
Rice Science ›› 2021, Vol. 28 ›› Issue (6): 594-604.DOI: 10.1016/j.rsci.2021.03.003
• Research Paper • Previous Articles
Anwen Xiao1,2, Danting Chen1, Wai Chin Li2( ), Zhihong Ye1(
), Zhihong Ye1( )
)
Received:2020-11-14
Accepted:2021-03-01
Online:2021-11-28
Published:2021-11-28
Anwen Xiao, Danting Chen, Wai Chin Li, Zhihong Ye. Root Morphology and Anatomy Affect Cadmium Translocation and Accumulation in Rice[J]. Rice Science, 2021, 28(6): 594-604.
Add to citation manager EndNote|Ris|BibTeX
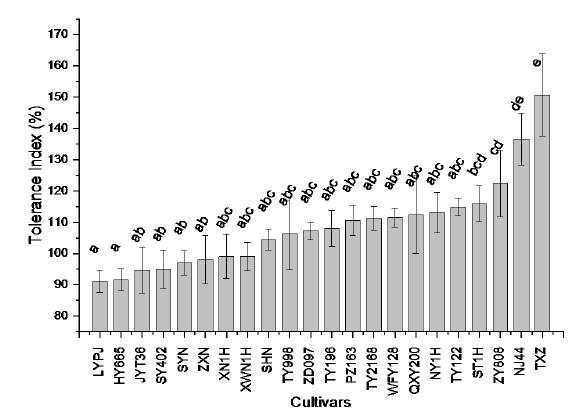
Fig. S1. Cd tolerance index of 22 rice cultivars grown in solution with treatment of 0.1 mg/L Cd for 3 weeks.LYPJ, Liangyoupeijiu; JYT36, Yinyou T36; SYN, Suyunuo; XN1H, Xinnuo 1; TY2168, Tianyou 2168; NY1H, Nuoyou 1; QXY200, Qianxiangyou 2000; TY122, Tianyou 122; NJ44, Nanjing 44; TY998, Tianyou 998; XWN1H, Xiangwannuo 1; WFY128, Wufengyou 128; TXZ, Texianzhan; ZY808, Zhongyou 808; SY402, Shanyou 402; PZ163, Peiyou 163; TY196, Tianyou 196; HY665, Huayou 665; ZD097, Zhongdao 097; SHN, Suihongnuo; ST1H, Shengtai 1; ZXN, Zixiangnuo.Data are Mean ± SE (n = 4). Different letters above the bars indicate significant difference between cultivars at P < 0.05.
| Cultivar | Control | 0.1 mg/L Cd treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant height (cm) | Longest root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | Plant height (cm) | Longest root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | ||
| JYT36 | 26.43 ± 1.25 | 9.25 ± 0.32 | 0.30 ± 0.04 | 0.23 ± 0.04 | 28.33 ± 0.88 | 8.67 ± 1.67 | 0.37 ± 0.09 | 0.31 ± 0.08 | |
| LYPJ | 19.63 ± 2.48 | 8.00 ± 0.74 | 0.22 ± 0.03 | 0.14 ± 0.02 | 20.50 ± 0.96 | 10.23 ± 0.65 | 0.21 ± 0.02 | 0.15 ± 0.02 | |
| NJ44 | 17.25 ± 0.52 | 9.55 ± 1.00 | 0.17 ± 0.01 | 0.14 ± 0.01 | 14.50 ± 0.89 | 5.83 ± 0.20 | 0.08 ± 0.01 | 0.10 ± 0.01 | |
| NY1H | 28.88 ± 0.66 | 6.00 ± 1.02 | 0.46± 0.05 | 0.32 ± 0.05 | 27.25 ± 2.07 | 8.38 ± 0.43 | 0.43 ± 0.09 | 0.33 ± 0.06 | |
| QXY200 | 23.13 ± 1.05 | 9.13 ± 0.66 | 0.17 ± 0.02 | 0. 11 ± 0.02 | 24.38 ± 3.02 | 5.25 ± 0.32 | 0.22 ± 0.06 | 0.12± 0.01 | |
| SYN | 23.38 ± 1.65 | 8.25 ± 0.43 | 0.29 ± 0.06 | 0.17 ± 0.03 | 23.75 ± 1.44 | 9.53 ± 1.03 | 0.34 ± 0.07 | 0.25 ± 0.04 | |
