
Rice Science ›› 2025, Vol. 32 ›› Issue (1): 107-117.DOI: 10.1016/j.rsci.2024.10.005
• Research Papers • Previous Articles Next Articles
Xu Liting,#, He Kaiwei,#, Guo Chunyu,#, Quan Cantao,#, Ma Yahuan, Zhang Wei, Ren Lifen, Wang Long, Song Li, Ouyang Qing, Yin Junjie, Zhu Xiaobo, Tang Yongyan, He Min, Chen Xuewei, Li Weitao( )
)
Received:2024-08-27
Accepted:2024-10-11
Online:2025-01-28
Published:2025-03-25
Contact:
Li Weitao
About author:First author contact:#These authors contributed equally to this work
Xu Liting, He Kaiwei, Guo Chunyu, Quan Cantao, Ma Yahuan, Zhang Wei, Ren Lifen, Wang Long, Song Li, Ouyang Qing, Yin Junjie, Zhu Xiaobo, Tang Yongyan, He Min, Chen Xuewei, Li Weitao. Spireoside Controls Blast Disease by Disrupting Membrane Integrity of Magnaporthe oryzae[J]. Rice Science, 2025, 32(1): 107-117.
Add to citation manager EndNote|Ris|BibTeX
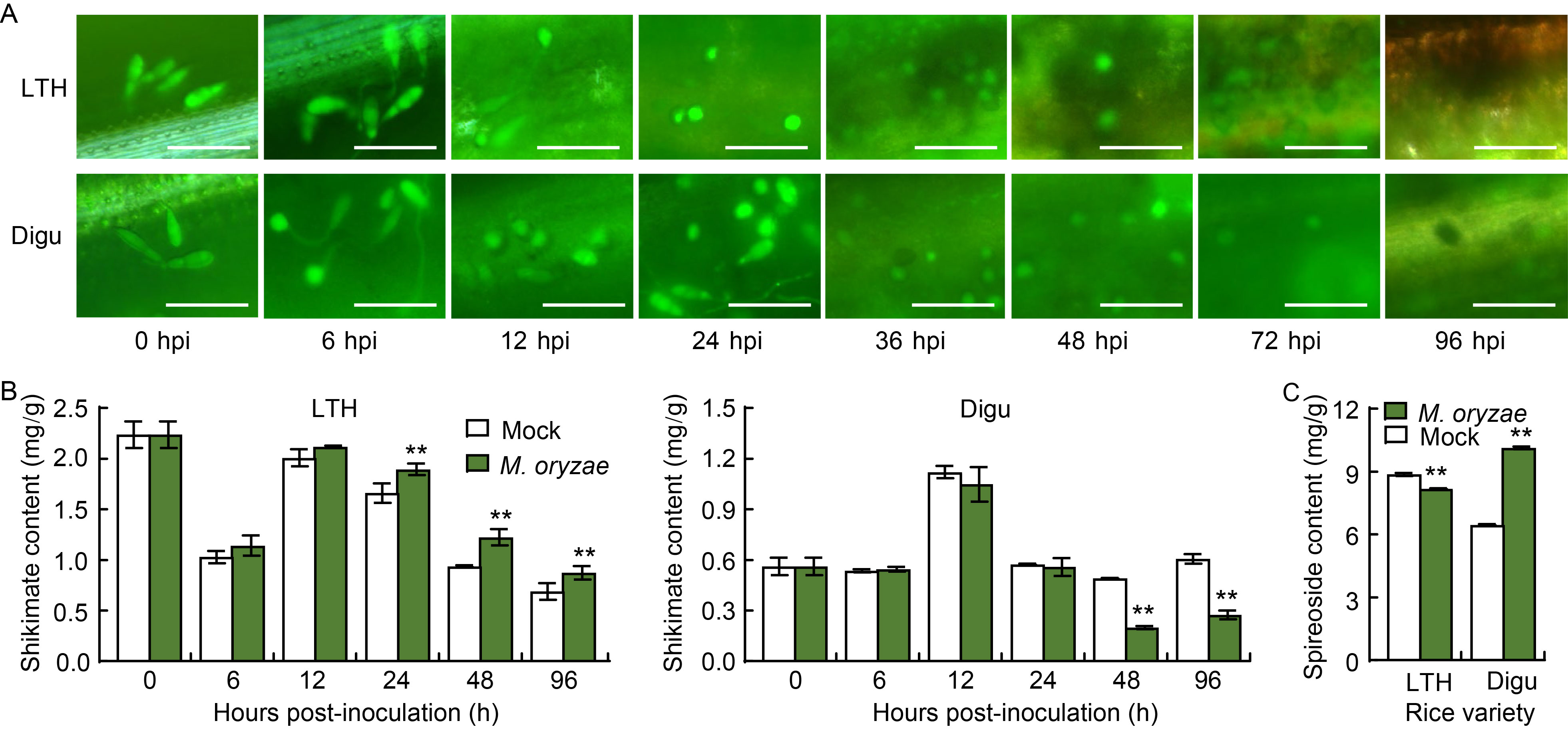
Fig. 1. Epifluorescent microscopy examination of Magnaporthe oryzae invading rice leaves, and determination of shikimate and spireoside contents. A, Epifluorescent microscopy examination of M. oryzae invading rice leaves of susceptible variety Lijiangxintuanheigu (LTH) and resistant variety Digu. The blast isolate Guy11 was tagged with eGFP. Scale bars, 45 μm. hpi, Hours post-inoculation. B, Shikimate content in LTH and Digu post-inoculation with M. oryzae. C, Spireoside content in LTH and Digu post-inoculation with M. oryzae. Data are Mean ± SD (n = 3). Statistical analysis was performed using a two-sided Student’s t-test. **, P < 0.01.
