
Rice Science ›› 2021, Vol. 28 ›› Issue (6): 579-593.DOI: 10.1016/j.rsci.2021.03.002
• Research Paper • Previous Articles Next Articles
Chunquan Zhu1,#, Wenjun Hu2,#, Xiaochuang Cao1, Lianfeng Zhu1, Yali Kong1, Qianyu Jin1, Guoxin Shen2, Weipeng Wang4, Hui Zhang3( ), Junhua Zhang1(
), Junhua Zhang1( )
)
Received:2020-10-12
Accepted:2021-03-01
Online:2021-11-28
Published:2021-11-28
About author:#These authors contributed equally to this work
Chunquan Zhu, Wenjun Hu, Xiaochuang Cao, Lianfeng Zhu, Yali Kong, Qianyu Jin, Guoxin Shen, Weipeng Wang, Hui Zhang, Junhua Zhang. Physiological and Proteomic Analyses Reveal Effects of Putrescine-Alleviated Aluminum Toxicity in Rice Roots[J]. Rice Science, 2021, 28(6): 579-593.
Add to citation manager EndNote|Ris|BibTeX
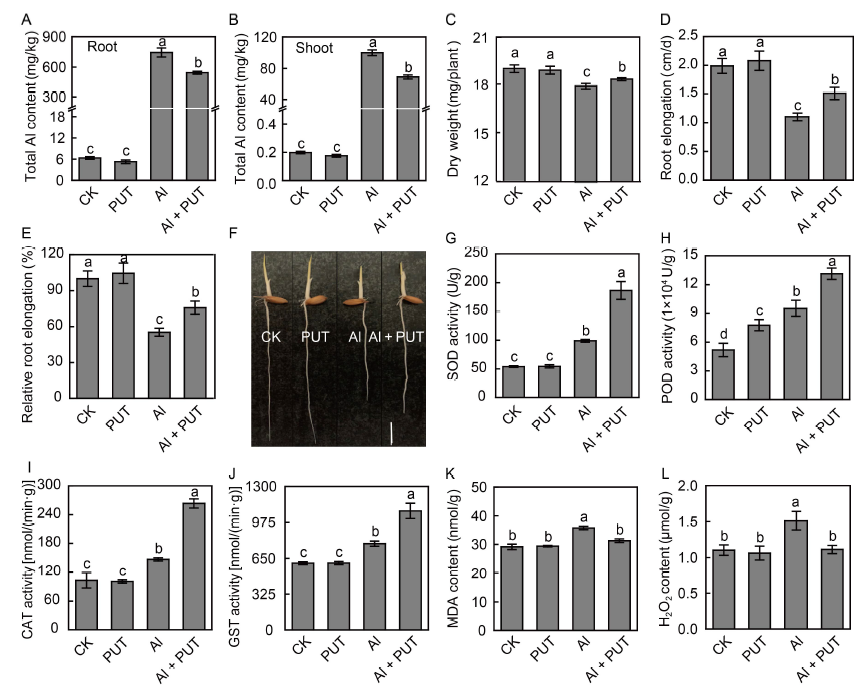
Fig. 1. Effects of putrescine (PUT) on rice response to aluminum (Al) toxicity.A, Total Al content in rice roots. B, Total Al content in rice shoots. C, Whole rice dry weight. D, Root elongation. E, Relative root elongation. F, Phenotype of rice roots under different treatments. Scale bar, 1 cm. G, Superoxide dismutase (SOD) activity in rice roots. H, Peroxidase (POD) activity in rice roots. I, Catalase (CAT) activity in rice roots. J, Glutathione S-transferase (GST) activity in rice roots. K, Malondialdehyde (MDA) content in rice roots. L, H2O2 content in rice roots.The rice seeds were placed in an incubator for 2 d at 30 ºC in darkness until the roots had grown to about 2 cm long, and then treated with Al (50 μmol/L) or PUT (0.1 mmol/L) for another 1 d. After that, the rice roots were collected for measurement. Data are Mean ± SD (n = 4). Columns with different lowercase letters are significantly different at P < 0.05.
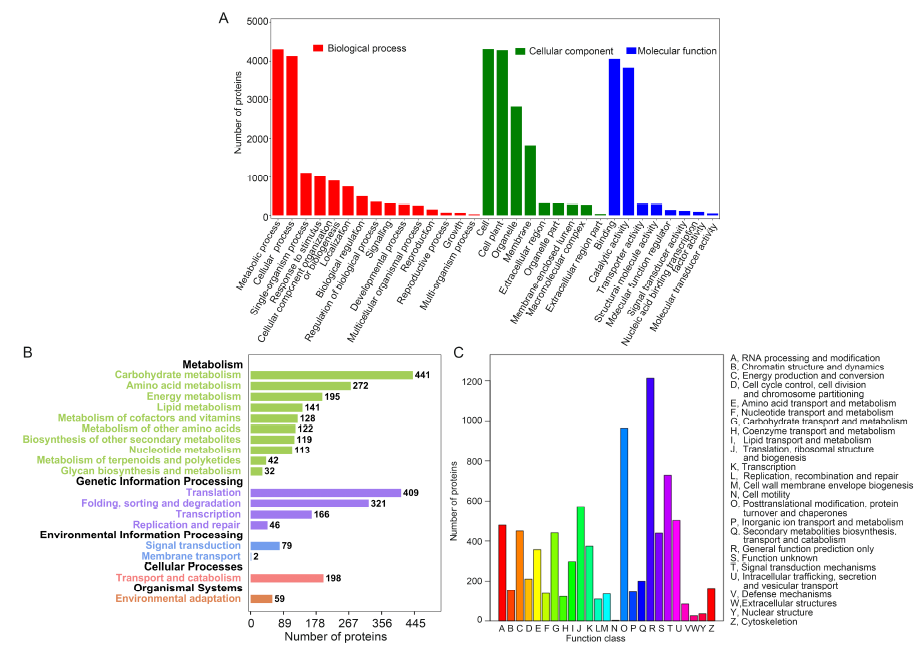
Fig. 2. Protein functional annotation.A, Gene Ontology analysis. B, Kyoto Encyclopedia of Genes and Genomes pathway annotation. C, EuKaryotic Orthologous Groups function classification of peptide sequences.The rice seeds were placed in an incubator for 2 d at 30 ºC in darkness until the roots had grown to about 2 cm long, and then treated with aluminum (50 μmol/L) or putrescine (0.1 mmol/L) for another 1 d. After that, the rice roots were collected for proteomic analyses.
| No. | ID | Protein name | Biology function | PUT/CK | P value | Al/CK | P value | Al + PUT /Al | P value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Os01t0323000-01 | Similar to Ser/Thr specific protein kinase-like protein | Protein metabolism | 1.02 | 0.64 | 0.01 | 0.76 | 85.94 | 0.03 |
| 2 | Os12t0428600-01 | Similar to E3 ubiquitin protein ligase UPL2 | Protein metabolism | 0.6 | 0.44 | 0.43 | 0.01 | 1.80 | 0.04 |
| 3 | Os02t0242900-00 | Similar to hydroquinone glucosyltransferase | Carbon metabolism | 1.39 | 0.71 | 1.54 | 0.01 | 0.80 | 0.01 |
| 4 | Os03t0243600-01 | Acyl-CoA-binding protein | Lipid metabolism | 0.99 | 0.42 | 0.62 | 0.06 | 1.75 | 0.02 |
| 5 | Os10t0361000-01 | Lipoxygenase | Lipid metabolism | 1.74 | 0.43 | 2.12 | 1.38E-04 | 0.63 | 8.74E-04 |
| 6 | Os01t0705200-01 | Late embryogenesis abundant protein repeat containing protein | Energy metabolism | 1.94 | 0.02 | 2.25 | 1.82E-04 | 0.67 | 0.02 |
| 7 | Os03t0296300-01 | Mitochondrial import inner membrane translocase subunit tim22 | Energy metabolism | 1.76 | 0.04 | 1.32 | 0.31 | 1.12 | 0.92 |
| 8 | Os05t0187100-02 | Similar to Hexokinase | Energy metabolism | 0.59 | 0.08 | 0.6 | 2.32E-04 | 1.34 | 0.04 |
| 9 | Os05t0593100-01 | Vacuolar ATP synthase subunit C | Energy metabolism | 0.76 | 1.02E-04 | 0.64 | 7.47E-06 | 1.06 | 0.23 |
| 10 | Os02t0208100-01 | Plastidic ATP/ADP-transporter | Energy metabolism | 0.65 | 0.03 | 0.62 | 6.92E-05 | 0.94 | 0.28 |
| 11 | Os05t0302700-01 | ATP/ADP carrier protein | Energy metabolism | 0.65 | 0.17 | 0.54 | 0.26 | 0.96 | 0.41 |
| 12 | Os03t0281600-01 | Ca2+-ATPase | Energy metabolism | 0.62 | 0.04 | 0.43 | 9.65E-04 | 1.15 | 0.17 |
| 13 | Os12t0638700-01 | Plasma membrane H+ ATPase | Energy metabolism | 0.69 | 1.81E-03 | 0.64 | 4.47E-07 | 0.94 | 0.80 |
| 14 | Os03t0725300-01 | Metallophosphoesterase domain containing protein | Mineral metabolism | 0.97 | 0.14 | 1.27 | 0.14 | 0.50 | 0.02 |
| 15 | Os06t0699200-01 | Metallophosphoesterase domain containing protein | Mineral metabolism | 1.77 | 0.04 | 1.22 | 0.14 | 1.07 | 0.51 |
| 16 | Os11t0151700-01 | Purple acid phosphatase | Mineral metabolism | 1.07 | 0.98 | 1.71 | 6.47E-05 | 0.78 | 9.75E-05 |
| 17 | Os03t0150600-01 | Pi transporter | Mineral metabolism | 0.7 | 0.12 | 0.6 | 3.60E-05 | 0.93 | 0.14 |
| 18 | Os03t0712400-02 | Similar to atypical receptor-like kinase MARK | Cell proliferation | 0.97 | 0.17 | 0.71 | 0.12 | 1.57 | 4.00E-03 |
| 19 | Os06t0594600-01 | BAHD acyltransferase | Cell wall synthesis | 0.78 | 0.55 | 0.58 | 0.05 | 1.79 | 9.32E-03 |
| 20 | Os06t0711800-01 | Pectinesterase inhibitor domain containing protein | Cell wall synthesis | 0.06 | 5.77E-05 | 2.01 | 0.59 | 1.79 | 2.00E-03 |