| TY122 | 24.00 ± 0.68 | 7.75 ± 0.25 | 0.23 ±0.01 | 0.15 ± 0.01 | 21.83 ± 0.93 | 7.17 ± 0.33 | 0.29 ± 0.03 | 0.20 ± 0.03 | |
| TY2168 | 23.00 ± 0.71 | 8.75 ± 0.63 | 0.19 ± 0.02 | 0.12 ± 0.01 | 20.83 ± 0.73 | 7.00 ± 0.58 | 0.19 ± 0.03 | 0.12 ± 0.03 | |
| YY998 | 23.25 ± 1.01 | 9.50 ± 0.65 | 0.29 ± 0.02 | 0.17 ± 0.01 | 22.00 ± 0.71 | 8.63 ± 1.46 | 0.24 ± 0.01 | 0.14 ± 0.01 | |
| WFY128 | 23.88 ± 0.13 | 10.38 ± 1.30 | 0.28 ± 0.02 | 0.14 ± 0.02 | 22.17 ± 2.62 | 8.50 ± 0.58 | 0.30 ± 0.08 | 0.21 ± 0.06 | |
| TXZ | 18.63 ± 0.55 | 10.38 ± 0.80 | 0.14 ± 0.01 | 0.09 ± 0.01 | 16.20 ± 3.00 | 6.83 ± 0.44 | 0.16 ± 0.06 | 0.10 ± 0.01 | |
| XWN1H | 21.75 ± 1.45 | 12.25 ± 1.27 | 0.25 ± 0.03 | 0.17 ± 0.02 | 20.17 ± 3.06 | 10.43 ± 0.64 | 0.33 ± 0.04 | 0.18 ± 0.02 | |
| XN1H | 20.75 ± 1.13 | 10.00 ± 1.24 | 0.24 ± 0.02 | 0.13 ± 0.01 | 18.50 ± 1.04 | 12.33 ± 0.93 | 0.21 ± 0.02 | 0.13 ± 0.01 | |
| ZY808 | 27.88 ± 0.66 | 7.88 ± 0.75 | 0.36 ± 0.04 | 0.20 ± 0.04 | 30.50 ± 1.26 | 8.33 ± 1.86 | 0.59 ± 0.05 | 0.33 ± 0.03 | |
| HY665 | 21.14 ± 0.51 | 7.50 ± 0.87 | 0.24 ± 0.04 | 0.15 ± 0.03 | 24.67 ± 2.32 | 8.67 ± 1.33 | 0.33 ± 0.07 | 0.20 ± 0.06 | |
| SHN | 16.88 ± 0.75 | 8.75 ± 0.95 | 0.13 ± 0.01 | 0.11 ± 0.01 | 12.33 ± 0.93 | 7.17 ± 1.01 | 0.11 ± 0.01 | 0.12 ± 0.02 | |
| SY402 | 25.38 ± 1.65 | 7.40 ± 0.37 | 0.27 ± 0.04 | 0.19 ± 0.02 | 23.17 ± 0.83 | 9.17 ± 1.01 | 0.29 ± 0.01 | 0.20 ± 0.02 | |
| ST1H | 16.20 ± 0.93 | 9.60 ± 1.00 | 0.10 ± 0.01 | 0.08 ± 0.01 | 18.17 ± 0.33 | 6.67 ± 0.93 | 0.15 ± 0.03 | 0.13 ± 0.03 | |
| TY196 | 22.75 ± 0.48 | 8.33 ± 0.58 | 0.23 ± 0.02 | 0.17 ± 0.02 | 25.83 ± 2.09 | 8.83 ± 0.73 | 0.36 ± 0.08 | 0.20 ± 0.04 | |
| PZ163 | 25.50 ± 1.26 | 9.38 ± 1.01 | 0.29 ± 0.04 | 0.17 ± 0.01 | 22.33 ± 1.64 | 7.50 ± 0.29 | 0.23 ± 0.04 | 0.15 ± 0.01 | |
| ZXN | 16.75 ± 1.20 | 9.50 ± 0.74 | 0.11 ± 0.02 | 0.11 ± 0.01 | 15.17 ± 1.36 | 9.50 ± 0.50 | 0.10 ± 0.02 | 0.10 ± 0.02 | |
| ZD097 | 15.75 ± 0.14 | 2.04 ± 0.36 | 0.14 ± 0.01 | 0.12 ± 0.00 | 15.17 ± 1.42 | 8.87 ± 0.70 | 0.11 ± 0.02 | 0.13 ± 0.02 | |
Table S1. Growing parameters of 22 rice cultivars grown in solution with treatments of 0 (control) and 0.1 mg/L Cd for 3 weeks.