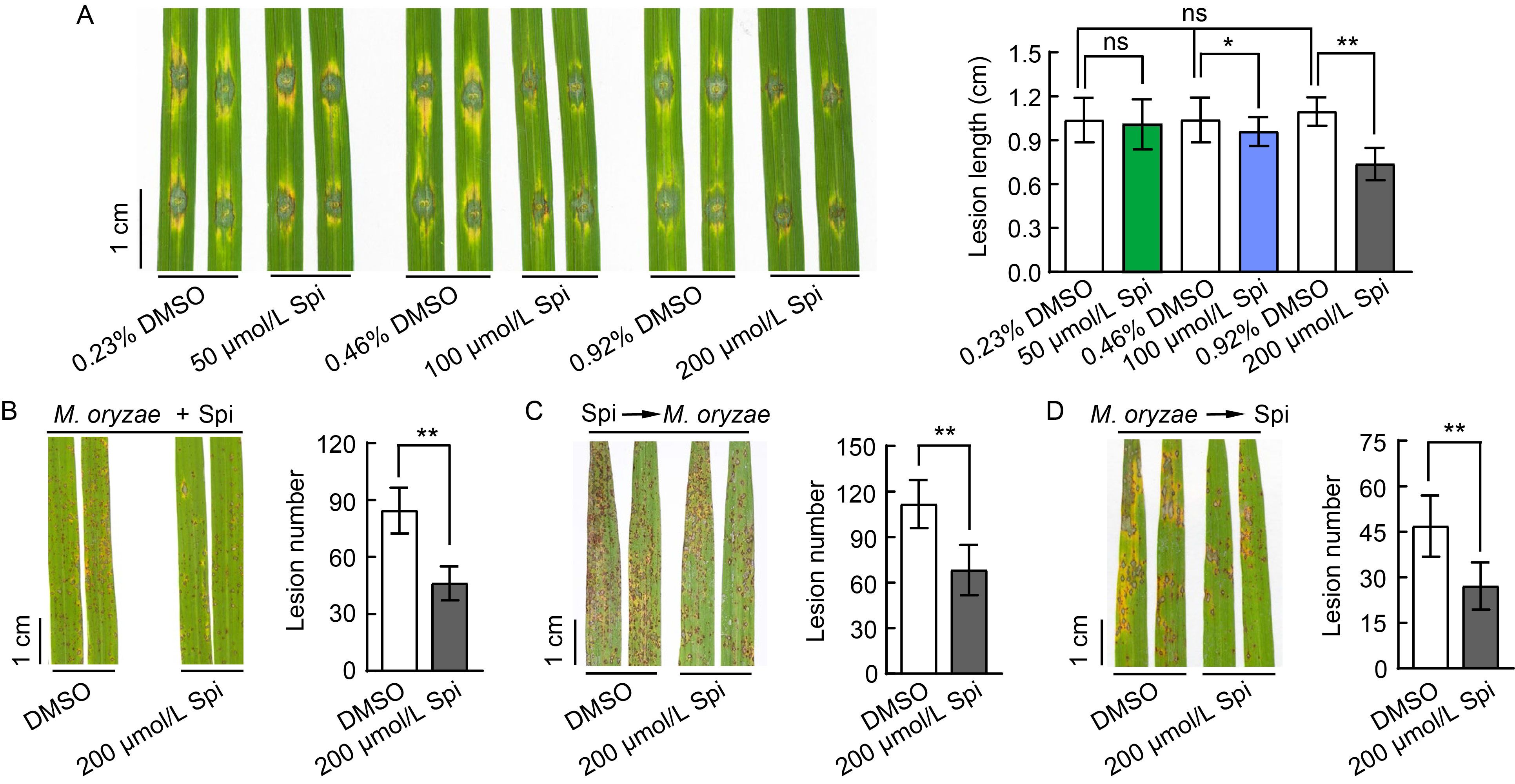
Fig. 2. Effect of spireoside on blast resistance. A, Rice leaves were punched and concurrently inoculated with 50, 100, and 200 μmol/L spireoside (Spi), respectively, along with Guy11 spores. The concentration of DMSO in the negative control was equivalent to that in the 50, 100, and 200 μmol/L spireoside treatments. B‒D, Rice leaves were treated with three different procedures in a field trial: punched and concurrently inoculated with 200 μmol/L spireoside and Guy11 spores (B), sprayed with 200 μmol/L spireoside 12 h before inoculation with Guy11 spores (C), and sprayed with 200 μmol/L spireoside 12 h after inoculation with Guy11 spores (D). The concentration of DMSO in the negative control was matched to that in the 200 μmol/L spireoside treatment. Lesion density was calculated from 5-cm segments of infected rice leaves. DMSO, Dimethyl sulfoxide. Data are Mean ± SD (n = 21‒30 for A, 16 for B, 13‒14 for C, and 15 for D). Statistical analysis was performed using a two-sided Student’s t-test. *, P < 0.05; **, P < 0.01; and ‘ns’ indicates no statistically significant difference at P > 0.05.