| 21 | Os06t0175500-01 | Epsin-like | Cell wall synthesis | 0.58 | 0.02 | 0.52 | 3.00E-03 | 1.81 | 0.05 |
| 22 | Os01t0687400-01 | Similar to Chitinase | Cell wall synthesis | 0.97 | 0.62 | 0.99 | 0.43 | 0.60 | 8.97E-04 |
| 23 | Os06t0696400-01 | Endotransglucosylase/hydrolase XTH5 | Cell wall synthesis | 1.60 | 0.03 | 1.53 | 4.15E-03 | 1.10 | 0.07 |
| 24 | Os05t0176100-05 | Similar to cellulose synthase BoCesA1 | Cell wall synthesis | 0.60 | 0.02 | 0.78 | 0.01 | 1.13 | 0.12 |
| 25 | Os11t0107000-01 | Xylan acetyltransferase | Cell wall synthesis | 0.52 | 0.01 | 0.53 | 0.11 | 1.39 | 0.96 |
| 26 | Os02t0529600-01 | Xyloglucan 6-xylosyltransferase | Cell wall synthesis | 0.43 | 3.18E-03 | 0.68 | 3.83E-03 | 1.08 | 0.08 |
| 27 | Os01t0597800-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.02 | 0.23 | 1.74 | 2.47E-15 | 0.79 | 2.65E-08 |
| 28 | Os05t0215300-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.31 | 0.78 | 1.55 | 5.85E-04 | 0.93 | 0.07 |
| 29 | Os01t0638000-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.14 | 1.74E-03 | 1.55 | 2.47E-17 | 0.84 | 1.81E-06 |
| 30 | Os11t0673600-00 | Similar to NB-ARC domain containing protein | Stress resistance | 0.53 | 0.97 | 0.16 | 0.02 | 4.81 | 0.05 |
| 31 | Os04t0111200-01 | Similar to ATP sulfurylase | Stress resistance | 2.02 | 4.00E-03 | 2.01 | 1.07E-03 | 1.01 | 0.11 |
| 32 | Os06t0216700-01 | Cupredoxin domain containing protein | Stress resistance | 1.95 | 7.00E-03 | 0.81 | 0.06 | 0.86 | 0.10 |
| 33 | Os03t0710800-01 | 14-3-3-like protein S94 | Stress resistance | 0.65 | 4.15E-04 | 0.46 | 2.87E-15 | 1.39 | 1.29E-05 |
| 34 | Os01t0120600-01 | Oxidoreductase NAD-binding domain containing protein | Antioxidation | 2.24 | 0.04 | 1.41 | 0.21 | 0.94 | 0.60 |
| 35 | Os08t0231400-01 | Germin-like protein 8-12 | Antioxidation | 1.58 | 5.13E-09 | 0.76 | 1.11E-04 | 0.77 | 0.16 |
| 36 | Os10t0528300-01 | Tau class GST protein 4 | Antioxidation | 0.95 | 2.09E-03 | 2.57 | 1.43E-28 | 0.76 | 1.32E-07 |
| 37 | Os09t0367700-01 | Similar to GST6 protein | Antioxidation | 0.81 | 0.48 | 2.44 | 1.67E-06 | 0.73 | 1.49E-13 |
| 38 | Os07t0638400-01 | Similar to 1-Cys peroxiredoxin | Antioxidation | 1.49 | 1.62E-17 | 2.14 | 2.68E-38 | 0.71 | 4.89E-27 |
| 39 | Os07t0104500-01 | Haem peroxidase | Antioxidation | 1.27 | 2.82E-05 | 1.62 | 4.62E-27 | 0.99 | 0.66 |
| 40 | Os07t0639000-01 | Class III peroxidase 46 | Antioxidation | 1.00 | 0.69 | 1.52 | 5.39E-14 | 0.88 | 0.49 |
| 41 | Os01t0667600-01 | Similar to GTP-binding protein | Signaling | 0.53 | 0.01 | 0.34 | 3.74E-06 | 1.96 | 6.37E-05 |
| 42 | Os01t0179700-01 | Similar to GTP-binding protein YPTM2 | Signaling | 0.59 | 2.97E-03 | 0.51 | 3.69E-08 | 1.77 | 1.00E-03 |
| 43 | Os02t0653800-01 | Similar to GTP-binding protein | Signaling | 1.06 | 0.91 | 0.76 | 0.24 | 1.55 | 0.05 |
| 44 | Os07t0239400-01 | Similar to Ethylene-responsive small GTP-binding protein | Signaling | 0.76 | 0.03 | 0.56 | 7.05E-12 | 1.62 | 0.02 |
| 45 | Os07t0564700-01 | Similar to ATMIN7 (Arabidopsis thaliana hopm interactor 7) | Signaling | 0.65 | 1.15E-03 | 0.39 | 0.01 | 2.10 | 0.03 |
| 46 | Os05t0149400-01 | ACC oxidase | Signaling | 1.32 | 0.07 | 1.95 | 2.72E-05 | 0.65 | 2.79E-04 |
| 47 | Os12t0555500-01 | Probenazole-inducible protein PBZ1 | Signaling | 1.12 | 0.02 | 1.54 | 3.61E-08 | 0.78 | 3.86E-06 |
| 48 | Os02t0669100-01 | Dehydrin family protein | Signaling | 1.33 | 2.36E-04 | 1.87 | 5.78E-05 | 0.78 | 8.81E-05 |
| 49 | Os09t0123300-01 | Similar to calmodulin-binding receptor-like kinase | Signaling | 0.95 | 0.08 | 0.68 | 7.26E-03 | 1.28 | 4.47E-03 |
| 50 | Os12t0597000-01 | Calcineurin B-like protein 2 | Signaling | 0.69 | 0.09 | 0.63 | 0.02 | 0.91 | 0.31 |
| 51 | Os03t0788500-01 | Calcium-dependent protein kinase | Signaling | 0.77 | 0.23 | 0.58 | 0.03 | 1.52 | 0.56 |
| 52 | Os11t0171500-01 | Calcium-dependent protein kinase | Signaling | 0.59 | 0.15 | 0.58 | 1.00E-03 | 1.02 | 0.30 |
| 53 | Os05t0587300-01 | Heat shock protein, Hsp40 | Reestablish protein | 0.86 | 0.58 | 1.43 | 0.29 | 1.73 | 0.03 |
| 54 | Os02t0761100-01 | Cyclophilin-40 | Reestablish protein | 1.60 | 1.00E-03 | 1.95 | 0.1 | 0.97 | 0.17 |
| 55 | Os08t0487800-01 | Similar to Heat shock protein precursor | Reestablish protein | 0.73 | 1.50E-03 | 0.63 | 1.88E-04 | 1.13 | 0.03 |
| 56 | Os04t0107900-04 | Similar to Heat shock protein 82 | Reestablish protein | 0.69 | 0.27 | 0.46 | 4.00E-03 | 1.43 | 0.17 |
| 57 | Os11t0620100-03 | Oligouridylate binding protein | Repair mRNAs | 1.12 | 0.82 | 1.51 | 0.01 | 0.70 | 0.04 |
| 58 | Os07t0563300-01 | B3 domain transcriptional repressor | Transcription | 1.24 | 0.71 | 0.86 | 0.94 | 1.59 | 0.03 |
| 59 | Os05t0367100-01 | Ribosome biogenesis protein Nop16 domain containing protein | Translation | 0.87 | 71.00 | 0.79 | 0.63 | 1.81 | 0.04 |
Table S1. Differentially expressed proteins in rice roots under different treatments.