| Cultivar | Control | 0.1 mg/L Cd treatment | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Plant height (cm) | Longest root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | Plant height (cm) | Longest root length (cm) | Shoot fresh weight (g) | Root fresh weight (g) | ||
| JYT36 | 26.43 ± 1.25 | 9.25 ± 0.32 | 0.30 ± 0.04 | 0.23 ± 0.04 | 28.33 ± 0.88 | 8.67 ± 1.67 | 0.37 ± 0.09 | 0.31 ± 0.08 | |
| LYPJ | 19.63 ± 2.48 | 8.00 ± 0.74 | 0.22 ± 0.03 | 0.14 ± 0.02 | 20.50 ± 0.96 | 10.23 ± 0.65 | 0.21 ± 0.02 | 0.15 ± 0.02 | |
| NJ44 | 17.25 ± 0.52 | 9.55 ± 1.00 | 0.17 ± 0.01 | 0.14 ± 0.01 | 14.50 ± 0.89 | 5.83 ± 0.20 | 0.08 ± 0.01 | 0.10 ± 0.01 | |
| NY1H | 28.88 ± 0.66 | 6.00 ± 1.02 | 0.46± 0.05 | 0.32 ± 0.05 | 27.25 ± 2.07 | 8.38 ± 0.43 | 0.43 ± 0.09 | 0.33 ± 0.06 | |
| QXY200 | 23.13 ± 1.05 | 9.13 ± 0.66 | 0.17 ± 0.02 | 0. 11 ± 0.02 | 24.38 ± 3.02 | 5.25 ± 0.32 | 0.22 ± 0.06 | 0.12± 0.01 | |
| SYN | 23.38 ± 1.65 | 8.25 ± 0.43 | 0.29 ± 0.06 | 0.17 ± 0.03 | 23.75 ± 1.44 | 9.53 ± 1.03 | 0.34 ± 0.07 | 0.25 ± 0.04 | |
| TY122 | 24.00 ± 0.68 | 7.75 ± 0.25 | 0.23 ±0.01 | 0.15 ± 0.01 | 21.83 ± 0.93 | 7.17 ± 0.33 | 0.29 ± 0.03 | 0.20 ± 0.03 | |
| TY2168 | 23.00 ± 0.71 | 8.75 ± 0.63 | 0.19 ± 0.02 | 0.12 ± 0.01 | 20.83 ± 0.73 | 7.00 ± 0.58 | 0.19 ± 0.03 | 0.12 ± 0.03 | |
| YY998 | 23.25 ± 1.01 | 9.50 ± 0.65 | 0.29 ± 0.02 | 0.17 ± 0.01 | 22.00 ± 0.71 | 8.63 ± 1.46 | 0.24 ± 0.01 | 0.14 ± 0.01 | |
| WFY128 | 23.88 ± 0.13 | 10.38 ± 1.30 | 0.28 ± 0.02 | 0.14 ± 0.02 | 22.17 ± 2.62 | 8.50 ± 0.58 | 0.30 ± 0.08 | 0.21 ± 0.06 | |
| TXZ | 18.63 ± 0.55 | 10.38 ± 0.80 | 0.14 ± 0.01 | 0.09 ± 0.01 | 16.20 ± 3.00 | 6.83 ± 0.44 | 0.16 ± 0.06 | 0.10 ± 0.01 | |
| XWN1H | 21.75 ± 1.45 | 12.25 ± 1.27 | 0.25 ± 0.03 | 0.17 ± 0.02 | 20.17 ± 3.06 | 10.43 ± 0.64 | 0.33 ± 0.04 | 0.18 ± 0.02 | |
| XN1H | 20.75 ± 1.13 | 10.00 ± 1.24 | 0.24 ± 0.02 | 0.13 ± 0.01 | 18.50 ± 1.04 | 12.33 ± 0.93 | 0.21 ± 0.02 | 0.13 ± 0.01 | |
| ZY808 | 27.88 ± 0.66 | 7.88 ± 0.75 | 0.36 ± 0.04 | 0.20 ± 0.04 | 30.50 ± 1.26 | 8.33 ± 1.86 | 0.59 ± 0.05 | 0.33 ± 0.03 | |
| HY665 | 21.14 ± 0.51 | 7.50 ± 0.87 | 0.24 ± 0.04 | 0.15 ± 0.03 | 24.67 ± 2.32 | 8.67 ± 1.33 | 0.33 ± 0.07 | 0.20 ± 0.06 | |
| SHN | 16.88 ± 0.75 | 8.75 ± 0.95 | 0.13 ± 0.01 | 0.11 ± 0.01 | 12.33 ± 0.93 | 7.17 ± 1.01 | 0.11 ± 0.01 | 0.12 ± 0.02 | |