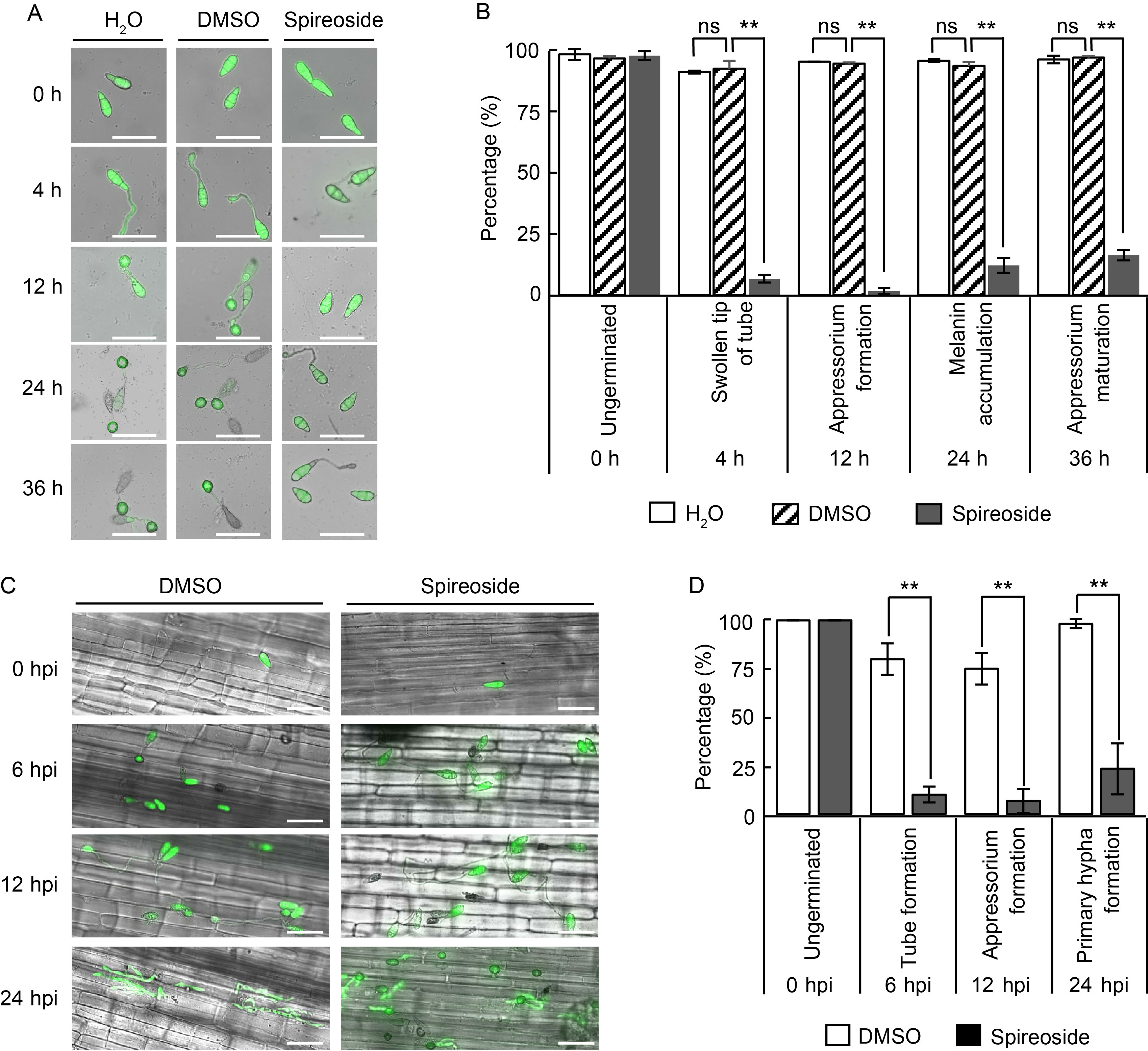
Fig. 3. Effect of spireoside on Magnaporthe oryzae. A, Representative microscopy images showing the effects of 200 μmol/L spireoside on conidial germination and appressorium formation in M. oryzae. The blast isolate Guy11 tagged with eGFP was cultured on hydrophobic coverslips at 26 ºC for 0, 4, 12, 24, and 36 h. Scale bars, 50 μm. B, Quantification of conidial germination and appressorium formation from the images in panel A. C, Representative laser scanning microscopy images of sheath cells from Lijiangxintuanheigu (LTH) infected by the eGFP-tagged blast isolate Guy11. LTH sheaths were concurrently inoculated with Guy11 spores and 200 μmol/L spireoside. Scale bars, 30 μm. D, Distribution of fungal infection progression in panel C. DMSO, Dimethyl sulfoxide; hpi, Hours post-inoculation. Data are Mean ± SD (n = 80 for B and 20 for D). Statistical analysis was performed using a two-sided Student’s t-test. **, P < 0.01; ‘ns’ indicates no statistical significance at P > 0.05.

