
Rice Science ›› 2021, Vol. 28 ›› Issue (6): 547-556.DOI: 10.1016/j.rsci.2021.09.003
• Research Paper • Previous Articles Next Articles
Dan Zeng1, Chunchao Wang1, Junpin Xie2, Fan Zhang1, Jialing Lu1, Xiaorong Shi2, Yingyao Shi2, Yongli Zhou1( )
)
Received:2020-07-01
Accepted:2020-10-12
Online:2021-11-28
Published:2021-11-28
Dan Zeng, Chunchao Wang, Junpin Xie, Fan Zhang, Jialing Lu, Xiaorong Shi, Yingyao Shi, Yongli Zhou. Stress-Activated Protein Kinase OsSAPK7 Regulates Salt- Stress Tolerance by Modulating Diverse Stress-Defensive Responses in Rice[J]. Rice Science, 2021, 28(6): 547-556.
Add to citation manager EndNote|Ris|BibTeX
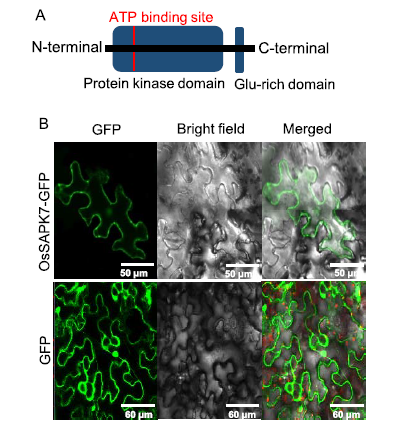
Fig. 1. Domain structure and subcellular localization of OsSAPK7.A, Schematic representation of the domain structure of OsSAPK7. The highly conserved kinase region at the N-terminal and the Glu-rich motif at the C-terminal are shown.B, Green fluorescence protein (GFP) signals arising from the OsSAPK7- GFP fusion proteins expressed in tobacco leaves were detected using a confocal microscope. Red signals represent chloroplast auto-fluorescence.The only GFP signal expressed by pCAMBIA-1300 in tobacco leaves served as a negative control.
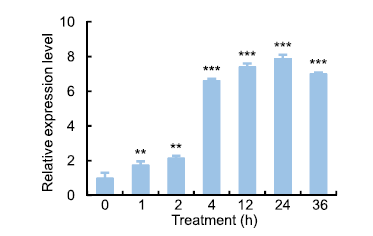
Fig. 2. Expression of OsSAPK7 in rice under salt-stress conditions.After 20-day-old rice seedlings were subjected to 100 mmol/L NaCl treatment, OsSAPK7 expression levels were assessed by qRT-PCR. Means and standard errors were obtained from three biological replicates. ** and *** indicate statistically significant differences at the 0.01 and 0.001 levels, respectively.
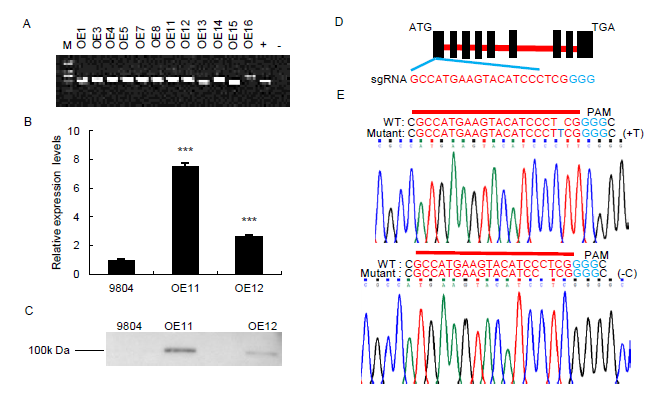
Fig. S1. Graphic representation of vector constructs and expression analysis of OsSAPK7 in transgenic plants.A, Positive OsSAPK7-overexpression transgenic plants was verified by PCR method. M, marker; +, positive; -, negative.B, Expression of OsSAPK7 in wild type 9804 and OsSAPK7-overexpression transgenic plants as determined by qRT-PCR. Actin gene was the control. Values are Mean ± SE (n = 3). ***, P < 0.001 according to the Student’s t-test.C, Protein expression of OsSAPK7 in 9804 and OsSAPK7-overexpressing transgenic plants as assessed by western blotting.D, A schematic description of the WT OsSAPK7 gene. Red letters represent the target sequence.E, Sanger sequencing results of mutations in OsSAPK7 knockouts. Above is the editing sequence of the target site, and below is the sequencing chromatogram.