| SY402 | 25.38 ± 1.65 | 7.40 ± 0.37 | 0.27 ± 0.04 | 0.19 ± 0.02 | 23.17 ± 0.83 | 9.17 ± 1.01 | 0.29 ± 0.01 | 0.20 ± 0.02 | |
| ST1H | 16.20 ± 0.93 | 9.60 ± 1.00 | 0.10 ± 0.01 | 0.08 ± 0.01 | 18.17 ± 0.33 | 6.67 ± 0.93 | 0.15 ± 0.03 | 0.13 ± 0.03 | |
| TY196 | 22.75 ± 0.48 | 8.33 ± 0.58 | 0.23 ± 0.02 | 0.17 ± 0.02 | 25.83 ± 2.09 | 8.83 ± 0.73 | 0.36 ± 0.08 | 0.20 ± 0.04 | |
| PZ163 | 25.50 ± 1.26 | 9.38 ± 1.01 | 0.29 ± 0.04 | 0.17 ± 0.01 | 22.33 ± 1.64 | 7.50 ± 0.29 | 0.23 ± 0.04 | 0.15 ± 0.01 | |
| ZXN | 16.75 ± 1.20 | 9.50 ± 0.74 | 0.11 ± 0.02 | 0.11 ± 0.01 | 15.17 ± 1.36 | 9.50 ± 0.50 | 0.10 ± 0.02 | 0.10 ± 0.02 | |
| ZD097 | 15.75 ± 0.14 | 2.04 ± 0.36 | 0.14 ± 0.01 | 0.12 ± 0.00 | 15.17 ± 1.42 | 8.87 ± 0.70 | 0.11 ± 0.02 | 0.13 ± 0.02 | |
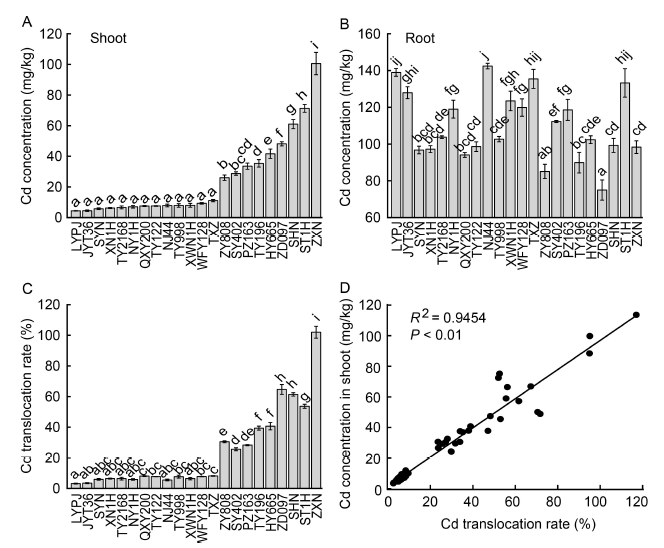
Fig. 1. Cd concentrations in shoots (A) and roots (B), Cd translocation rate (shoot/root) (C), and correlation between Cd concentration in shoots and Cd translocation rates (shoot/root) (D) of 22 rice cultivars with 0.1 mg/L Cd treatment for 3 weeks.LYPJ, Liangyoupeijiu; JYT36, Jinyou T36; SYN, Suyunuo; XN1H, Xinnuo 1; TY2168, Tianyou 2168; NY1H, Nuoyou 1; QXY200, Qianxiangyou 2000; TY122, Tianyou 122; NJ44, Nanjing 44; TY998, Tianyou 998; XWN1H, Xiangwannuo 1; WFY128, Wufengyou 128; TXZ, Texianzhan; ZY808, Zhongyou 808; SY402, Shanyou 402; PZ163, Peiyou 163; TY196, Tianyou 196; HY665, Huayou 665; ZD097, Zhongdao 097; SHN, Suihongnuo; ST1H, Shengtai 1; ZXN, Zixiangnuo.Data are Mean ± SE (n = 4). Different lowercase letters indicate significant differences among the cultivars at P < 0.05.
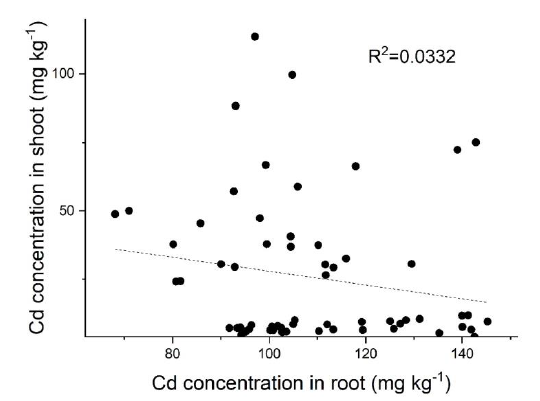
Fig. S2. Correlation between Cd concentration in shoots and roots of 22 rice cultivars with treatment of 0.1 mg/L Cd for 3 weeks.Data are Mean ± SE (n = 4).
| Measured parameter | Cd concentration (mg/L) | Cultivar | |
|---|---|---|---|
| JYT36 | ZXN | ||
| Root length (cm) | 0 | 16.67 ± 0.44 b | 14.63 ± 0.75 b |
| 0.5 | 16.17 ± 0.73 b | 13.17 ± 0.60 ab | |
| 2.5 | 9.67 ± 0.67 a | 12.50 ± 0.35 a | |
| Shoot height (cm) | 0 | 47.67 ± 0.88 c | 21.33 ± 3.11 b |
| 0.5 | 38.83 ± 0.93 b | 17.67 ± 0.60 ab | |
| 2.5 | 20.00 ± 1.44 a | 12.00 ± 0.58 a | |
Table S2. Effects of Cd stress on growth of two rice cultivars with treatments of 0.5 and 2.5 mg/L Cd for 4 weeks.