Fig. 4. Transcriptome analysis of Magnaporthe oryzae at 6 h under 200 μmol/L spireoside treatment. A, Differentially expressed genes (DEGs) under 200 μmol/L spireoside treatment. padj, Adjusted P-value. B, Gene Ontology (GO) analysis for all DEGs under 200 μmol/L spireoside treatment. C, Relative expression levels of random DEGs by qRT-PCR. The concentration of DMSO in the negative control was matched with that in the 200 μmol/L spireoside treatment. DMSO, Dimethyl sulfoxide. Values are Mean ± SD (n = 3). Statistical analysis was performed using a two-sided Student’s t-test. **, P < 0.01.
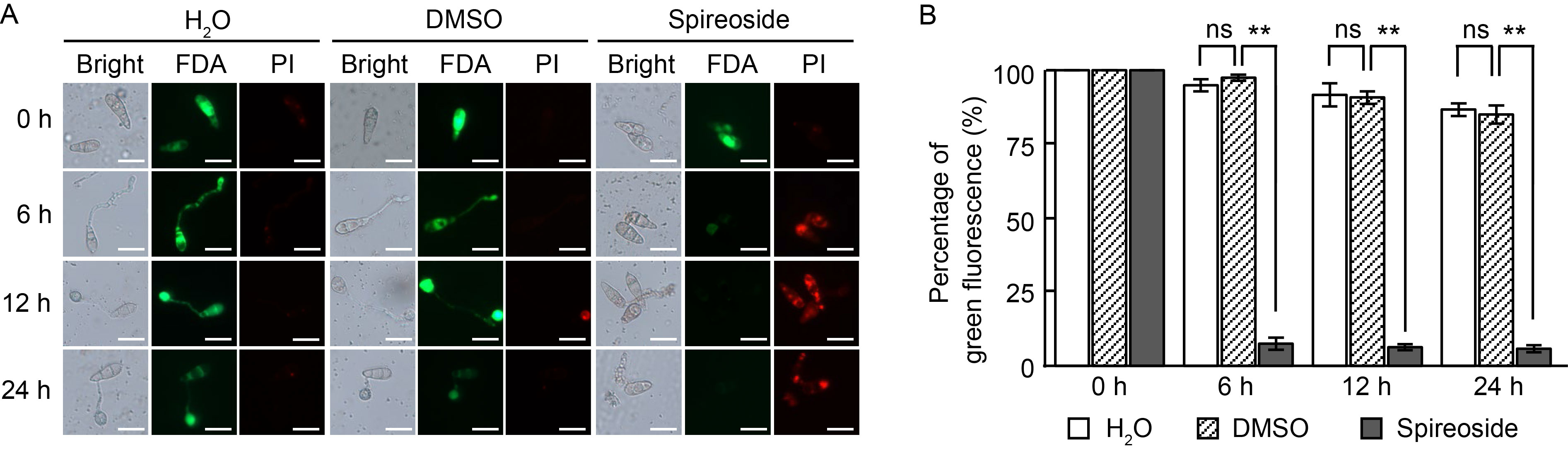
Fig. 5. Effect of spireoside on membrane integrity of Magnaporthe oryzae. A, Guy11 spores were inoculated on hydrophobic slides treated with H2O, 0.92% DMSO, and 200 μmol/L spireoside, respectively. Conidial membrane integrity was stained by fluorescein diacetate (FDA) and propidium iodide (PI). Red fluorescence indicates disrupted membranes, whereas green fluorescence indicates intact membranes. Scale bars, 20 μm. B, Quantification of conidia with green fluorescence in panel A. DMSO, Dimethyl sulfoxide. Values are Mean ± SD (n = 80). The data represent the means from three biologically independent replicates. **, P < 0.01; ‘ns’ indicates no statistical significance at P > 0.05, as determined by a two-sided Student’s t-test.