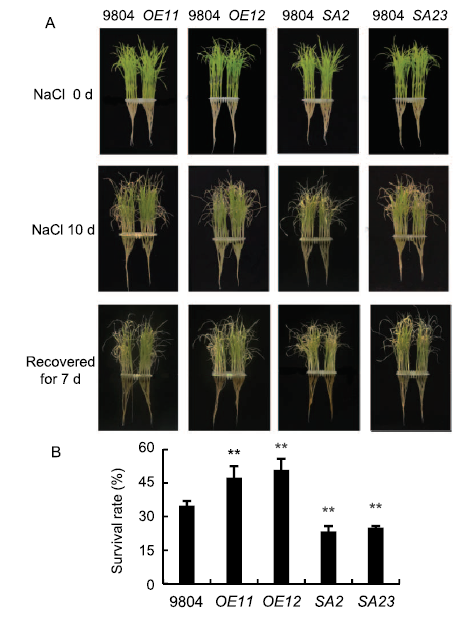
Fig. 3. Phenotypic reactions of OsSAPK7-overexpression, OsSAPK7 knockout and wild type (WT) 9804 lines under salt-stress conditions.A, Phenotypic comparison of seedings grown under salt stress at the seedling stage. OsSAPK7-overexpression lines (OE11 and OE12), OsSAPK7 knockout lines (SA2 and SA23) and 9804 plants under normal conditions for 20-day-old seedlings were transferred to Hoagland's nutrient solution supplemented with 100 mmol/L NaCl for 10 d and then recovered for 7 d.B, Survival rates of OE11, OE12, SA2, SA23 and 9804 plants after recovered for 7 d. Values are Mean ± SE (n = 3). **, P < 0.05 according to the Student's t-test.
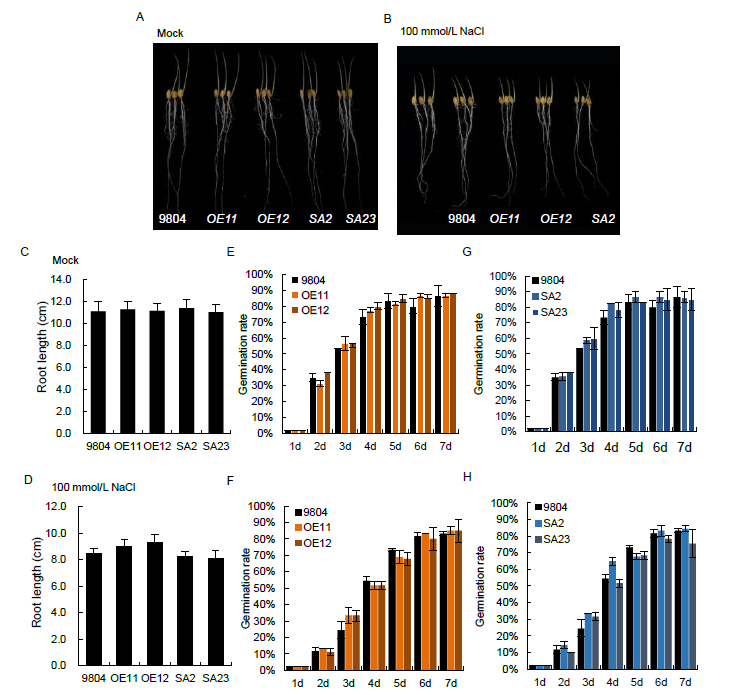
Fig. S2. Root growth and germination rates of OsSAPK7 transgenic lines and wild type 9804 under salt-stress conditions.A and B, Phenotype of OsSAPK7-overexpression (OE11 and OE12), OsSAPK7 knockout (SA2 and SA23) and wild type 9804 plants grown in distilled water (A) or in distilled water supplemented with 100 mmol/L NaCl solution (B).C and D, Root lengths of OsSAPK7-overexpression, OsSAPK7 knockout and 9804 plants grown in distilled water (C) or in distilled water supplemented with 100 mmol/L NaCl solution (D).E to H, Seed germination rates of OsSAPK7-overexpression, OsSAPK7 knockout and wild type 9804 plants grown in distilled water or in distilled water supplemented with 100 mmol/L NaCl solution.Values are Mean ± SE (n = 3). *, P < 0.05 according to the Student’s t-test.