| No. | ID | Protein name | Biology function | PUT/CK | P value | Al/CK | P value | Al + PUT /Al | P value |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Os01t0323000-01 | Similar to Ser/Thr specific protein kinase-like protein | Protein metabolism | 1.02 | 0.64 | 0.01 | 0.76 | 85.94 | 0.03 |
| 2 | Os12t0428600-01 | Similar to E3 ubiquitin protein ligase UPL2 | Protein metabolism | 0.6 | 0.44 | 0.43 | 0.01 | 1.80 | 0.04 |
| 3 | Os02t0242900-00 | Similar to hydroquinone glucosyltransferase | Carbon metabolism | 1.39 | 0.71 | 1.54 | 0.01 | 0.80 | 0.01 |
| 4 | Os03t0243600-01 | Acyl-CoA-binding protein | Lipid metabolism | 0.99 | 0.42 | 0.62 | 0.06 | 1.75 | 0.02 |
| 5 | Os10t0361000-01 | Lipoxygenase | Lipid metabolism | 1.74 | 0.43 | 2.12 | 1.38E-04 | 0.63 | 8.74E-04 |
| 6 | Os01t0705200-01 | Late embryogenesis abundant protein repeat containing protein | Energy metabolism | 1.94 | 0.02 | 2.25 | 1.82E-04 | 0.67 | 0.02 |
| 7 | Os03t0296300-01 | Mitochondrial import inner membrane translocase subunit tim22 | Energy metabolism | 1.76 | 0.04 | 1.32 | 0.31 | 1.12 | 0.92 |
| 8 | Os05t0187100-02 | Similar to Hexokinase | Energy metabolism | 0.59 | 0.08 | 0.6 | 2.32E-04 | 1.34 | 0.04 |
| 9 | Os05t0593100-01 | Vacuolar ATP synthase subunit C | Energy metabolism | 0.76 | 1.02E-04 | 0.64 | 7.47E-06 | 1.06 | 0.23 |
| 10 | Os02t0208100-01 | Plastidic ATP/ADP-transporter | Energy metabolism | 0.65 | 0.03 | 0.62 | 6.92E-05 | 0.94 | 0.28 |
| 11 | Os05t0302700-01 | ATP/ADP carrier protein | Energy metabolism | 0.65 | 0.17 | 0.54 | 0.26 | 0.96 | 0.41 |
| 12 | Os03t0281600-01 | Ca2+-ATPase | Energy metabolism | 0.62 | 0.04 | 0.43 | 9.65E-04 | 1.15 | 0.17 |
| 13 | Os12t0638700-01 | Plasma membrane H+ ATPase | Energy metabolism | 0.69 | 1.81E-03 | 0.64 | 4.47E-07 | 0.94 | 0.80 |
| 14 | Os03t0725300-01 | Metallophosphoesterase domain containing protein | Mineral metabolism | 0.97 | 0.14 | 1.27 | 0.14 | 0.50 | 0.02 |
| 15 | Os06t0699200-01 | Metallophosphoesterase domain containing protein | Mineral metabolism | 1.77 | 0.04 | 1.22 | 0.14 | 1.07 | 0.51 |
| 16 | Os11t0151700-01 | Purple acid phosphatase | Mineral metabolism | 1.07 | 0.98 | 1.71 | 6.47E-05 | 0.78 | 9.75E-05 |
| 17 | Os03t0150600-01 | Pi transporter | Mineral metabolism | 0.7 | 0.12 | 0.6 | 3.60E-05 | 0.93 | 0.14 |
| 18 | Os03t0712400-02 | Similar to atypical receptor-like kinase MARK | Cell proliferation | 0.97 | 0.17 | 0.71 | 0.12 | 1.57 | 4.00E-03 |
| 19 | Os06t0594600-01 | BAHD acyltransferase | Cell wall synthesis | 0.78 | 0.55 | 0.58 | 0.05 | 1.79 | 9.32E-03 |
| 20 | Os06t0711800-01 | Pectinesterase inhibitor domain containing protein | Cell wall synthesis | 0.06 | 5.77E-05 | 2.01 | 0.59 | 1.79 | 2.00E-03 |
| 21 | Os06t0175500-01 | Epsin-like | Cell wall synthesis | 0.58 | 0.02 | 0.52 | 3.00E-03 | 1.81 | 0.05 |
| 22 | Os01t0687400-01 | Similar to Chitinase | Cell wall synthesis | 0.97 | 0.62 | 0.99 | 0.43 | 0.60 | 8.97E-04 |
| 23 | Os06t0696400-01 | Endotransglucosylase/hydrolase XTH5 | Cell wall synthesis | 1.60 | 0.03 | 1.53 | 4.15E-03 | 1.10 | 0.07 |
| 24 | Os05t0176100-05 | Similar to cellulose synthase BoCesA1 | Cell wall synthesis | 0.60 | 0.02 | 0.78 | 0.01 | 1.13 | 0.12 |
| 25 | Os11t0107000-01 | Xylan acetyltransferase | Cell wall synthesis | 0.52 | 0.01 | 0.53 | 0.11 | 1.39 | 0.96 |
| 26 | Os02t0529600-01 | Xyloglucan 6-xylosyltransferase | Cell wall synthesis | 0.43 | 3.18E-03 | 0.68 | 3.83E-03 | 1.08 | 0.08 |
| 27 | Os01t0597800-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.02 | 0.23 | 1.74 | 2.47E-15 | 0.79 | 2.65E-08 |
| 28 | Os05t0215300-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.31 | 0.78 | 1.55 | 5.85E-04 | 0.93 | 0.07 |
| 29 | Os01t0638000-01 | UDP-glucuronosyl/UDP-glucosyltransferase family protein | Cell wall synthesis | 1.14 | 1.74E-03 | 1.55 | 2.47E-17 | 0.84 | 1.81E-06 |
| 30 | Os11t0673600-00 | Similar to NB-ARC domain containing protein | Stress resistance | 0.53 | 0.97 | 0.16 | 0.02 | 4.81 | 0.05 |
| 31 | Os04t0111200-01 | Similar to ATP sulfurylase | Stress resistance | 2.02 | 4.00E-03 | 2.01 | 1.07E-03 | 1.01 | 0.11 |
| 32 | Os06t0216700-01 | Cupredoxin domain containing protein | Stress resistance | 1.95 | 7.00E-03 | 0.81 | 0.06 | 0.86 | 0.10 |
| 33 | Os03t0710800-01 | 14-3-3-like protein S94 | Stress resistance | 0.65 | 4.15E-04 | 0.46 | 2.87E-15 | 1.39 | 1.29E-05 |
| 34 | Os01t0120600-01 | Oxidoreductase NAD-binding domain containing protein | Antioxidation | 2.24 | 0.04 | 1.41 | 0.21 | 0.94 | 0.60 |
| 35 | Os08t0231400-01 | Germin-like protein 8-12 | Antioxidation | 1.58 | 5.13E-09 | 0.76 | 1.11E-04 | 0.77 | 0.16 |
| 36 | Os10t0528300-01 | Tau class GST protein 4 | Antioxidation | 0.95 | 2.09E-03 | 2.57 | 1.43E-28 | 0.76 | 1.32E-07 |
| 37 | Os09t0367700-01 | Similar to GST6 protein | Antioxidation | 0.81 | 0.48 | 2.44 | 1.67E-06 | 0.73 | 1.49E-13 |
| 38 | Os07t0638400-01 | Similar to 1-Cys peroxiredoxin | Antioxidation | 1.49 | 1.62E-17 | 2.14 | 2.68E-38 | 0.71 | 4.89E-27 |
| 39 | Os07t0104500-01 | Haem peroxidase | Antioxidation | 1.27 | 2.82E-05 | 1.62 | 4.62E-27 | 0.99 | 0.66 |
| 40 | Os07t0639000-01 | Class III peroxidase 46 | Antioxidation | 1.00 | 0.69 | 1.52 | 5.39E-14 | 0.88 | 0.49 |
| 41 | Os01t0667600-01 | Similar to GTP-binding protein | Signaling | 0.53 | 0.01 | 0.34 | 3.74E-06 | 1.96 | 6.37E-05 |
| 42 | Os01t0179700-01 | Similar to GTP-binding protein YPTM2 | Signaling | 0.59 | 2.97E-03 | 0.51 | 3.69E-08 | 1.77 | 1.00E-03 |
| 43 | Os02t0653800-01 | Similar to GTP-binding protein | Signaling | 1.06 | 0.91 | 0.76 | 0.24 | 1.55 | 0.05 |
| 44 | Os07t0239400-01 | Similar to Ethylene-responsive small GTP-binding protein | Signaling | 0.76 | 0.03 | 0.56 | 7.05E-12 | 1.62 | 0.02 |
| 45 | Os07t0564700-01 | Similar to ATMIN7 (Arabidopsis thaliana hopm interactor 7) | Signaling | 0.65 | 1.15E-03 | 0.39 | 0.01 | 2.10 | 0.03 |
| 46 | Os05t0149400-01 | ACC oxidase | Signaling | 1.32 | 0.07 | 1.95 | 2.72E-05 | 0.65 | 2.79E-04 |