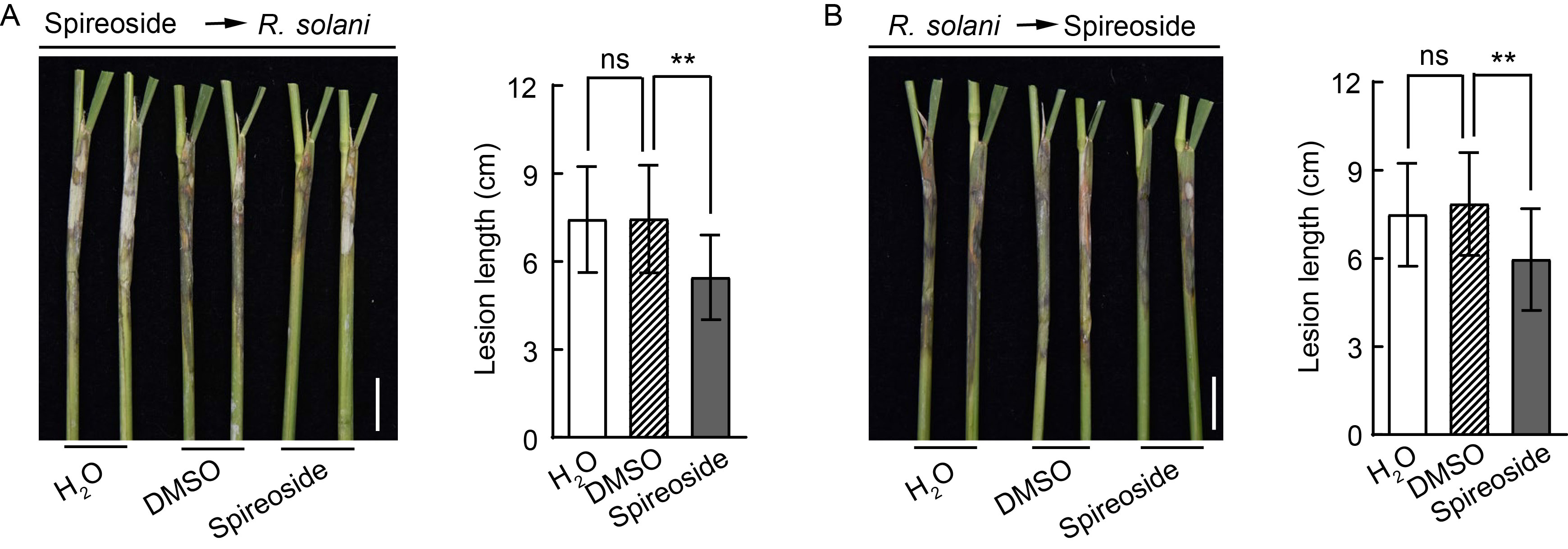
Fig. 6. Effect of spireoside on rice sheath blight resistance. A and B, Rice sheaths were treated with 200 μmol/L spireoside 12 h before inoculation (A) and 12 h after inoculation (B) with Rhizoctonia solani isolate AG-1-IA in a field trial. Representative leaf sheaths (left panel) and lesion length data (right panel) are shown. Scale bars, 2 cm. The dimethyl sulfoxide (DMSO) concentration in the negative control was equivalent to that in the 200 μmol/L spireoside treatment. Values are Mean ± SD (n = 40‒59 for A and 31‒46 for B). **, P < 0.01; ‘ns’ indicates no statistical significance at P > 0.05, as determined by a two-sided Student’s t-test.
| [1] | Akatsuka T, Kodama O, Sekido H, et al. 1985. Novel phytoalexins (oryzalexins A, B and C) isolated from rice blast leaves infected with Pyricularia oryzae: Part I. Isolation, characterization and biological activities of oryzalexins. Agric Biol Chem, 49(6): 1689-1694. |
| [2] | An X Y, Zhang H, Li J L, et al. 2022. The level of endogenous JA is critical for activation of SA- and JA-defensive signaling pathway in japonica rice cultivar Ziyu44 upon Magnaporthe oryzae infection. J Plant Pathol, 104(2): 619-629. |
| [3] | An Y L, Chen X H, Huang Y M, et al. 1999. A study on repression effects of some anthraquinoneson fungus phytop athogens. J Southwest For Coll, 19(2): 122-125. (in Chinese with English abstract) |
| [4] | Bai J R, Wu Y P, Liu X Y, et al. 2015. Antibacterial activity of shikimic acid from pine needles of Cedrus deodara against Staphylococcus aureus through damage to cell membrane. Int J Mol Sci, 16(11): 27145-27155. |
| [5] | Bao J D, Chen M L, Zhong Z H, et al. 2017. PacBio sequencing reveals transposable elements as a key contributor to genomic plasticity and virulence variation in Magnaporthe oryzae. Mol Plant, 10(11): 1465-1468. |
| [6] | Carvalho M F N N. 2022. Synthesis and biological activity of antimicrobial agents. Antibiotics, 11(3): 337. |
| [7] | Deleu M, Paquot M, Nylander T. 2005. Fengycin interaction with lipid monolayers at the air-aqueous interface: Implications for the effect of fengycin on biological membranes. J Colloid Interface Sci, 283(2): 358-365. |
| [8] | Du Fall L A, Solomon P S. 2011. Role of cereal secondary metabolites involved in mediating the outcome of plant-pathogen interactions. Metabolites, 1(1): 64-78. |
| [9] | Durmaz L, Kiziltas H, Karagecili H, et al. 2023. Potential antioxidant, anticholinergic, antidiabetic and antiglaucoma activities and molecular docking of spiraeoside as a secondary metabolite of onion (Allium cepa). Saudi Pharm J, 31(10): 101760. |
| [10] | Fukuoka S, Saka N, Koga H, et al. 2009. Loss of function of a proline-containing protein confers durable disease resistance in rice. Science, 325: 998-1001. |
| [11] | Gao X W, Wang Y C, Sun J H, et al. 2020. Field efficacy trials of tea saponin & berberine 7.5% SL against rice blast. Pestic Sci Adm, 41(8): 39-45. (in Chinese with English abstract) |
| [12] | González-Jaramillo L M, Aranda F J, Teruel J A, et al. 2017. Antimycotic activity of fengycin C biosurfactant and its interaction with phosphatidylcholine model membranes. Colloid Surf B: Biointerfaces, 156: 114-122. |
| [13] | Guo Z Q, Liu X Y, Wang N, et al. 2023. Membrane component ergosterol builds a platform for promoting effector secretion and virulence in Magnaporthe oryzae. New Phytol, 237(3): 930-943. |
| [14] | Hafeez R, Guo J N, Ahmed T, et al. 2024. Integrative transcriptomic and metabolomic analyses reveals the toxicity and mechanistic insights of bioformulated chitosan nanoparticles against Magnaporthe oryzae. Chemosphere, 356: 141904. |
| [15] | Hasegawa M, Mitsuhara I, Seo S, et al. 2014. Analysis on blast fungus-responsive characters of a flavonoid phytoalexin sakuranetin; accumulation in infected rice leaves, antifungal activity and detoxification by fungus. Molecules, 19(8): 11404-11418. |
| [16] | He M, Xu Y P, Chen J H, et al. 2018. MoSnt2-dependent deacetylation of histone H3 mediates MoTor-dependent autophagy and plant infection by the rice blast fungus Magnaporthe oryzae. Autophagy, 14(9): 1543-1561. |