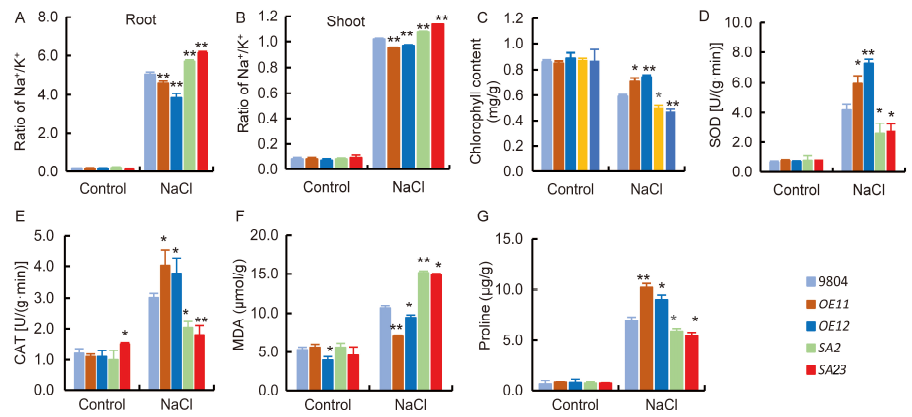
Fig. 4. Responses of 20-day-old transgenic lines and wild type 9804 at 6 d after exposure to 100 mmol/L NaCl treatment.A, Na+/K+ ratio in roots. B, Na+/K+ ratio in shoots. C, Chlorophyll content in the leaves under control and salt-stress conditions for 6 d. D, Superoxide dismutase (SOD) activity in leaves. E, Catalase (CAT) activity in leaves. F, Malomdiadehyde (MDA) content of leaves. G, Proline content of leaves.OE11 and OE12 are the OsSAPK7-overexpression lines. SA2 and SA23 are the OsSAPK7 knockout lines. Values are Mean ± SE (n = 3). * and ** indicate significant differences at the 0.05 and 0.01 levels according to the Student's t-test compared with 9804, respectively.