| Measured parameter | Cd concentration (mg/L) | Cultivar | |
|---|---|---|---|
| JYT36 | ZXN | ||
| Root length (cm) | 0 | 16.67 ± 0.44 b | 14.63 ± 0.75 b |
| 0.5 | 16.17 ± 0.73 b | 13.17 ± 0.60 ab | |
| 2.5 | 9.67 ± 0.67 a | 12.50 ± 0.35 a | |
| Shoot height (cm) | 0 | 47.67 ± 0.88 c | 21.33 ± 3.11 b |
| 0.5 | 38.83 ± 0.93 b | 17.67 ± 0.60 ab | |
| 2.5 | 20.00 ± 1.44 a | 12.00 ± 0.58 a | |
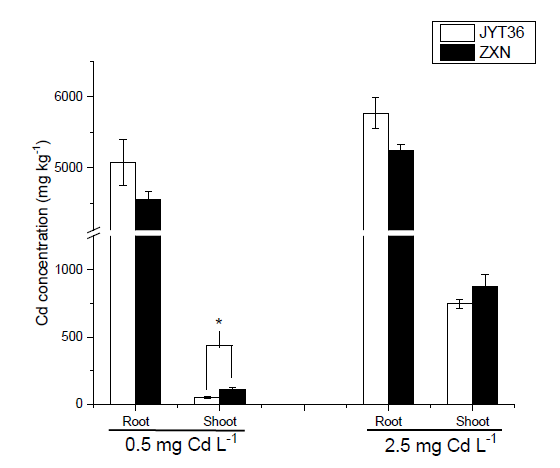
Fig. S3. Cd concentration in roots and shoots of two rice cultivars with 0.5 and 2.5 mg/L Cd treatments for 4 weeks.Data are Mean ± SE (n = 4). * indicate significant difference between cultivars at P < 0.05.
| Cultivar | Vmax [nmol/(g∙h)] | Km (μmol/L) | R2 |
|---|---|---|---|
| ZXN | 35.12 ± 2.32 | 0.55 ± 0.03 | 0.91 |
| JYT36 | 82.29 ± 5.17 | 0.98 ± 0.06 | 0.98 |
Table S3. Kinetic parameters for Cd influx into rice roots of cultivars ZXN and JYT36.
| Cultivar | Vmax [nmol/(g∙h)] | Km (μmol/L) | R2 |
|---|---|---|---|
| ZXN | 35.12 ± 2.32 | 0.55 ± 0.03 | 0.91 |
| JYT36 | 82.29 ± 5.17 | 0.98 ± 0.06 | 0.98 |
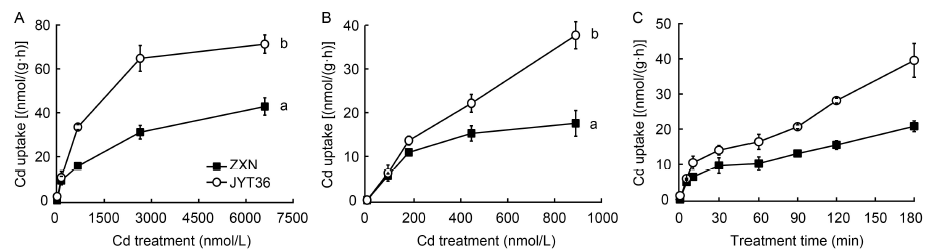
Fig. 2. Concentration-dependent and time-dependent kinetics for Cd uptake by rice roots of cultivars Zixiangnuo (ZXN) and Jinyou T36 (JYT36).A, High-affinity uptake kinetics of Cd in the two cultivars.B, Low-affinity uptake kinetics of Cd in the two cultivars.C, Time-dependent kinetics of Cd in the two cultivars.The 10 d old intact seedlings were treated for 30 min in A and B, and treated with 180 nmol/L Cd in C. Data are Mean ± SE (n = 3). Data with different lowercase letters mean significant differences between the treatments at P < 0.001 (A) and P < 0.05 (B).
| Parameter | Cd concentration (mg/L) | Cultivar | Significant | |
|---|---|---|---|---|
| ZXN | JYT36 | |||
| No. of root tips per cm2 | 0.0 | 90.14 ± 6.72 c | 49.40 ± 5.33 b | *** |
| 0.5 | 62.67 ± 4.36 b | 27.24 ± 2.86 b | *** | |
| 2.5 | 29.51 ± 3.30 a | 25.98 ± 3.89 a | ||
| Average diameter (mm) | 0.0 | 0.26 ± 0.02 a | 0.38 ± 0.02 a | *** |
| 0.5 | 0.32 ± 0.02 ab | 0.41 ± 0.02 b | ** | |
| 2.5 | 0.34 ± 0.02 b | 0.30 ± 0.02 a | ||
Table 1 Parameters of root morphology in rice with treatments of 0.5 and 2.5 mg/L Cd for 4 weeks.