| [17] | Hiruma K, Onozawa-Komori M, Takahashi F, et al. 2010. Entry mode-dependent function of an indole glucosinolate pathway in Arabidopsis for nonhost resistance against anthracnose pathogens. Plant Cell, 22(7): 2429-2443. |
| [18] | Kai K, Wang R, Bi W L, et al. 2021. Chlorogenic acid induces ROS-dependent apoptosis in Fusarium fujikuroi and decreases the postharvest rot of cherry tomato. World J Microbiol Biotechnol, 37(6): 93. |
| [19] | Katanić J, Boroja T, Stanković N, et al. 2015. Bioactivity, stability and phenolic characterization of Filipendula ulmaria (L.) Maxim. Food Funct, 6(4): 1164-1175. |
| [20] | Kato H, Kodama O, Akatsuka T. 1993. Oryzalexin E, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry, 33(1): 79-81. |
| [21] | Kato H, Kodama O, Akatsuka T. 1994. Oryzalexin F, a diterpene phytoalexin from UV-irradiated rice leaves. Phytochemistry, 36(2): 299-301. |
| [22] | Kodama O, Li W X, Tamogami S, et al. 1992. Oryzalexin S, a novel stemarane-type diterpene rice phytoalexin. Biosci Biotechnol Biochem, 56(6): 1002-1003. |
| [23] | Koga J, Ogawa N, Yamauchi T, et al. 1997. Functional moiety for the antifungal activity of phytocassane E, a diterpene phytoalexin from rice. Phytochemistry, 44(2): 249-253. |
| [24] | Kühnau J. 1976. The flavonoids. A class of semi-essential food components: Their role in human nutrition. World Rev Nutr Diet, 24: 117-191. |
| [25] | Lee J, Lee D G. 2015. Antimicrobial peptides (AMPs) with dual mechanisms: Membrane disruption and apoptosis. J Microbiol Biotechnol, 25(6): 759-764. |
| [26] | Li A P, Zhao Z M, Zhang S Y, et al. 2021. Fungicidal activity and mechanism of action of glabridin from Glycyrrhiza glabra L. Int J Mol Sci, 22(20): 10966. |
| [27] | Li W T, Zhu Z W, Chern M, et al. 2017. A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell, 170(1): 114-126.e15. |
| [28] | Li W T, Chern M, Yin J J, et al. 2019. Recent advances in broad- spectrum resistance to the rice blast disease. Curr Opin Plant Biol, 50: 114-120. |
| [29] | Mabrouk S B, Reis M, Sousa M L, et al. 2020. The marine seagrass Halophila stipulacea as a source of bioactive metabolites against obesity and biofouling. Mar Drugs, 18(2): 88. |
| [30] | Maeda H, Dudareva N. 2012. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu Rev Plant Biol, 63: 73-105. |
| [31] | Murota K, Mitsukuni Y, Ichikawa M, et al. 2004. Quercetin-4ʹ- glucoside is more potent than quercetin-3-glucoside in protection of rat intestinal mucosa homogenates against iron ion-induced lipid peroxidation. J Agric Food Chem, 52(7): 1907-1912. |
| [32] | Ngo M T, Han J W, Yoon S, et al. 2019. Discovery of new triterpenoid saponins isolated from Maesa japonica with antifungal activity against rice blast fungus Magnaporthe oryzae. J Agric Food Chem, 67(27): 7706-7715. |
| [33] | Nile A, Gansukh E, Park G S, et al. 2021. Novel insights on the multi-functional properties of flavonol glucosides from red onion (Allium cepa L) solid waste: In vitro and in silico approach. Food Chem, 335: 127650. |
| [34] | Norvienyeku J, Lin L L, Waheed A, et al. 2021. Bayogenin 3-O- cellobioside confers non-cultivar-specific defence against the rice blast fungus Pyricularia oryzae. Plant Biotechnol J, 19(3): 589-601. |
| [35] | Ogi K, Sumitani H. 2019. Elucidation of an α-glucosidase inhibitor from the peel of Allium cepa by principal component analysis. Biosci Biotechnol Biochem, 83(4): 751-754. |
| [36] | Piyo A, Udomsilp J, Khang-Khun P, et al. 2009. Antifungal activity of essential oils from basil (Ocimum basilicum Linn.) and sweet fennel (Ocimum gratissimum Linn.): Alternative strategies to control pathogenic fungi in organic rice. Asian J Food Agro-Ind, 2: S2-S9. |
| [37] | Qi Z Q, Xue Y F, Zhang M, et al. 2015. Effect of osthol on the invasion of Magnaporthe oryzae. Jiangsu J Agric Sci, 31(6): 1265-1269. (in Chinese with English abstract) |