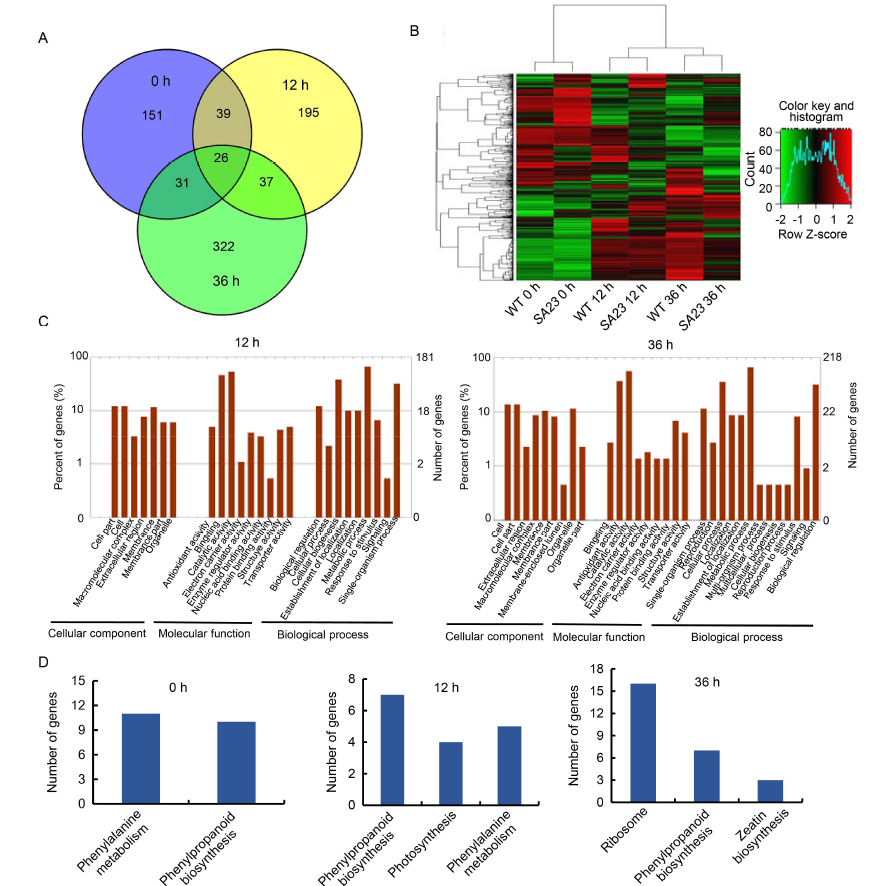
Fig. 5. Transcriptome profiling analysis of OsSAPK7 knockout line SA23 and wild type (WT, 9804) under salt stress conditions.A, Venn diagram of differentially expressed genes (DEGs) in WT vs SA23 at 0, 12 and 36 h under salt-stress conditions.B, Hierarchical clustering of DEGs between WT and SA23 at 12 and 36 h under salt stress. The color scale represents log2 of the fragment per kilo base of exon model per million mapped fragments (FPKM).C, Gene Ontology analysis of DEGs in WT vs SA23 at 12 and 36 h under salt-stress conditions.D, Kyoto Encyclopedia of Genes and Genomes pathway analysis of DEGs in WT vs SA23.
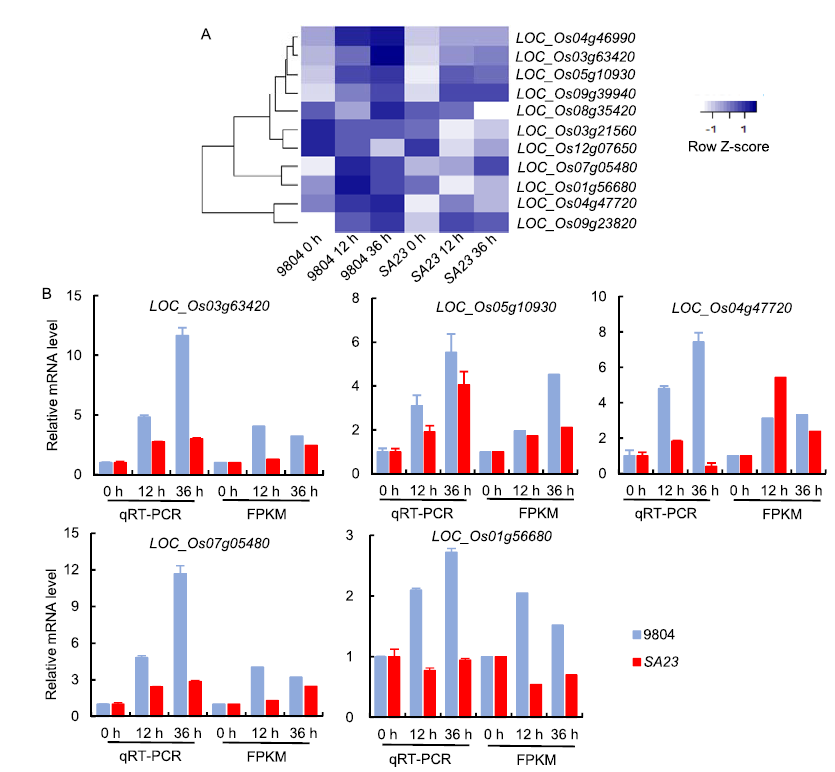
Fig. 6. Expression patterns of differentially expressed genes (DEGs) in OsSAPK7 knockout line SA23 compared with wild type 9804.A, Hierarchical clustering of DEGs associated with electron carrier activity, photosynthesis and zeatin synthesis pathway regulated by OsSAPK7 after salt-stress treatment. Color scale represents the log2 of the fragment per kilo base of exon model per million mapped fragments (FPKM).B, Expression patterns of key genes in 9804 and SA23 assessed by qRT-PCR and FPKM. Values are Mean ± SE (n = 3).