| 47 | Os12t0555500-01 | Probenazole-inducible protein PBZ1 | Signaling | 1.12 | 0.02 | 1.54 | 3.61E-08 | 0.78 | 3.86E-06 |
| 48 | Os02t0669100-01 | Dehydrin family protein | Signaling | 1.33 | 2.36E-04 | 1.87 | 5.78E-05 | 0.78 | 8.81E-05 |
| 49 | Os09t0123300-01 | Similar to calmodulin-binding receptor-like kinase | Signaling | 0.95 | 0.08 | 0.68 | 7.26E-03 | 1.28 | 4.47E-03 |
| 50 | Os12t0597000-01 | Calcineurin B-like protein 2 | Signaling | 0.69 | 0.09 | 0.63 | 0.02 | 0.91 | 0.31 |
| 51 | Os03t0788500-01 | Calcium-dependent protein kinase | Signaling | 0.77 | 0.23 | 0.58 | 0.03 | 1.52 | 0.56 |
| 52 | Os11t0171500-01 | Calcium-dependent protein kinase | Signaling | 0.59 | 0.15 | 0.58 | 1.00E-03 | 1.02 | 0.30 |
| 53 | Os05t0587300-01 | Heat shock protein, Hsp40 | Reestablish protein | 0.86 | 0.58 | 1.43 | 0.29 | 1.73 | 0.03 |
| 54 | Os02t0761100-01 | Cyclophilin-40 | Reestablish protein | 1.60 | 1.00E-03 | 1.95 | 0.1 | 0.97 | 0.17 |
| 55 | Os08t0487800-01 | Similar to Heat shock protein precursor | Reestablish protein | 0.73 | 1.50E-03 | 0.63 | 1.88E-04 | 1.13 | 0.03 |
| 56 | Os04t0107900-04 | Similar to Heat shock protein 82 | Reestablish protein | 0.69 | 0.27 | 0.46 | 4.00E-03 | 1.43 | 0.17 |
| 57 | Os11t0620100-03 | Oligouridylate binding protein | Repair mRNAs | 1.12 | 0.82 | 1.51 | 0.01 | 0.70 | 0.04 |
| 58 | Os07t0563300-01 | B3 domain transcriptional repressor | Transcription | 1.24 | 0.71 | 0.86 | 0.94 | 1.59 | 0.03 |
| 59 | Os05t0367100-01 | Ribosome biogenesis protein Nop16 domain containing protein | Translation | 0.87 | 71.00 | 0.79 | 0.63 | 1.81 | 0.04 |
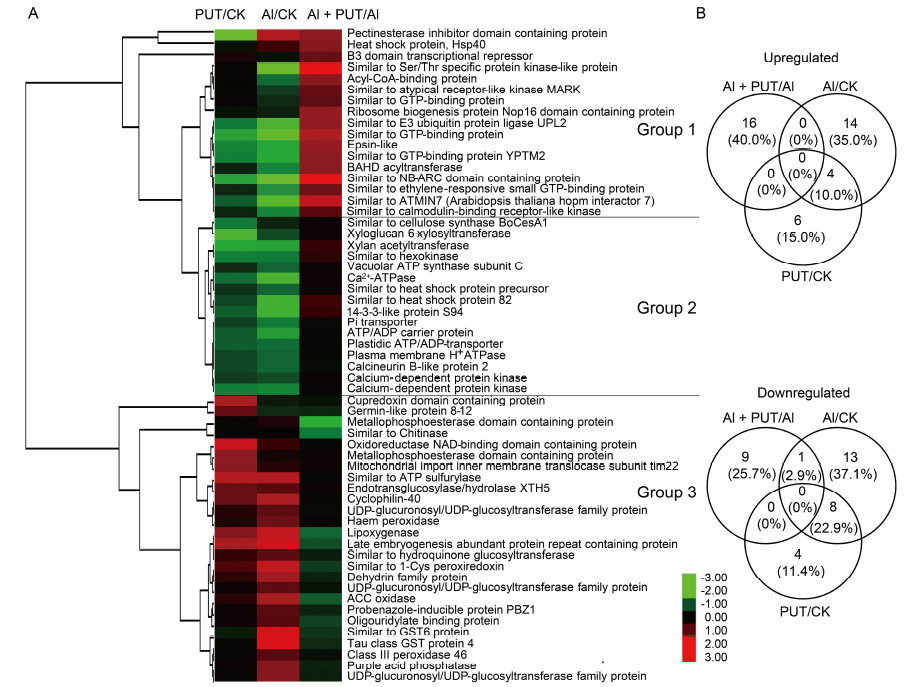
Fig. 3. Hierarchical clustering analysis (A) and Venn diagram (B) for selected differentially expressed proteins of rice roots in Al/CK, Al + PUT/Al and PUT/CK sets.Upregulated and downregulated proteins are indicated in red and green, respectively, in A. The intensity of the colors indicates the abundance of the protein, as shown in the bar. The rice seeds were placed in an incubator for 2 d at 30 ºC in darkness until the roots had grown to about 2 cm long, and then treated with Al (50 μmol/L) or PUT (0.1 mmol/L) for another 1 d. After that, the rice roots were collected for proteomic analyses. Three independent biological replicates were tested. Al, Aluminum; PUT, Putrescine.
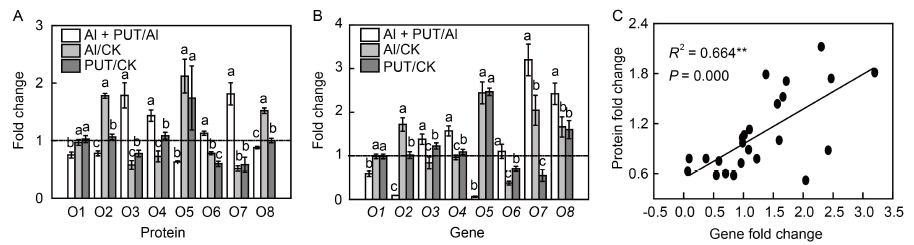
Fig. 4. Comparison between protein and mRNA expression changes of eight randomly selected proteins.A, Comparison of expression changes at protein level. The data of protein fold change are from the results of data-independent acquisition proteomics. B, Comparison of expression changes at mRNA level. Total RNA was extracted from rice root after 24 h of treatment with the aluminum (Al) concentration in the solution of 50 μmol/L and putrescine (PUT) concentration of 0.1 mmol/L. The relative expression of genes was defined CK as ‘1’. The OsHistone gene was used as a reference gene. C, Correlation between proteins and genes.O1, Os11t0702100-01, class III chitinase homologue; O2, Os11t0151700-01, purple acid phosphatase; O3, Os06t0594600-01, BAHD acyltransferase; O4, Os07t0194500-01, 2OG-Fe(II) oxygenase domain containing protein; O5, Os10t0361000-01, lipoxygenase; O6, Os05t0176100-05, cellulose synthase BoCesA1; O7, Os06t0175500-01, epsin-like; O8, Os07t0639000-01, class III peroxidase 46. The results of selected genes's relevant expression are calculated in the form of Al + PUT/Al, Al/CK and PUT/CK. Data are Mean ± SD (n = 4). Columns with different lowercase letters are significantly different at P < 0.05.
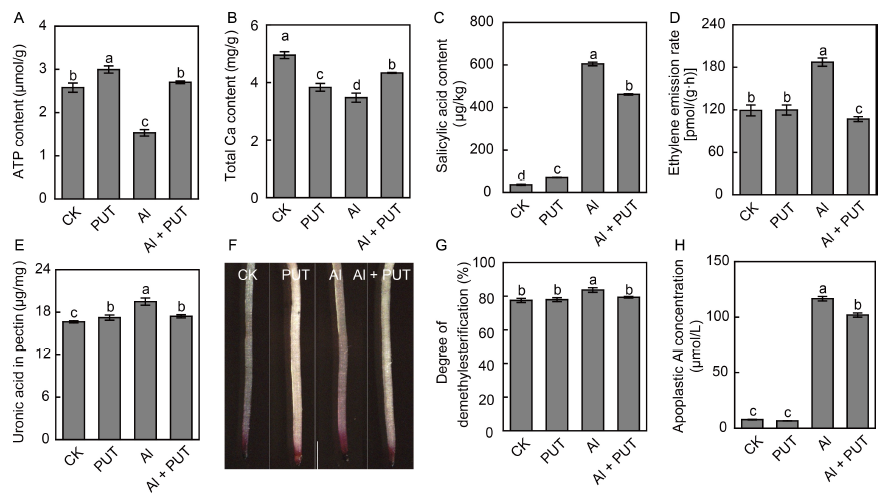
Fig. 5. Effects of putrescine (PUT) on rice response to aluminum (Al) toxicity.A, ATP content in rice roots. B, Ca content in rice roots. C, Salicylic acid content in rice roots. D, Ethylene emission rate in rice roots. E, Pectin content in rice roots. F, Pectin content on root surface. The pectin content on root surface was indicated by red color. Scale bar is 1 mm. G, Degree of pectin demethylesterification in rice roots. H, Apoplast Al concentration in rice roots.The rice seeds were placed in an incubator for 2 d at 30 ºC in darkness until the roots had grown to about 2 cm long, and then treated with Al (50 μmol/L) or PUT (0.1 mmol/L) for another 1 d. After that, the rice roots were collected for measurement. Data are Mean ± SD (n = 4). Columns with different lowercase letters are significantly different at P < 0.05.

Fig. 6. Effects of putrescine (PUT) on relative abundance of lipoxygenase and ATP synthase proteins.The rice seeds were placed in an incubator for 2 d at 30 ºC in darkness until the roots had grown to about 2 cm long, and then treated with aluminum (Al, 50 μmol/L) or PUT (0.1 mmol/L) for another 1 d. After that, the rice roots were collected for western blot. The values are compared with CK and indicate the band intensities. There were four replicates for each experiment, and only one experiment was displayed.
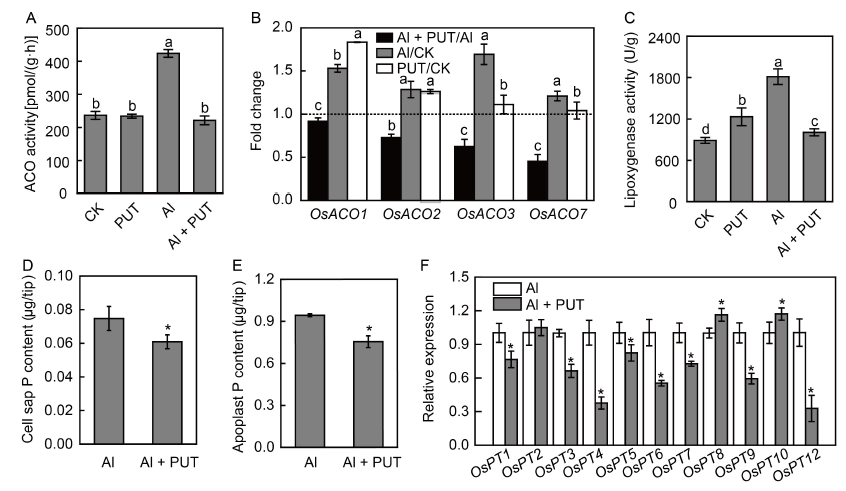
Fig. 7. Effects of putrescine (PUT) on ethylene emission and phosphorus (P) uptake in rice roots.A, 1-aminocyclopropane-1-carboxylic acid oxidase (ACO) activity. B, Comparison of OsACOs expression changes in the form of Al + PUT/Al, Al/CK and PUT/CK. Total RNA was extracted from rice roots after 24 h of treatment. C, Lipoxygenase activity. D, Cell sap P content. E, Apoplast P content. F, Relative expression of OsPTs under aluminum (Al) conditions. Total RNA was extracted from rice roots after 24 h of treatment. Relative expression of OsPTs under Al treatment was defined as ‘1’, and the OsHistone gene was used as a reference gene.Al concentration in the solution is 50 μmol/L and PUT concentration is 0.1 mmol/L. Data are Mean ± SD (n = 4). Columns with different lowercase letters (A-C) or stars (D-F) are significantly different at P < 0.05.