| [38] | Riedlmeier M, Ghirardo A, Wenig M, et al. 2017. Monoterpenes support systemic acquired resistance within and between plants. Plant Cell, 29(6): 1440-1459. |
| [39] | Sato H, Feix J B. 2006. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic alpha-helical antimicrobial peptides. Biochim Biophys Acta, 1758(9): 1245-1256. |
| [40] | Savina T, Lisun V, Feduraev P, et al. 2023. Variation in phenolic compounds, antioxidant and antibacterial activities of extracts from different plant organs of meadowsweet (Filipendula ulmaria (L.) Maxim.). Molecules, 28(8): 3512. |
| [41] | Schlaeppi K, Abou-Mansour E, Buchala A, et al. 2010. Disease resistance of Arabidopsis to Phytophthora brassicae is established by the sequential action of indole glucosinolates and camalexin. Plant J, 62(5): 840-851. |
| [42] | Sekido H, Endo T, Suga R, et al. 1986. Oryzalexin D (3,7-dihydroxy- (+)-sandaracopimaradiene), a new phytoalexin isolated from blast- infected rice leaves. J Pestic Sci, 11(3): 369-372. |
| [43] | Sharma A, Kashyap D, Sak K, et al. 2018. Therapeutic charm of quercetin and its derivatives: A review of research and patents. Pharm Pat Anal, 7(1): 15-32. |
| [44] | Sianglum W, Saeloh D, Tongtawe P, et al. 2018. Early effects of rhodomyrtone on membrane integrity in methicillin-resistant Staphylococcus aureus. Microb Drug Resist, 24(7): 882-889. |
| [45] | Stotz H U, Sawada Y, Shimada Y, et al. 2011. Role of camalexin, indole glucosinolates, and side chain modification of glucosinolate- derived isothiocyanates in defense of Arabidopsis against Sclerotinia sclerotiorum. Plant J, 67(1): 81-93. |
| [46] | Vasconcelos-Cardoso M, Batista-Almeida D, Rios-Barros L V, et al. 2022. Cellular and molecular mechanisms underlying plasma membrane functionality and integrity. J Cell Sci, 135(13): jcs259806. |
| [47] | Wang J H, Lou J F, Luo C, et al. 2012. Phenolic compounds from Halimodendron halodendron (Pall.) voss and their antimicrobial and antioxidant activities. Int J Mol Sci, 13(9): 11349-11364. |
| [48] | Want E J, Masson P, Michopoulos F, et al. 2013. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat Protoc, 8(1): 17-32. |
| [49] | Wu S C, Yang Z Q, Liu F, et al. 2019. Antibacterial effect and mode of action of flavonoids from licorice against methicillin- resistant Staphylococcus aureus. Front Microbiol, 10: 2489. |
| [50] | Zeng H L, He K W, He Q, et al. 2024. Exogenous indole-3-acetic acid suppresses rice infection of Magnaporthe oryzae by affecting plant resistance and fungal growth. Phytopathology, 114(5): 1050-1056. |
| [51] | Zhang B, Dong C J, Shang Q M, et al. 2013. New insights into membrane-active action in plasma membrane of fungal hyphae by the lipopeptide antibiotic bacillomycin L. Biochim Biophys Acta, 1828(9): 2230-2237. |
| [52] | Zhang M L. 2016. Effects of the extraction of Ginkgo biloba L. leaves and quercetin on control of postharvest blue mould in kiwifruit. Shaanxi, China: Northwest A & F University. (in Chinese with English abstract) |
| [53] | Zhang Q, Liu F Y, Zeng M, et al. 2022. Antifungal activity of sodium new houttuyfonate against Aspergillus fumigatus in vitro and in vivo. Front Microbiol, 13: 856272. |
| [54] | Zhao Y T, Wang X E, Zheng B L, et al. 2022. Current situation and prospects of screening research of plant-derived fungicides. Biol Disaster Sci, 45(4): 400-404. (in Chinese with English abstract) |
| [55] | Zhong L Y, Lin Y J, Wang C, et al. 2022. Chemical profile, antimicrobial and antioxidant activity assessment of the crude extract and its main flavonoids from Tartary buckwheat sprouts. Molecules, 27(2): 374. |
| [56] | Zhu Z W, Yin J J, Chern M, et al. 2020. New insights into bsr-d1- mediated broad-spectrum resistance to rice blast. Mol Plant Pathol, 21(7): 951-960. |
| [57] | Zhu Z W, Xiong J, Shi H, et al. 2023. Magnaporthe oryzae effector MoSPAB1 directly activates rice Bsr-d1 expression to facilitate pathogenesis. Nat Commun, 14: 8399. |
| [1] | Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight [J]. Rice Science, 2025, 32(1): 118-130. |