| [1] | Anderberg R J, Walker-Simmons M K. 1992. Isolation of a wheat cDNA clone for an abscisic acid-inducible transcript with homology to protein kinases. Proc Natl Acad Sci USA, 89(21): 10183-10187. |
| [2] | Ashraf M A, Ashraf M, Ali Q. 2010. Response of two genetically diverse wheat cultivars to salt stress at different growth stages: Leaf lipid peroxidation and phenolic contents. Pak J Bot, 42(1): 559-565. |
| [3] | Bhatt T, Sharma A, Puri S, Minhas A P. 2020. Salt tolerance mechanisms and approaches: Future scope of halotolerant genes and rice landraces. Rice Sci, 27(5): 368-383. |
| [4] | Boudsocq M, Barbier-Brygoo H, Lauriere C. 2004. Identification of nine sucrose nonfermenting 1-related protein kinases 2 activated by hyperosmotic and saline stresses in Arabidopsis thaliana. J Biol Chem, 279: 41758-41766. |
| [5] | Civello P M, Martinez G A, Chaves A R, Anon M C. 1995. Peroxidase from strawberry fruit (Fragaria ananassa Duch): Partial purification and determination of some properties. J Agric Food Chem, 43(10): 2596-2601. |
| [6] | Curtis M D, Grossniklaus U. 2003. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol, 133(2): 462-469. |
| [7] | Dey A, Samanta M K, Gayen S, Maiti M K. 2016. The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression. BMC Plant Biol, 16: 158. |
| [8] | Diédhiou C J, Popova O V, Dietz K J, Golldack D. 2008. The SNF1-type serine-threonine protein kinase SAPK4 regulates stress-responsive gene expression in rice. BMC Plant Biol, 8: 49. |
| [9] | Garg R, Jhanwar S, Tyagi A K, Jain M. 2010. Genome-wide survey and expression analysis suggest diverse roles of glutaredoxin gene family members during development and sesponse to various stimuli in rice. DNA Res, 17(6): 353-367. |
| [10] | Horie T, Karahara I, Katsuhara M. 2015. Salinity tolerance mechanisms in glycophytes: An overview with the central focus on rice plants. Rice, 5(1): 11. |
| [11] | Kim B. 2017. Western blot techniques. In: Espina V. Molecular Profiling: Methods and Protocols, Methods in Molecular Biology. Springer Science and Business Media LLC: 1606: 133-139. |
| [12] | Kobayashi Y, Yamamoto S, Minami H, Kagaya Y, Hattori T. 2004. Differential activation of the rice sucrose nonfermenting1-related protein kinase2 family by hyperosmotic stress and abscisic acid. Plant Cell, 16(5): 1163-1677. |
| [13] | Kulik A, Wawer I, Krzywińska E, Bucholc M, Dobrowolska G. 2011. SnRK2 protein kinases: Key regulators of plant response to abiotic stresses. OMICS: J Interg Biol, 15(12): 859-872. |
| [14] | Lee H J, Park Y J, Seo P J, Kim J H, Sim H J, Kim S G, Park C M. 2015. Systemic immunity requires SnRK28-mediated nuclear import of NPR1 in Arabidopsis. Plant Cell, 27(12): 3425-3438. |
| [15] | Li B, Huang J, Wang L, Li J, Liang Y Y, Chen J. 2020. A review on how plant hormones and environment factors are involved in rice root hair development. Chin J Rice Sci, 34(4): 287-299. (in Chinese with English abstract) |
| [16] | Lou D J, Wang H P, Yu D Q. 2018. The sucrose non-fermenting-1- related protein kinases SAPK1 and SAPK2 function collaboratively as positive regulators of salt stress tolerance in rice. BMC Plant Biol, 18: 203-219. |