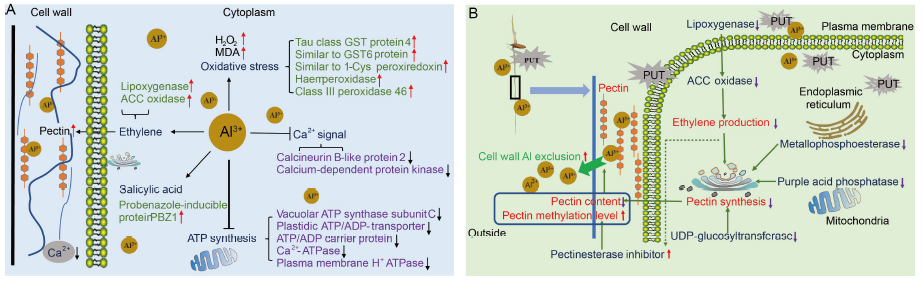
Fig. 8. Schematic models for rice response to aluminum (Al) toxicity and putrescine (PUT) alleviate Al toxicity.A, Response mechanism of rice roots to Al toxicity. The presence of Al inhibited the synthesis of ATP, disturbed the Ca2+ signal, aggravated the oxidative stress, induced ethylene emissions and increased the pectin content in rice. The rice plants increased peroxidase activity and the accumulation of salicylic acid to resist Al toxicity.B, A hypothetical model displaying the pathway of PUT-alleviated Al toxicity in rice through an increased pectin methylation degree and decreased pectin content to remove cell wall Al content. Application of PUT under the Al toxicity conditions decreased ethylene emissions by decreasing the lipoxygenase, 1-aminocyclopropane-1-carboxylic acid (ACC) oxidase and aminotransferase protein contents. It inhibited pectin synthesis and increased the pectin methylation level in rice root cell walls. In addition, application of PUT decreased metallophosphoesterase, purple acid phosphatase and UDP-glucosyltransferase to inhibit pectin synthesis in rice roots. All the above processes improved the cell wall Al exclusion and ultimately alleviated Al toxicity in rice.
| Primer name | Sequence (5′-3′) |
|---|---|
| Os07t0194500-01-F | CTCTTTCAGGCATTCCATTGATG |
| Os07t0194500-01-R | CAACACCTTGTCAGCTTTCAAGC |
| Os11t0702100-01-F | CCTCTACACCACCGTCATCATCT |
| Os11t0702100-01-R | TGGCAGTGCTTGATGTCGG |
| Os11t0151700-01-F | CGTGGTACAACACGAACGAG |
| Os11t0151700-01-R | CTTGATGAACTTGAGAGCAAGG |
| Os06t0594600-01-F | GACCCGTTCCAGATGACGTT |
| Os06t0594600-01-R | GATGAGGTCGCAGTTCACCA |
| Os10t0361000-01-F | GCCTGCTTCCTCTTCTTCC |
| Os10t0361000-01-R | CGTAGCTCACGTACCACCC |
| Os05t0176100-05-F | GGCATACCACCCTCAACG |
| Os05t0176100-05-R | CAGACCCAAGAGCCCAAC |
| Os06t0175500-01-F | CGAATACAAGAAAGAGGCAGAG |
| Os06t0175500-01-R | GAGCGTGAGCAAGTCCAGTC |
| Os07t0639000-01-F | GCCCTCCAACAAGTGCTAC |
| Os07t0639000-01-R | CAAACATAATCCGTTCGTCA |
| Os12t0597000-01-F | GCAGGGACATCACCACTA |
| Os12t0597000-01-R | CAATCCAACAACCCAATA |
| OsACO1-F | GCCTCGCTCGCTCTGTTCTG |
| OsACO1-R | AGGGACTTGCTATGACACGG |
| OsACO2-F | CATCGCCACCGCTTGATA |
| OsACO2-R | GCCCGTTACACACACTTGAG |
| OsACO3-F | GGCGAGACGTATCCCAAGTT |
| OsACO3-R | AACGCGAGCTGAGTAGCTGA |
| OsACO7-F | GACTACTACCAGGGCACCAA |
| OsACO7-R | CGATTGATTCAAACCAAACA |
| OsPT1-F | AGCGTTCGGGTTCCTGTA |
| OsPT1-R | CGTTCTTGATGCCGATCC |
| OsPT2-F | GACGAGACCGCCCAAGAAG |
| OsPT2-R | TTTTCAGTCACTCACGTCGAGAC |
| OsPT3-F | GTGCTCATGGTGGTGTGCT |
| OsPT3-R | GAGCCAGAACCGGAAGAAG |
| OsPT4-F | GGAGAAGGCTGACGAGGTC |
| OsPT4-R | CCCATGGCGTCTCAAAAA |
| OsPT5-F | GGCGAGAACGAAATGGAG |
| OsPT5-R | GACGGTCTGCCTGTAGGAGT |
| OsPT6-F | TATAACTGATCGATCGAGACCAGAG |
| OsPT6-R | TGGATAGCCAGGCCAGTTATATATC |
| OsPT7-F | GCTTCCTCCTCACCTTCCTT |
| OsPT7-R | TTCTCCCGTGACATCTCCTC |
| OsPT8-F | AGAAGGCAAAAGAAATGTGTGTTAAAT |
| OsPT8-R | AAAATGTATTCGTGCCAAATTGCT |
| OsPT9-F | CATAGGCTTGTCATCCTTTGG |
| OsPT9-R | CACTGTAAATAAATCCGCGTTTC |
| OsPT10-F | GAGCTCGCACCTCAGCAT |
| OsPT10-R | GAGTTCACTCACACGGAGACC |
| OsPT12-F | AAATCGAGGTGGAGGAGGAG |
| OsPT12-R | CGAGAAGAGGCCGTAGTCC |
| OsHistone-F | GGTCAACTTGTTGATTCCCCTCT |
| OsHistone-R | AACCGCAAAATCCAAAGAACG |
Table S2. List of primers used.
| Primer name | Sequence (5′-3′) |
|---|---|
| Os07t0194500-01-F | CTCTTTCAGGCATTCCATTGATG |
| Os07t0194500-01-R | CAACACCTTGTCAGCTTTCAAGC |
| Os11t0702100-01-F | CCTCTACACCACCGTCATCATCT |
| Os11t0702100-01-R | TGGCAGTGCTTGATGTCGG |
| Os11t0151700-01-F | CGTGGTACAACACGAACGAG |
| Os11t0151700-01-R | CTTGATGAACTTGAGAGCAAGG |
| Os06t0594600-01-F | GACCCGTTCCAGATGACGTT |
| Os06t0594600-01-R | GATGAGGTCGCAGTTCACCA |
| Os10t0361000-01-F | GCCTGCTTCCTCTTCTTCC |
| Os10t0361000-01-R | CGTAGCTCACGTACCACCC |
| Os05t0176100-05-F | GGCATACCACCCTCAACG |
| Os05t0176100-05-R | CAGACCCAAGAGCCCAAC |
| Os06t0175500-01-F | CGAATACAAGAAAGAGGCAGAG |
| Os06t0175500-01-R | GAGCGTGAGCAAGTCCAGTC |
| Os07t0639000-01-F | GCCCTCCAACAAGTGCTAC |
| Os07t0639000-01-R | CAAACATAATCCGTTCGTCA |
| Os12t0597000-01-F | GCAGGGACATCACCACTA |
| Os12t0597000-01-R | CAATCCAACAACCCAATA |
| OsACO1-F | GCCTCGCTCGCTCTGTTCTG |
| OsACO1-R | AGGGACTTGCTATGACACGG |
| OsACO2-F | CATCGCCACCGCTTGATA |
| OsACO2-R | GCCCGTTACACACACTTGAG |
| OsACO3-F | GGCGAGACGTATCCCAAGTT |
| OsACO3-R | AACGCGAGCTGAGTAGCTGA |
| OsACO7-F | GACTACTACCAGGGCACCAA |
| OsACO7-R | CGATTGATTCAAACCAAACA |
| OsPT1-F | AGCGTTCGGGTTCCTGTA |
| OsPT1-R | CGTTCTTGATGCCGATCC |
| OsPT2-F | GACGAGACCGCCCAAGAAG |
| OsPT2-R | TTTTCAGTCACTCACGTCGAGAC |
| OsPT3-F | GTGCTCATGGTGGTGTGCT |
| OsPT3-R | GAGCCAGAACCGGAAGAAG |
| OsPT4-F | GGAGAAGGCTGACGAGGTC |
| OsPT4-R | CCCATGGCGTCTCAAAAA |
| OsPT5-F | GGCGAGAACGAAATGGAG |
| OsPT5-R | GACGGTCTGCCTGTAGGAGT |
| OsPT6-F | TATAACTGATCGATCGAGACCAGAG |
| OsPT6-R | TGGATAGCCAGGCCAGTTATATATC |
| OsPT7-F | GCTTCCTCCTCACCTTCCTT |
| OsPT7-R | TTCTCCCGTGACATCTCCTC |
| OsPT8-F | AGAAGGCAAAAGAAATGTGTGTTAAAT |
| OsPT8-R | AAAATGTATTCGTGCCAAATTGCT |
| OsPT9-F | CATAGGCTTGTCATCCTTTGG |
| OsPT9-R | CACTGTAAATAAATCCGCGTTTC |
| OsPT10-F | GAGCTCGCACCTCAGCAT |
| OsPT10-R | GAGTTCACTCACACGGAGACC |
| OsPT12-F | AAATCGAGGTGGAGGAGGAG |
| OsPT12-R | CGAGAAGAGGCCGTAGTCC |
| OsHistone-F | GGTCAACTTGTTGATTCCCCTCT |
| OsHistone-R | AACCGCAAAATCCAAAGAACG |
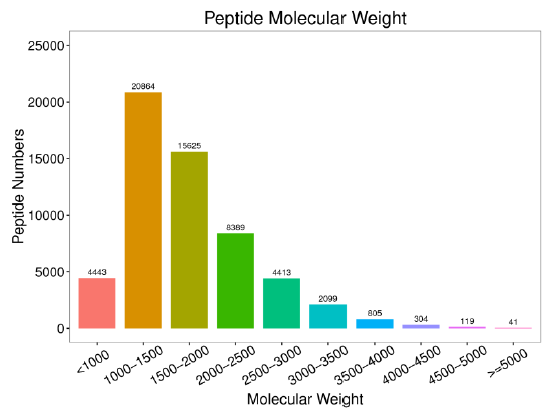
Fig. S1. Numbers of different peptide molecular weights from the database.When constructing the protein database, we obtained 78 436 precursors, 57 102 peptides, 6 928 protein groups, and 8 226 proteins. Most of the peptide molecular weights ranged from 1 000-1 500 and 1 500-2 000.