| Parameter | Cd concentration (mg/L) | Cultivar | Significant | |
|---|---|---|---|---|
| ZXN | JYT36 | |||
| No. of root tips per cm2 | 0.0 | 90.14 ± 6.72 c | 49.40 ± 5.33 b | *** |
| 0.5 | 62.67 ± 4.36 b | 27.24 ± 2.86 b | *** | |
| 2.5 | 29.51 ± 3.30 a | 25.98 ± 3.89 a | ||
| Average diameter (mm) | 0.0 | 0.26 ± 0.02 a | 0.38 ± 0.02 a | *** |
| 0.5 | 0.32 ± 0.02 ab | 0.41 ± 0.02 b | ** | |
| 2.5 | 0.34 ± 0.02 b | 0.30 ± 0.02 a | ||
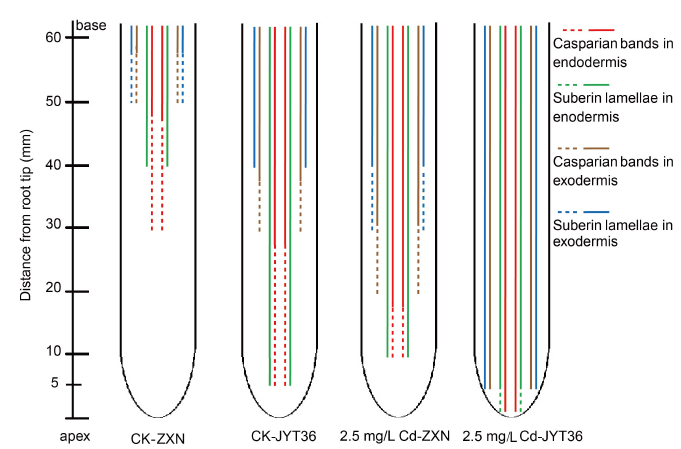
Fig. 3. Schematic representation of endodermal and exodermal apoplastic barriers of two rice cultivars in control (CK) and Cd (2.5 mg/L) treatments.ZXN, Zixiangnuo; JYT36, Jinyou T36. Casparian bands and suberin lamellae in endodermis are represented by red and green lines, respectively. Casparian bands and suberin lamellae in exodermis are represented by brown and blue lines, respectively. The dotted lines indicate the early and immature deposition of the barriers and solid lines represent the Casparian bands or suberin lamellae which have developed maturely in the zone.
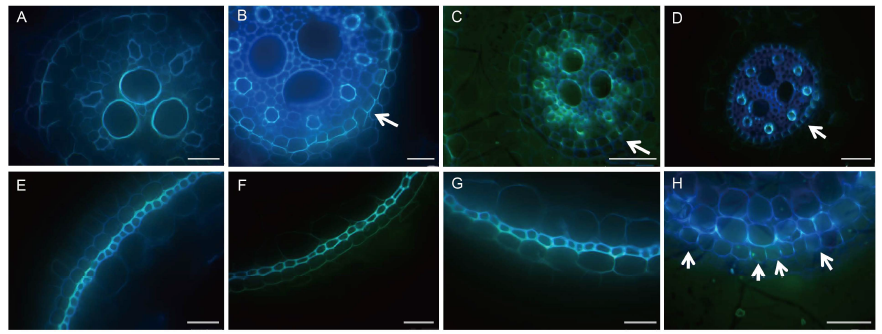
Fig. 4. Comparison of development of Casparian bands at 20 mm distance from rice root tips with treatments of control and 2.5 mg/L Cd for 4 weeks.A to D are endodermis sections, and E to H are exodermis sections; A, C, E and G are cultivar Zixiangnuo, and B, D, F and H are cultivar Jinyou T36; A, B, E and F are in the control treatment, and C, D, G and H are in 2.5 mg/L Cd treatment. Arrow heads refer to the Casparian bands. Scale bars are 20 μm in A, B, E, F and G, and 50 μm in C, D and H.
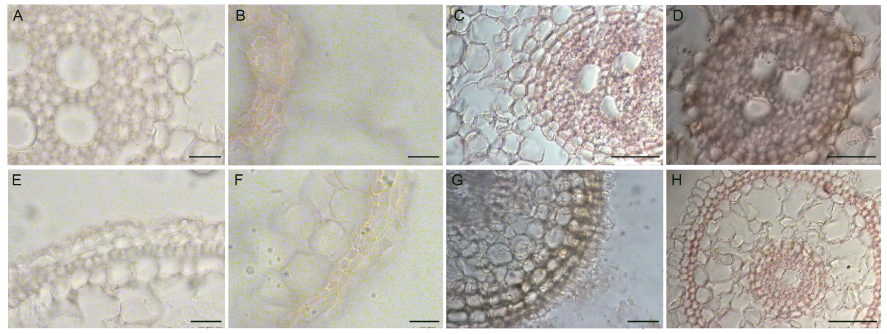
Fig. 5. Comparison of development of suberin lamellae at 20 mm distance from rice root tips with treatments of control and 2.5 mg/L Cd for 4 weeks.A to D are endodermis sections, and E to H are exodermis sections; A, C, E and G are cultivar Zixiangnuo, and B, D, F and H are cultivar Jinyou T36; A, B, E and F are in control treatment, and C, D, G and H are in 2.5 mg/L Cd treatment. Scale bars are 20 μm in A, B, E and F, and 50 μm in C, D, G and H.