| [17] | Ma X L, Zhang Q Y, Zhu Q L, Liu W, Chen Y, Qiu R, Wang B, Yang Z F, Li H Y, Lin Y R, Xie Y Y, Shen R X, Chen S F, Wang Z, Chen Y L, Guo J X, Chen L T, Zhao X C, Dong Z C, Liu Y G. 2005. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot. Mol Plant, 8(8): 1274-1284. |
| [18] | Munns R, Tester M. 2008. Mechanisms of salinity tolerance. Annu Rev Plant Biol, 59: 651-681. |
| [19] | Passricha N, Saifi S K, Kharb P, Tuteja N. 2019. Marker-free transgenic rice plant overexpressing pea LecRLK imparts salinity tolerance by inhibiting sodium accumulation. Plant Mol Biol, 99(3): 265-281. |
| [20] | Rodo A P, Brugière N, Vankova R, Malbeck J, Olson J M, Haines S C, Martin R C, Habben J E, Mok D W S, Mok M C. 2008. Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tasselseed formation. J Exp Bot, 59(10): 2673-2686. |
| [21] | Schmidt R, Mieulet D, Hubberten H M, Obata T, Hoefgen R, Fernie A R, Fisahn J, San B S, Guiderdoni E, Schippers J H M, Mueller-Roeber B. 2013. Salt-responsive ERF1 regulates reactive oxygen species-dependent signaling during the initial response to salt stress in rice. Plant Cell, 25(6): 2115-2131. |
| [22] | Tezvergil-Mutluay A, Agee K A, Hoshika T, Carrilho M, Breschi L, Tjäderhane L, Nishitani Y, Carvalho R M, Looney S, Tay F R, Pashley D H. 2010. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Plant Soil, 26(11): 1059-1067. |
| [23] | Trapnell C, Williams B A, Pertea G, Mortazavi A, Kwan G, van Baren M J, Salzberg S L, Word B J, Pachter L. 2010. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol, 28(5): 511-515. |
| [24] | Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente R M. 2017. Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep, 36(6): 1971-1984. |
| [25] | Wang Y Q, Song F H, Zhu J W, Zhang S S, Yang Y D, Chen T T, Tang B X, Dong L L, Ding N, Zhang Q, Bai Z X, Dong X N, Chen H X, Sun M Y, Zhai S, Sun Y B, Yu L, Lan L, Xiao J F, Fang X D, Lei H X, Zhang Z, Zhao W M. 2017. GSA: Genome sequence archive. Genom Proteom Bioinf, 15(1): 14-18. |
| [26] | Xu J, Duan X G, Yang J, Beeching J R, Zhang P. 2013. Reactive oxygen species scavenging by overproduction of superoxide dismutase and catalase delays postharvest physiological deterioration of cassava storage roots. Plant Physiol, 161(3): 1517-1528. |
| [27] | Xu M R, Huang L Y, Zhang F, Zhu L H, Zhou Y L, Li Z K. 2013. Genome-wide phylogenetic analysis of stress-activated protein kinase genes in rice (OsSAPKs) and expression profiling in response to Xanthomonas oryzae pv oryzicola infection. Plant Mol Biol Rep, 31(4): 877-885. |
| [28] | Ying S, Zhang D F, Li H Y, Liu Y H, Shi Y S, Song Y C, Wang T Y, Li Y. 2011. Cloning and characterization of a maize SnRK2 protein kinase gene confers enhanced salt tolerance in transgenic Arabidopsis. Plant Cell Rep, 30(9): 1683-1699. |
| [29] | Yoshida S, Forno D A, Cock J H, Gomez K A. 1976. Laboratory Manual for Physiological Studies of Rice. Manila, the Philippines: International Rice Research Institute: 61-64. |
| [30] | Zhang M, Smith J A, Harberd N P, Jiang C F. 2016. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol Biol, 91(6): 651-659. |
