K, et al. 2013. QTL-seq: Rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J, 74(1): 174-183. |
| [57] | Thai Meteorological Department. 2021. Climatic Information. [2024-01-05]. https://www.tmd.go.th. |
| [58] | Timabud T, Yin X J, Pongdontri P, et al. 2016. Gel-free/label-free proteomic analysis of developing rice grains under heat stress. J Proteomics, 133: 1-19. |
| [59] | Trivedi D K, Ansari M W, Tuteja N. 2013. Multiple abiotic stress responsive rice cyclophilin: (OsCYP-25) mediates a wide range of cellular responses. Commun Integr Biol, 6: e25260. |
| [60] | Varinruk B. 2017. Thailand rice production and rice R&D on climate change. In: Strengthening APEC Cooperation on Food Security and Climate Change. Hanoi, Veitnam, 19-21 April 2017. |
| [61] | Varshney R K, Graner A, Sorrells M E. 2005. Genomics-assisted breeding for crop improvement. Trends Plant Sci, 10(12): 621-630. |
| [62] | Visakh R L, Anand S, Arya S N, et al. 2024. Rice heat tolerance breeding: A comprehensive review and forward gaze. Rice Sci, 31(4): 375-400. |
| [63] | Wang Y L, Wang L, Zhou J X, et al. 2019. Research progress on heat stress of rice at flowering stage. Rice Sci, 26(1): 1-10. |
| [64] | Wassmann R, Jagadish S V K, Sumfleth K, et al. 2009. Regional vulnerability of climate change impacts on Asian rice production and scope for adaptation. Adv Agron, 102: 91-133. |
| [65] | Wolfe D, Dudek S, Ritchie M D, et al. 2013. Visualizing genomic information across chromosomes with PhenoGram. BioData Min, 6(1): 18. |
| [66] | Xie W J, Li Y H, Li Y Z, et al. 2021. Application of γ-aminobutyric acid under low light conditions: Effects on yield, aroma, element status, and physiological attributes of fragrant rice. Ecotoxicol Environ Saf, 213: 111941. |
| [67] | Xu Y F, Chu C C, Yao S G. 2021. The impact of high-temperature stress on rice: Challenges and solutions. Crop J, 9(5): 963-976. |
| [68] | Ye C R, Tenorio F A, Redoña E D, et al. 2015a. Fine-mapping and validating qHTSF4.1 to increase spikelet fertility under heat stress at flowering in rice. Theor Appl Genet, 128(8): 1507-1517. |
| [69] | Ye C R, Tenorio F A, Argayoso M A, et al. 2015b. Identifying and confirming quantitative trait loci associated with heat tolerance at flowering stage in different rice populations. BMC Genet, 16: 41. |
| [70] | Yoshida S. 1981. Fundamentals of rice crops science. Los Banos, the Philippines: International Rice Research Institute. |
| [71] | Yoshida S, Hara T. 1977. Effects of air temperature and light on grain filling of an indica and a japonica rice (Oryza sativa L.) under controlled environmental conditions. Soil Sci Plant Nutr, 23(1): 93-107. |
| [72] | Yu A M, Li P, Tang T, et al. 2015. Roles of Hsp70s in stress responses of microorganisms, plants, and animals. Biomed Res Int, 2015: 510319. |
| [73] | Zhao Y, Mette M F, Gowda M, et al. 2014. Bridging the gap between marker-assisted and genomic selection of heading time and plant height in hybrid wheat. Heredity, 112(6): 638-645. |
| [74] | Zhu C L, Xiao Y H, Wang C M, et al. 2005. Mapping QTLs for heat tolerance during grain filling in rice. Chin J Rice Sci, 19(2): 117-121. (in Chinese with English abstract) |
| [1] | Wang Mingyue, Zhao Weibo, Feng Xiaoya, Chen Yi, Li Junhao, Fu Jinmei, Yan Yingchun, Chu Zhaohui, Huang Wenchao. Disruption of Energy Metabolism and Reactive Oxygen Species Homeostasis in Honglian Type-Cytoplasmic Male Sterility (HL-CMS) Rice Pollen [J]. Rice Science, 2025, 32(1): 81-93. |