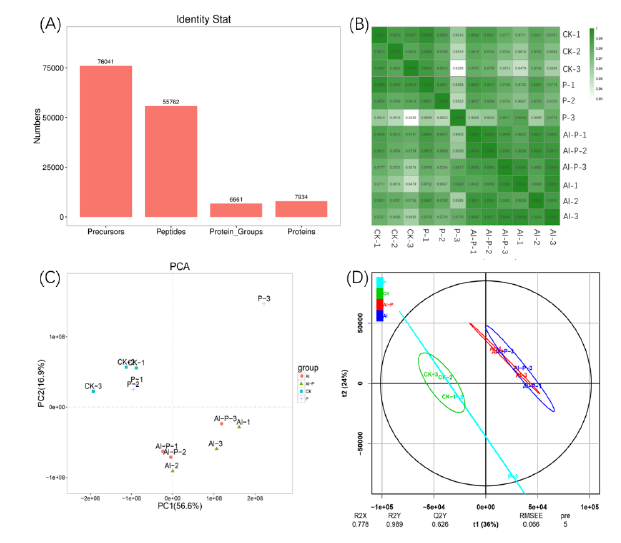
Fig. S3. Values of identity statistics (A), Pearson’s correlation coefficient (B), principal component analysis (PCA) (C) and partial least squares discriminant analysis (PLS-DA) (D) of samples from four treatments.CK, Without Al treatment and putrescine application; P, 0.1 mmol/L putrescine; Al, 50 μmol/ Al; Al-P, 0.1 mmol/ putrescine together with 50 μmol/ Al.From the samples, we identified 76 041 precursors, 55 762 peptides, 6 661 protein groups and 7 934 proteins (Fig. S3-A).The results of the Pearson correlation coefficient analysis showed that the correlation between any two samples was higher than 0.90, indicating that the replicates were reliable (Fig. S3-B). In the principal component analysis (PCA) plot, the three control samples clustered tightly and were distanced from the Al and Al + PUT treatments, indicating the different protein responses under the treatments (Fig. S3-C). Using the partial least squares-discriminant analysis (PLS-DA) modeling, a decent separation between the control and Al and Al + PUT was observed. However, the distance between the control and PUT groups and Al and Al + PUT groups in the model was small, implying that exposure to 0.1 mmol/L putrescine under no Al or 50 μmol/L Al conditions caused significant, but not extensive, changes to the protein profiles (Fig. S3-D). It should be noted that the spread of replicates in the putrescine groups indicated a high degree of biological variation (Fig. S3-C and -D). The combined use of PCA, PLS-DA and Pearson’s correlation coefficient analysis has helped to ensure a more reliable result.
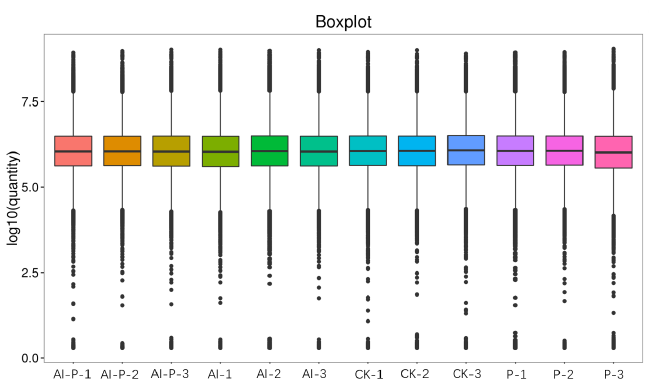
Fig. S5. Box diagram of all samples after normalization.The local normalization method in the Pulsar software was used to normalize the overall sample peak strength to ensure the sample loading consistency. The results showed that the distribution of the normalized quantitative value of the peptide segments and the signal intensity of most samples achieved basically the same response intensity.
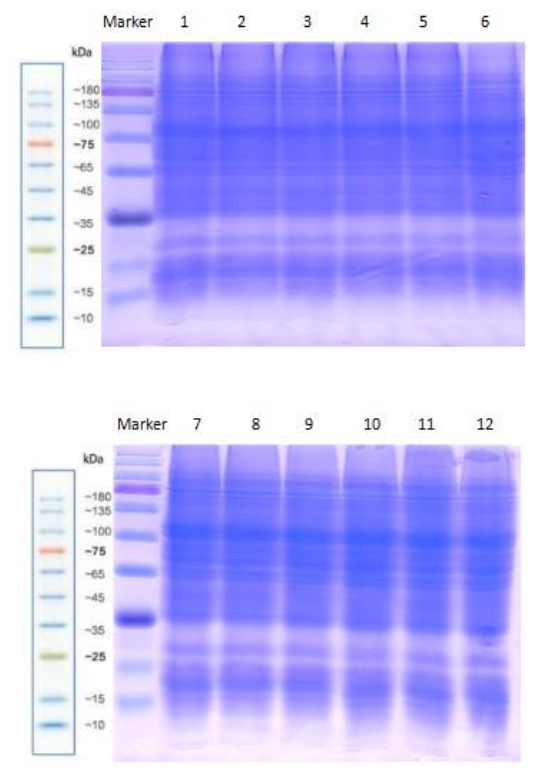
Fig. S7. Protein quality examined by SDS-PAGE.Lanes 1?3, CK; Lanes 4?6: 0.1 mmol/L putrescine treatment; Lanes 7?9: 50 μmol/L Al treatment; Lanes 10?12: 0.1 mmol/L putrescine together with 50 μmol/L Al treatment.