| [31] | Zheng Z F, Xu X P, Crosley R A, Greenwalt S A, Sun Y J, Blakeslee B, Wang L Z, Ni W T, Sopko M S, Yao C L, Yau K, Burton S, Zhuang M B, McCaskill D G, Gachotte D, Thompson M, Greene T W. 2010. The protein kinase SnRK2. 6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol, 153(1): 99-113. |
| [32] | Zhou Y B, Liu C, Tang D Y, Yan L, Wang D, Yang Y Z, Gui J S, Zhao X Y, Li L G, Tang X D, Yu F, Li J L, Liu L L, Zhu Y H, Lin J Z, Liu X M. 2018. The receptor-like cytoplasmic kinase STRK1 phosphorylates and activates CatC, thereby regulating H2O2 homeostasis and improving salt tolerance in rice. Plant Cell, 30(5): 1100-1118. |
| [33] | Zhu J K. 2003. Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol, 6(5): 441-445. |
| [1] | Prathap V, Suresh KUMAR, Nand Lal MEENA, Chirag MAHESHWARI, Monika DALAL, Aruna TYAGI. Phosphorus Starvation Tolerance in Rice Through a Combined Physiological, Biochemical and Proteome Analysis [J]. Rice Science, 2023, 30(6): 8-. |
| [2] | Serena REGGI, Elisabetta ONELLI, Alessandra MOSCATELLI, Nadia STROPPA, Matteo Dell’ANNO, Kiril PERFANOV, Luciana ROSSI. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Rice Engineered Lines [J]. Rice Science, 2023, 30(6): 6-. |
| [3] | Sundus ZAFAR, XU Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 4-. |
| [4] | Kankunlanach KHAMPUANG, Nanthana CHAIWONG, Atilla YAZICI, Baris DEMIRER, Ismail CAKMAK, Chanakan PROM-U-THAI. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 9-. |
| [5] | FAN Fengfeng, CAI Meng, LUO Xiong, LIU Manman, YUAN Huanran, CHENG Mingxing, Ayaz AHMAD, LI Nengwu, LI Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Rice Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 14-. |
| [6] | LIN Shaodan, YAO Yue, LI Jiayi, LI Xiaobin, MA Jie, WENG Haiyong, CHENG Zuxin, YE Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 10-. |
| [7] | Md. Forshed DEWAN, Md. AHIDUZZAMAN, Md. Nahidul ISLAM, Habibul Bari SHOZIB. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and South-East Asia: A Review [J]. Rice Science, 2023, 30(6): 5-. |
| [8] | Raja CHAKRABORTY, Pratap KALITA, Saikat SEN. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Chakhao Poireiton (A Pigmented Black Rice) in High-Fat High-Sugar induced Rats [J]. Rice Science, 2023, 30(6): 11-. |
| [9] | LI Qianlong, FENG Qi, WANG Heqin, KANG Yunhai, ZHANG Conghe, DU Ming, ZHANG Yunhu, WANG Hui, CHEN Jinjie, HAN Bin, FANG Yu, WANG Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 7-. |
| [10] | JI Dongling, XIAO Wenhui, SUN Zhiwei, LIU Lijun, GU Junfei, ZHANG Hao, Tom Matthew HARRISON, LIU Ke, WANG Zhiqin, WANG Weilu, YANG Jianchang. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 12-. |
| [11] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [12] | Monica Ruffini Castiglione, Stefania Bottega, Carlo Sorce, Carmelina SpanÒ. Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa [J]. Rice Science, 2023, 30(5): 449-458. |
| [13] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| [14] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [15] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||