| [2] | Intan Farahanah, Shariza Sahudin, Hannis Fadzillah Mohsin, Siti Alwani Ariffin, Liyana Dhamirah Aminuddin. Understanding Investigational Perspective of Antioxidant and Antibacterial Properties of Rice [J]. Rice Science, 2025, 32(1): 15-31. |
| [3] | Jeberlin Prabina Bright, Hemant S. Maheshwari, Sugitha Thangappan, Kahkashan Perveen, Najat A. Bukhari, Debasis Mitra, Riyaz Sayyed, Andrea Mastinu. Biofilmed-PGPR: Next-Generation Bioinoculant for Plant Growth Promotion in Rice under Changing Climate [J]. Rice Science, 2025, 32(1): 94-106. |
| [4] | Wang Haoran, Chen Guoqing, Feng Guozhong. Expanding Viral Diversity in Rice Fields by Next-Generation Sequencing [J]. Rice Science, 2025, 32(1): 44-51. |
| [5] | Durga Prasad Mullangie, Kalaimagal Thiyagarajan, Manonmani Swaminathan, Jagadeesan Ramalingam, Sritharan Natarajan, Senthilkumar Govindan. Breeding Resilience: Exploring Lodging Resistance Mechanisms in Rice [J]. Rice Science, 2024, 31(6): 659-672. |
| [6] | Fu Yiwei, Wu Jiayelu, Wu Mingming, Ye Shenghai, Zhai Rongrong, Ye Jing, Zhu Guofu, Yu Faming, Lu Yanting, Zhang Xiaoming. Progress on Molecular Mechanism of Heat Tolerance in Rice [J]. Rice Science, 2024, 31(6): 673-687. |
| [7] | Yang Yigang, Xu Ya’nan, Bai Yeran, Zhang Yuanpei, Han Wei, Makoto Saito, Lü Guohua, Song Jiqing, Bai Wenbo. Mixed-Oligosaccharides Promote Seedling Growth of Direct-Seeded Rice under Salt and Alkaline Stress [J]. Rice Science, 2024, 31(6): 712-724. |
| [8] | Ren Jian, Hu Kelin, Feng Puyu, William D. Batchelor, Liu Haitao, Lü Shihua. Simulating Responses of Rice Yield and Nitrogen Fates to Ground Cover Rice Production System under Different Types of Precipitation Years [J]. Rice Science, 2024, 31(6): 725-739. |
| [9] | Tao Yi, Xiao Deshun, Ye Chang, Liu Kancheng, Tang Xinxin, Ma Hengyu, Chu Guang, Yu Kai, Xu Chunmei, Wang Danying. Compound Microbial Agent Improves Soil Redox Status to Reduce Methane Emissions from Paddy Fields [J]. Rice Science, 2024, 31(6): 740-750. |
| [10] | Sitthikorn Bodeerath, Jeeraporn Veeradittakit, Sansanee Jamjod, Chanakan Prom-U-Thai. Applying Boron Fertilizer at Different Growth Stages Promotes Boron Uptake and Productivity in Rice [J]. Rice Science, 2024, 31(6): 751-760. |
| [11] | Kunhikrishnan Hemalatha Dhanyalakshmi, Reshma Mohan, Sasmita Behera, Uday Chand Jha, Debashis Moharana, Ahalya Behera, Sini Thomas, Preman Rejitha Soumya, Rameswar Prasad Sah, Radha Beena. Next Generation Nutrition: Genomic and Molecular Breeding Innovations for Iron and Zinc Biofortification in Rice [J]. Rice Science, 2024, 31(5): 526-544. |
| [12] | Zhang Youliang, Zhu Kaican, Tang Yongqi, Feng Shaoyuan. Rice Cultivation under Film Mulching Can Improve Soil Environment and Be Beneficial for Rice Production in China [J]. Rice Science, 2024, 31(5): 545-555. |
| [13] | Chirag Maheshwari, Nitin Kumar Garg, Archana Singh, Aruna Tyagi. Ameliorative Effects of Paclobutrazol via Physio-Biochemical and Molecular Manifestation in Rice under Water Deficit Stress [J]. Rice Science, 2024, 31(5): 603-616. |
| [14] | Hong Weiyuan, Li Ziqiu, Feng Xiangqian, Qin Jinhua, Wang Aidong, Jin Shichao, Wang Danying, Chen Song. Estimating Key Phenological Dates of Multiple Rice Accessions Using Unmanned Aerial Vehicle-Based Plant Height Dynamics for Breeding [J]. Rice Science, 2024, 31(5): 617-628. |
| [15] | Liyana Sara, Sompop Saeheng, Panupong Puttarak, Lompong Klinnawee. Changes in Metabolites and Allelopathic Effects of Non-Pigmented and Black-Pigmented Lowland Indica Rice Varieties in Phosphorus Deficiency [J]. Rice Science, 2024, 31(4): 434-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||