| [1] | Ballance S, Kristiansen K A, Skogaker N T, Tvedt K E, Christensen B E. 2012. The localisation of pectin in Sphagnum moss leaves and its role in preservation. Carbohydr Polym, 87: 1326-1332. |
| [2] | Beauchamp C, Fridovich I. 1971. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem, 44: 276-287. |
| [3] | Boscolo P R S, Menossi M, Jorge R A. 2003. Aluminum-induced oxidative stress in maize. Phytochemistry, 62: 181-189. |
| [4] | Bradford M M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 72: 248-254. |
| [5] | Chen J, Wang W H, Wu F H, You C Y, Liu T W, Dong X J, He J X, Zheng H L. 2013. Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil, 362: 301-318. |
| [6] | Chen J, Duan R X, Hu W J, Zhang N N, Lin X Y, Zhang J H, Zheng H L. 2019. Unravelling calcium-alleviated aluminium toxicity in Arabidopsis thaliana: Insights into regulatory mechanisms using proteomics. J Proteomics, 199: 15-30. |
| [7] | Chen Q F, Pan Z Z, Zhao M, Wang Q, Qiao C, Miao L Y, Ding X S. 2017. High cholesterol in lipid rafts reduces the sensitivity to EGFR-TKI therapy in non-small cell lung cancer. J Cell Physiol, 233: 6722-6732. |
| [8] | Chen W H, Xu C M, Zhao B, Wang X D, Wang Y C. 2008. Improved Al tolerance of saffron (Crocus sativus L.) by exogenous polyamines. Acta Physiol Plant, 30: 121-127. |
| [9] | Dhindsa R S, Plumbdhindsa P, Thorpe T A. 1981. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J Exp Bot, 32: 93-101. |
| [10] | Elizabeth M C, Yu C T, Janet B. 2005. Handling calcium signaling: Arabidopsis CaMs and CMLs. Trends Plant Sci, 10: 383-389. |
| [11] | Etinba-Gen A. 2020. Putrescine modifies the pollen tube growth of tea (Camellia sinensis) by affecting actin organization and cell wall structure. Protoplasma, 257: 89-101. |
| [12] | Fageria N K. 2007. Yield of rice. J Plant Nutr, 30: 843-879. |
| [13] | Farinati S, DalCorso G, Bona E, Corbella M, Lampis S, Cecconi D, Furini A. 2009. Proteomic analysis of Arabidopsis halleri shoots in response to the heavy metals cadmium and zinc and rhizosphere microorganisms. Proteomics, 9: 4837-4850. |
| [14] | Foy C D. 1988. Plant adaptation to acid aluminum-toxic soils. Commun Soil Sci Plant Anal, 19: 959-987 |
| [15] | Frova C. 2003. The plant glutathione transferase gene family: Genomic structure, functions, expression and evolution. Physiol Plant, 119(4): 469-479. |
| [16] | Gachomo E W, Jimenez-Lopez J C, Baptiste L J, Kotchoni S O. 2014. GIGANTUS1 (GTS1), a member of transducin/WD40 protein superfamily, controls seed germination, growth and biomass accumulation through ribosome-biogenesis protein interactions in Arabidopsis thaliana. BMC Plant Biol, 14: 37. |
| [17] | Gaudet P, Livstone M S, Lewis S E, Thomas P D. 2011. Phylogenetic- based propagation of functional annotations within the Gene Ontology consortium. Briefings Bioinf, 12: 449-462. |
| [18] | Horst W J, Wang Y, Eticha D. 2010. The role of the root apoplast in aluminium-induced inhibition of root elongation and in aluminium resistance of plants: A review. Ann Bot, 106: 185. |
| [19] | Hossain M A, Ashrafuzzaman M, Hossain A K, Ismail M R, Koyama H. 2014. Role of accumulated calcium in alleviating aluminum injury in wheat plants. Sci World J, 2014: 457187. |
| [20] | Hu W J, Chen J, Liu TW, Wu Q, Wang W H, Liu X, Shen Z J, Simon M, Wu F H, Pei Z M. 2014. Proteome and calcium- related gene expression in Pinus massoniana needles in response to acid rain under different calcium levels. Plant Soil, 380: 285-303. |
| [21] | Hu W J, Wu Q, Liu X, Shen Z J, Chen J, Liu T W, Chen J, Zhu C Q, Wu F H, Chen L. 2015. Comparative proteomic analysis reveals the effects of exogenous calcium against acid rain stress in Liquidambar formosana Hance leaves. J Proteome Res, 15: 216-228. |
| [22] | Illes P, Schlicht M, Pavlovkin J, Lichtscheidl I, Baluska F, Ovecka M. 2006. Aluminium toxicity in plants: Internalization of aluminium into cells of the transition zone in Arabidopsis root apices related to changes in plasma membrane potential, endosomal behaviour, and nitric oxide production. J Exp Bot, 57: 4201-4213. |
| [23] | Ingram G C, Waites R. 2006. Keeping it together: Co-ordinating plant growth. Curr Opin Plant Biol, 9: 12-20. |
| [24] | Jang J C, León P, Zhou L, Sheen J. 1997. Hexokinase as a sugar sensor in higher plants. Plant Cell, 9: 5-19. |
| [25] | Joo J H, Shiyu W, Chen J G, Jones A M, Fedoroff N V. 2005. Different signaling and cell death roles of heterotrimeric G protein α and β subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell, 17: 957-970. |
| [26] | Köpnick C, Grübe M, Stock J, Senula A, Mock H P, Nagel M. 2018. Changes of soluble sugars and ATP content during DMSO droplet freezing and PVS3 droplet vitrification of potato shoot tips. Cryobiology, 85: 79-86. |
| [27] | Laemmli U K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227: 680-685. |
| [28] | Liu K, Luan S. 2001. Internal aluminum block of plant inward K+ channels. Plant Cell, 13: 1453-1465. |
| [29] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods, 25: 402-408. |
| [30] | Ma X Y, Liu M, Li Z P. 2015. Changes in microbial properties and community composition in acid soils receiving wastewater from concentrated animal farming operations. Appl Soil Ecol, 90: 11-17. |
| [31] | Maejima E, Watanabe T, Osaki M, Wagatsuma T. 2014. Phosphorus deficiency enhances aluminum tolerance of rice (Oryza sativa) by changing the physicochemical characteristics of root plasma membranes and cell walls. J Plant Physiol, 171: 9-15. |
| [32] | Mandal C, Ghosh N, Maiti S, Das K, Gupta S, Dey N, Adak M K. 2013. Antioxidative responses of Salvinia (Salvinia natans Linn.) to aluminium stress and it's modulation by polyamine. Physiol Mol Biol Plants, 19: 91-103. |
| [33] | Manevich Y, Sweitzer T, Pak J H, Feinstein S I, Muzykantov V, Fisher A B. 2002. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci USA, 99: 11599-11604. |
| [34] | Matange N, Podobnik M, Visweswariah S S. 2015. Metallo- phosphoesterases: Structural fidelity with functional promiscuity. Biochem J, 467: 201-216. |
| [35] | Matsumoto H. 2000. Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol, 200: 1-46. |
| [36] | Mayinger P, Meyer D I. 1993. An ATP transporter is required for protein translocation into the yeast endoplasmic reticulum. EMBO J, 12(2): 659-666. |
| [37] | Mitchell R A, Dupree P, Shewry P R. 2007. A novel bioinformatics approach identifies candidate genes for the synthesis and feruloylation of arabinoxylan. Plant Physiol, 144: 43-53. |
| [38] | Paumard P. 2002. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J, 21(3): 221-230. |
| [39] | Rasmussen J T, Færgeman N J, Kristiansen K, Knudsen J. 1994. Acyl-CoA-binding protein (ACBP) can mediate intermembrane acyl-CoA transport and donate acyl-CoA for β-oxidation and glycerolipid synthesis. Biochem J, 299: 165-170. |
| [40] | Rengel Z. 2004. Aluminium cycling in the soil-plant-animal-human continuum. Biometals, 17: 669-689. |
| [41] | Rengel Z, Reid R J. 1997. Uptake of Al across the plasma membrane of plant cells. Plant Soil, 192: 31-35. |
| [42] | Riaz M, Yan L, Wu X, Hussain S, Aziz O, Wang Y, Imran M, Jiang C. 2018. Boron alleviates the aluminum toxicity in trifoliate orange by regulating antioxidant defense system and reducing root cell injury. J Environ Manage, 208: 149-158. |
| [43] | Ridley B L, O'Neill M A, Mohnen D. 2001. Pectins: Structure, biosynthesis, and oligogalacturonide-related signaling. Phytochemistry, 57: 929-967. |
| [44] | Rose J K C, Janet B, Fry S C, Kazuhiko N. 2003. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol, 43: 1421-1435. |
| [45] | Ryan P R, Tyerman S D, Sasaki T, Furuichi T, Yamamoto Y, Zhang W H, Delhaize E. 2011. The identification of aluminium- resistance genes provides opportunities for enhancing crop production on acid soils. J Exp Bot, 62: 9-20. |
| [46] | Schmitt M, Watanabe T, Jansen S. 2016. The effects of aluminium on plant growth in a temperate and deciduous aluminium accumulating species. Aob Plants, 8: plw065. |
| [47] | Shen R, Ma J F, Kyo M, Iwashita T. 2002. Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moenc. Planta, 215(3): 394-398. |
| [48] | Shen Z J, Chen J, Kabir G, Hu W J, Gao G F, Luo M R, Li Z, Martin S, Zhu X Y, Zheng H L. 2018. Proteomic analysis on mangrove plant Avicennia marina leaves reveals nitric oxide enhances the salt tolerance by up-regulating photosynthetic and energy metabolic protein expression. Tree Physiol, 38(11): 1605-1622. |
| [49] | Shu K, Yang W. 2017. E3 ubiquitin ligases: Ubiquitous actors in plant development and abiotic stress responses. Plant Cell Physiol, 58: 1461-1476. |
| [50] | Siedow J N. 1991. Plant lipoxygenase: Structure and function. Ann Rev Plant Biol, 42: 145-188. |
| [51] | Sorenson R, Bailey-Serres J. 2014. Selective mRNA sequestration by OLIGOURIDYLATE-BINDING PROTEIN 1 contributes to translational control during hypoxia in Arabidopsis. Proc Natl Acad Sci USA, 111: 2373-2378. |