| [2] | Durga Prasad Mullangie, Kalaimagal Thiyagarajan, Manonmani Swaminathan, Jagadeesan Ramalingam, Sritharan Natarajan, Senthilkumar Govindan. Breeding Resilience: Exploring Lodging Resistance Mechanisms in Rice [J]. Rice Science, 2024, 31(6): 659-672. |
| [3] | Li Wei, Zhang Mengchen, Yang Yaolong, Weng Lin, Hu Peisong, Wei Xinghua. Molecular Evolution of Rice Blast Resistance Gene bsr-d1 [J]. Rice Science, 2024, 31(6): 700-711. |
| [4] | Hou Xinyue, Wang Yuping, Qian Qian, Ren Deyong. Molecular Mechanism of Rice Necrotic Lesion for Optimized Yield and Disease Resistance [J]. Rice Science, 2024, 31(3): 285-299. |
| [5] | Gao Ningning, Ye Shuifeng, Zhang Yu, Zhou Liguo, Ma Xiaosong, Yu Hanxi, Li Tianfei, Han Jing, Liu Zaochang, Luo Lijun. A β-Carotene Ketolase Gene NfcrtO from Subaerial Cyanobacteria Confers Drought Tolerance in Rice [J]. Rice Science, 2024, 31(1): 62-76. |
| [6] | Li Chao, Li He, Zhang Xianduo, Yang Zhimin. A Pleiotropic Drug Resistance Family Protein Gene Is Required for Rice Growth, Seed Development and Zinc Homeostasis [J]. Rice Science, 2023, 30(2): 127-137. |
| [7] | Liu Yantong, Li Ting, Jiang Zhishu, Zeng Chuihai, He Rong, Qiu Jiao, Lin Xiaoli, Peng Limei, Song Yongping, Zhou Dahu, Cai Yicong, Zhu Changlan, Fu Junru, He Haohua, Xu Jie. Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding [J]. Rice Science, 2022, 29(6): 522-534. |
| [8] | Fabiano T. P. K. Távora, Anne Cécile Meunier, Aurore Vernet, Murielle Portefaix, Joëlle Milazzo, Henri Adreit, Didier Tharreau, Octávio L. Franco, Angela Mehta. CRISPR/Cas9-Targeted Knockout of Rice Susceptibility Genes OsDjA2 and OsERF104 Reveals Alternative Sources of Resistance to Pyricularia oryzae [J]. Rice Science, 2022, 29(6): 535-544. |
| [9] | Suhas Gorakh Karkute, Vishesh Kumar, Mohd Tasleem, Dwijesh Chandra Mishra, Krishna Kumar Chaturvedi, Anil Rai, Amitha Mithra Sevanthi, Kishor Gaikwad, Tilak Raj Sharma, Amolkumar U. Solanke. Genome-Wide Analysis of von Willebrand Factor A Gene Family in Rice for Its Role in Imparting Biotic Stress Resistance with Emphasis on Rice Blast Disease [J]. Rice Science, 2022, 29(4): 375-384. |
| [10] | Liu Kai, Li Minjuan, Zhang Bin, Yin Xuming, Xia Xinjie, Wang Manling, Cui Yanchun. Poaceae Orthologs of Rice OsSGL, DUF1645 Domain-Containing Genes, Positively Regulate Drought Tolerance, Grain Length and Weight in Rice [J]. Rice Science, 2022, 29(3): 257-267. |
| [11] | Zhou Ying, Wan Tao, Yuan Bin, Lei Fang, Chen Meijuan, Wang Qiong, Huang Ping, Kou Shuyan, Qiu Wenxiu, Liu Li. Improving Rice Blast Resistance by Mining Broad-Spectrum Resistance Genes at Pik Locus [J]. Rice Science, 2022, 29(2): 133-142. |
| [12] | R. Abdul Fiyaz, D. Shivani, K. Chaithanya, K. Mounika, M. Chiranjeevi, G. S. Laha, B. C. Viraktamath, L. V. Subba Rao, R. M. Sundaram. Genetic Improvement of Rice for Bacterial Blight Resistance: Present Status and Future Prospects [J]. Rice Science, 2022, 29(2): 118-132. |
| [13] | Muduli Lakesh, Kumar Pradhan Sukanta, Mishra Abinash, Nath Bastia Debendra, Chandra Samal Kailash, Kumar Agrawal Pawan, Dash Manasi. Understanding Brown Planthopper Resistance in Rice: Genetics, Biochemical and Molecular Breeding Approaches [J]. Rice Science, 2021, 28(6): 532-546. |
| [14] | Saichompoo Uthomphon, Narumol Possawat, Nakwilai Pawat, Thongyos Peeranut, Nanta Aekchupong, Tippunya Patompong, Ruengphayak Siriphat, Itthisoponkul Teerarat, Bueraheng Niranee, Cheabu Sulaiman, Malumpong Chanate. Breeding Novel Short Grain Rice for Tropical Region to Combine Important Agronomical Traits, Biotic Stress Resistance and Cooking Quality in Koshihikari Background [J]. Rice Science, 2021, 28(5): 479-792. |
| [15] | Balija Vishalakshi, Bangale Umakanth, Ponnuvel Senguttuvel, Makarand Barbadikar Kalyani, Prasad Madamshetty Srinivas, Rao Durbha Sanjeeva, Yadla Hari, Madhav Maganti Sheshu. Improvement of Upland Rice Variety by Pyramiding Drought Tolerance QTL with Two Major Blast Resistance Genes for Sustainable Rice Production [J]. Rice Science, 2021, 28(5): 493-500. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||