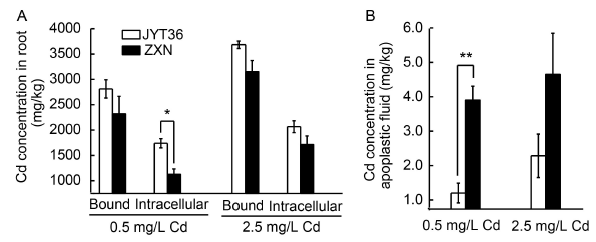
Fig. 6. Distribution of Cd in rice roots with treatments of 0.5 and 2.5 mg/L Cd for 4 weeks.A, Cd bound in cell wall and intracellular Cd.B, Cd concentration in the apoplastic fluid.ZXN, Zixiangnuo; JYT36, Jinyou T36. Data are Mean ± SE (n = 3). * and ** mean significant differences between the cultivars in the same treatment at the 0.05 and 0.01 levels, respectively.
| Cultivar | Abbreviation | Subspecies |
|---|---|---|
| Liangyoupeijiu | LYPJ | Indica |
| Huayou 665 | HY665 | Indica |
| Shanyou 402 | SY402 | Indica |
| Jinyou T36 | JYT36 | Indica |
| Tianyou 998 | TY998 | Indica |
| Tianyou 196 | TY196 | Indica |
| Peiyou 163 | PZ163 | Indica |
| Tianyou 2168 | TY2168 | Indica |
| Wufengyou 128 | WFY128 | Indica |
| Qianxiangyou 2000 | QXY200 | Indica |
| Nuoyou 1 | NY1H | Indica |
| Tianyou 122 | TY122 | Indica |
| Zhongyou 808 | ZY808 | Indica |
| Texianzhan | TXZ | Indica |
| Suihongnuo | SHN | Indica |
| Xiangwannuo 1 | XWN1H | Indica |
| Shengtai 1 | ST1H | Indica |
| Zhongdao 097 | ZD097 | Japonica |
| Nanjing 44 | NJ44 | Japonica |
| Suyunuo | SYN | Japonica |
| Zixiangnuo | ZXN | Japonica |
| Xinnuo 1 | XN1H | Japonica |
Table S4. Rice cultivars used.
| Cultivar | Abbreviation | Subspecies |
|---|---|---|
| Liangyoupeijiu | LYPJ | Indica |
| Huayou 665 | HY665 | Indica |
| Shanyou 402 | SY402 | Indica |
| Jinyou T36 | JYT36 | Indica |
| Tianyou 998 | TY998 | Indica |
| Tianyou 196 | TY196 | Indica |
| Peiyou 163 | PZ163 | Indica |
| Tianyou 2168 | TY2168 | Indica |
| Wufengyou 128 | WFY128 | Indica |
| Qianxiangyou 2000 | QXY200 | Indica |
| Nuoyou 1 | NY1H | Indica |
| Tianyou 122 | TY122 | Indica |
| Zhongyou 808 | ZY808 | Indica |
| Texianzhan | TXZ | Indica |
| Suihongnuo | SHN | Indica |
| Xiangwannuo 1 | XWN1H | Indica |
| Shengtai 1 | ST1H | Indica |
| Zhongdao 097 | ZD097 | Japonica |
| Nanjing 44 | NJ44 | Japonica |
| Suyunuo | SYN | Japonica |
| Zixiangnuo | ZXN | Japonica |
| Xinnuo 1 | XN1H | Japonica |
| [1] | Armstrong J, Armstrong W. 2005. Rice: Sulfide-induced barriers to root radial oxygen loss, Fe2+ and water uptake, and lateral root emergence. Ann Bot, 96(4): 625-638. |
| [2] | Boominathan R, Doran P M. 2003. Cadmium tolerance and antioxidative defenses in hairy roots of the cadmium hyperaccumulator, Thlaspi caerulescens. Biotechnol Bioeng, 83(2): 158-167. |
| [3] | Chi Y, Li F, Tam N F Y, Liu C, Ouyang Y, Qi X, Li W C, Ye Z H. 2018. Variations in grain cadmium and arsenic concentrations and screening for stable low-accumulating rice cultivars from multi-environment trials. Sci Total Environ, 643(1): 1314-1324. |
| [4] | Chiao W T, Syu C H, Chen B C, Juang K W. 2019. Cadmium in rice grains from a field trial in relation to model parameters of Cd-toxicity and -absorption in rice seedlings. Ecotox Environ Safe, 169: 837-847. |
| [5] | Chiao W T, Chen B C, Syu C H, Juang K W. 2020. Aspects of cultivar variation in physiological traits related to Cd distribution in rice plants with a short-term stress. Bot Stud, 61: 27. |
| [6] | Grant C A, Clarke J M, Duguid S, Chaney R L. 2008. Selection and breeding of plant cultivars to minimize cadmium accumulation. Sci Total Environ, 390: 301-310. |
| [7] | Hoagland D R, Arnon D I. 1938. The water culture method for growing plants without soil. Calif Agric Exp Stn, 347: 1-39. |
| [8] | Huang G, Ding C, Guo F, Li X G, Zhou Z G, Zhang T L, Wang X X. 2017. The role of node restriction on cadmium accumulation in the brown rice of 12 Chinese rice (Oryza sativa L.) cultivars. J Agric Food Chem, 65(47): 10157-10164. |
| [9] | Huang L, Li W C, Tam N F, Ye Z H. 2019. Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.). J Environ Sci, 75: 296-306. |
| [10] | Kotula L, Ranathunge K, Schreiber L, Steudle E. 2009. Functional and chemical comparison of apoplastic barriers to radial oxygen loss in roots of rice (Oryza sativa L.) grown in aerated or deoxygenated solution. J Exp Bot, 60(7): 2155-2167. |