| [52] | Sun P, Tian Q Y, Zhao M G, Dai X Y, Huang J H, Li L H, Zhang W H. 2007. Aluminum-induced ethylene production is associated with inhibition of root elongation in Lotus japonicus L. Plant Cell Physiol, 48: 1229-1235. |
| [53] | Sun P, Tian QY, Chen J, Zhang W H. 2010. Aluminium-induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin. J Exp Bot, 61: 347-356. |
| [54] | Suzuki M, McCarty D R. 2008. Functional symmetry of the B3 network controlling seed development. Curr Opin Plant Biol, 11: 548-553. |
| [55] | Takayoshi I, Shigemi S, Ichiro M, Yuko O. 2007. Probenazole- induced accumulation of salicylic acid confers resistance to Magnaporthe grisea in adult rice plants. Plant Cell Physiol, 48: 915-924. |
| [56] | Ullah H, Chen J G, Young J C, Im K H, Sussman M R, Jones A M. 2001. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science, 292: 2066-2069. |
| [57] | Vaddepalli P, Fulton L, Batoux M, Yadav R K, Schneitz K. 2011. Structure-function analysis of STRUBBELIG, an Arabidopsis atypical receptor-like kinase involved in tissue morphogenesis. PLoS One, 6: e19730. |
| [58] | van Ooijen G, Mayr G, Kasiem M M, Albrecht M, Cornelissen B J, Takken F L. 2008. Structure-function analysis of the NB-ARC domain of plant disease resistance proteins. J Exp Bot, 59: 1383-1397. |
| [59] | Vuttipongchaikij S, Brocklehurst D, Steele-King C, Ashford D A, Gomez L D, Mcqueen-Mason S J. 2012. Arabidopsis GT34 family contains five xyloglucan α-1,6-xylosyltransferases. New Phytol, 195: 585-595. |
| [60] | Wang H H, Liang W H, Huang J J. 2013. Putrescine mediates aluminum tolerance in red kidney bean by modulating aluminum- induced oxidative stress. Crop Sci, 53: 2120-2128. |
| [61] | Wang H H, Hou J J, Li Y, Zhang Y Y, Huang J J, Liang W H. 2017. Nitric oxide-mediated cytosolic glucose-6-phosphate dehydrogenase is involved in aluminum toxicity of soybean under high aluminum concentration. Plant Soil, 416: 39-52. |
| [62] | Wang W X, Vinocur B, Shoseyov O, Altman A. 2004. Role of plant heat-shock proteins and molecular chaperones in the abiotic stress response. Trends Plant Sci, 9: 244-252. |
| [63] | Wehenkel A, Bellinzoni M, Graña M, Duran R, Villarino A, Fernandez P, Andre-Leroux G, England P, Takiff H, Cerveñansky C. 2008. Mycobacterial Ser/Thr protein kinases and phosphatases: Physiological roles and therapeutic potential. Biochim Biophys Acta, 1784: 193-202. |
| [64] | Welinder K G. 1992. Superfamily of plant, fungal and bacterial peroxisases. Curr Opin Struct Biol, 2: 388-393. |
| [65] | Wu Y S, Yang Z L, How J Y, Xu H N, Chen L M, Li K Z. 2017. Overexpression of a peroxidase gene (AtPrx64) of Arabidopsis thaliana in tobacco improves plant's tolerance to aluminum stress. Plant Mol Biol, 95: 1-12. |
| [66] | Xia J X, Yamaji N, Kasai T, Ma J F. 2010. Plasma membrane- localized transporter for aluminum in rice. Proc Natl Acad Sci USA, 107: 18381-18385. |
| [67] | Xia J X, Yamaji N, Ma J F. 2013. A plasma membrane-localized small peptide is involved in rice aluminum tolerance. Plant J, 76: 345-355. |
| [68] | Yang J L, Li Y Y, Zhang Y J, Zhang S S, Wu Y R, Wu P, Zheng S J. 2008. Cell wall polysaccharides are specifically involved in the exclusion of aluminum from the rice root apex. Plant Physiol, 146: 602-611. |
| [69] | Yang J L, Zhu X F, Peng Y X, Zheng C, Li G X, Liu Y, Shi Y Z, Zheng S J. 2011. Cell wall hemicellulose contributes significantly to aluminum adsorption and root growth in Arabidopsis. Plant Physiol, 155: 1885-1892. |
| [70] | Yang Q S, Wu J H, Li C Y, Wei Y R, Sheng O, Hu C H, Kuang R B, Huang Y H, Peng X X, McCardle J A, Chen W, Yang Y, Rose J K C, Zhang S, Yi G J. 2012. Quantitative proteomic analysis reveals that antioxidation mechanisms contribute to cold tolerance in plantain (Musa paradisiaca L.; ABB group) seedlings. Mol Cell Proteomics, 11: 1853-1869. |
| [71] | Yang Y J, Cheng L M, Liu Z H. 2007. Rapid effect of cadmium on lignin biosynthesis in soybean roots. Plant Sci, 172: 632-639. |
| [72] | Yokoyama M, Inomata S, Seto S, Yanagi M. 1990. Effects of sugars on the glucosylation of exogenous hydroquinone by Catharanthus roseus cells in suspension culture. Plant Cell Physiol, 31: 551-555. |
| [73] | Yu Y, Jin C W, Sun C L, Wang J H, Ye Y Q, Lu L L, Lin X Y. 2015. Elevation of arginine decarboxylase-dependent putrescine production enhances aluminum tolerance by decreasing aluminum retention in root cell walls of wheat. J Hazard Mater, 299: 280-288. |
| [74] | Yu Y, Jin C W, Sun C L, Wang J H, Ye Y, Q Zhou W W, Lu L L, Lin X Y. 2016. Inhibition of ethylene production by putrescine alleviates aluminium-induced root inhibition in wheat plants. Sci Rep, 6: 18888. |
| [75] | Zhang P, Ding Z R, Zhong Z Z, Tong H H. 2019. Transcriptomic analysis for indica and japonica rice varieties under aluminum toxicity. Int J Mol Sci, 20(4): 997. |
| [76] | Zhao J J, Wang Y, Zhao D, Zhang L Z, Chen P J, Xu X. 2020. Integration of metabolomics and proteomics to reveal the metabolic characteristics of high-intensity interval training. Analyst, 145: 6500-6510. |
| [77] | Zhong R Q, Cui D T, Dasher R L, Ye Z H. 2018. Biochemical characterization of rice xylan O-acetyltransferases. Planta, 247(6): 1-10. |
| [78] | Zhu C Q, Zhang J H, Sun L M, Zhu L F, Abliz B, Hu W J, Zhong C, Bai Z G, Sajid H, Cao X C, Jin Q Y. 2018a. Hydrogen sulfide alleviates aluminum toxicity via decreasing apoplast and symplast Al contents in rice. Front Plant Sci, 9: 294. |
| [79] | Zhu C Q, Zhang J H, Zhu L F, Abliz B, Zhong C, Bai Z G, Hu W J, Sajid H, James A B, Cao X C. 2018b. NH4+ facilitates iron reutilization in the cell walls of rice (Oryza sativa) roots under iron-deficiency conditions. Environ Exp Bot, 15: 21-31. |
| [80] | Zhu C Q, Cao X C, Bai Z G, Zhu L F, Hu W J, Hu A Y, Abliz B, Zhong C, Liang Q D, Huang J, Zhang J H, Jin Q Y. 2019a. Putrescine alleviates aluminum toxicity in rice(Oryza sativa) by reducing cell wall Al contents in an ethylene-dependent manner. Physiol Plant, 167(4): 471-487. |
| [81] | Zhu C Q, Cao X C, Zhu L F, Hu W J, Hu A Y, Abliz B, Bai Z G, Huang J, Liang Q D, Sajid H, Li Y F, Wang L P, Jin Q Y, Zhang J H. 2019b. Boron reduces cell wall aluminum content in rice (Oryza sativa) roots by decreasing H2O2 accumulation. Plant Physiol Biochem, 138: 80-90. |
| [82] | Zhu C Q, Hu W J, Cao X C, Zhu L F, Bai Z G, Huang J, Liang Q D, Jin Q Y, Zhang J H. 2020. Role of salicylic acid in alleviating the inhibition of root elongation by suppressing ethylene emission in rice under Al toxicity conditions. Plant Growth Regul, 90: 475-487. |
| [83] | Zhu F Y, Chan W L, Chen M X, Kong R P W, Cai C X, Wang Q M, Zhang J H, Lo C. 2016. SWATH-MS quantitative proteomic investigation reveals a role of jasmonic acid during lead response in Arabidopsis. J Proteome Res, 15(10): 3528-3539. |
| [1] | Prathap V, Suresh KUMAR, Nand Lal MEENA, Chirag MAHESHWARI, Monika DALAL, Aruna TYAGI. Phosphorus Starvation Tolerance in Rice Through a Combined Physiological, Biochemical and Proteome Analysis [J]. Rice Science, 2023, 30(6): 8-. |
| [2] | Serena REGGI, Elisabetta ONELLI, Alessandra MOSCATELLI, Nadia STROPPA, Matteo Dell’ANNO, Kiril PERFANOV, Luciana ROSSI. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Rice Engineered Lines [J]. Rice Science, 2023, 30(6): 6-. |
| [3] | Sundus ZAFAR, XU Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 4-. |
| [4] | Kankunlanach KHAMPUANG, Nanthana CHAIWONG, Atilla YAZICI, Baris DEMIRER, Ismail CAKMAK, Chanakan PROM-U-THAI. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 9-. |
| [5] | FAN Fengfeng, CAI Meng, LUO Xiong, LIU Manman, YUAN Huanran, CHENG Mingxing, Ayaz AHMAD, LI Nengwu, LI Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Rice Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 14-. |
| [6] | LIN Shaodan, YAO Yue, LI Jiayi, LI Xiaobin, MA Jie, WENG Haiyong, CHENG Zuxin, YE Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 10-. |
| [7] | Md. Forshed DEWAN, Md. AHIDUZZAMAN, Md. Nahidul ISLAM, Habibul Bari SHOZIB. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and South-East Asia: A Review [J]. Rice Science, 2023, 30(6): 5-. |
| [8] | Raja CHAKRABORTY, Pratap KALITA, Saikat SEN. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Chakhao Poireiton (A Pigmented Black Rice) in High-Fat High-Sugar induced Rats [J]. Rice Science, 2023, 30(6): 11-. |
| [9] | LI Qianlong, FENG Qi, WANG Heqin, KANG Yunhai, ZHANG Conghe, DU Ming, ZHANG Yunhu, WANG Hui, CHEN Jinjie, HAN Bin, FANG Yu, WANG Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 7-. |
| [10] | JI Dongling, XIAO Wenhui, SUN Zhiwei, LIU Lijun, GU Junfei, ZHANG Hao, Tom Matthew HARRISON, LIU Ke, WANG Zhiqin, WANG Weilu, YANG Jianchang. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 12-. |
| [11] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [12] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| [13] | Monica Ruffini Castiglione, Stefania Bottega, Carlo Sorce, Carmelina SpanÒ. Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa [J]. Rice Science, 2023, 30(5): 449-458. |
| [14] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [15] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||