| [11] | Kreszies T, Schreiber L, Ranathunge K. 2018. Suberized transport barriers in Arabidopsis, barley and rice roots: From the model plant to crop species. J Plant Physiol, 227: 75-83. |
| [12] | Liu J G, Liang J S, Li K Q, Zhang Z J, Yu B Y, Lu X L, Yang J C, Zhu Q S. 2003. Correlations between cadmium and mineral nutrients in absorption and accumulation in various genotypes of rice under cadmium stress. Chemosphere, 52(9): 1467-1473. |
| [13] | Liu J G, Qian M, Cai G L, Yang J C, Zhu Q S. 2007. Uptake and translocation of Cd in different rice cultivars and the relation with Cd accumulation in rice grain. J Hazard Mater, 143(1/2): 443-447. |
| [14] | Loix C, Huybrechts M, Vangronsveld J, Gielen M, Keunen E, Cuypers A. 2017. Reciprocal interactions between cadmium- induced cell wall responses and oxidative stress in plants. Front Plant Sci, 8: 1867. |
| [15] | Lux A, Sottniková A, Opatrná J, Greger M. 2004. Differences in structure of adventitious roots in Salix clones with contrasting characteristics of cadmium accumulation and sensitivity. Physiol Plant, 120(4): 537-545. |
| [16] | Lux A, Morita S, Abe J, Ito K. 2005. An improved method for clearing and staining free-hand sections and whole-mount samples. Ann Bot, 96(6): 989-996. |
| [17] | Lux A, Martinka M, Vaculik M, White P J. 2011. Root responses to cadmium in the rhizosphere: A review. J Exp Bot, 62(1): 21-37. |
| [18] | Ma F, Peterson C A. 2003. Current insights into the development, structure, and chemistry of the endodermis and exodermis of roots. Can J Bot, 81: 405-421. |
| [19] | Maksimović I, Kastori R, Krstić L, Luković J. 2007. Steady presence of cadmium and nickel affects root anatomy, accumulation and distribution of essential ions in maize seedlings. Biol Plant, 51(3): 589-592. |
| [20] | Man Y, Zhao Y Y, Ye R, Lin J X, Jing Y P. 2018. In vivo cytological and chemical analysis of Casparian strips using stimulated Raman scattering microscopy. J Plant Physiol, 220: 136-144. |
| [21] | Moore C A, Bowen H C, Scrase-Field S, Knight M R, White P J. 2002. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J, 30(4): 457-466. |
| [22] | Nordberg G, Jin T Y, Bernard A, Fierens S, Buchet J P, Ye T T, Kong Q H, Wang H F. 2002. Low bone density and renal dysfunction following environmental cadmium exposure in China. Ambio, 31(6): 478-481. |
| [23] | Parrotta L, Guerriero G, Sergeant K, Cai G, Hausman J F. 2015. Target or barrier? The cell wall of early- and later-diverging plants vs cadmium toxicity: Differences in the response mechanisms. Front Plant Sci, 6: 133. |
| [24] | Qi X L, Tam N F, Li W C, Ye Z H. 2020. The role of root apoplastic barriers in cadmium translocation and accumulation in cultivars of rice (Oryza sativa L.) with different Cd- accumulating characteristics. Environ Pollut, 264: 114736. |
| [25] | Redjala T, Zelko I, Sterckeman T, Legué V, Lux A. 2011. Relationship between root structure and root cadmium uptake in maize. Environ Exp Bot, 71(2): 241-248. |
| [26] | Rodda M S, Li G, Reid R J. 2011. The timing of grain Cd accumulation in rice plants: The relative importance of remobilisation within the plant and root Cd uptake post- flowering. Plant Soil, 347: 105-114. |
| [27] | Rout G R, Das P. 2002. Rapid hydroponic screening for molybdenum tolerance in rice through morphological and biochemical analysis. Rostl Výroba, 48: 505-512. |
| [28] | Schreiber L, Hartmann K, Skrabs M, Zeier J. 1999. Apoplastic barriers in roots: Chemical composition of endodermal and hypodermal cell walls. J Exp Bot, 50: 1267-1280. |
| [29] | Shi Y J, Xu Y F, Ni Z Y, Wang J W, Li D, Zhang M K. 2019. Difference of Cd accumulation in main crops in Hangzhou and its influencing factors. Zhejiang Agric Sci, 60(7): 1230-1233. (in Chinese with English abstract) |
| [30] | Tao Q, Jupa R, Luo J P, Lux A, Kovac J, Wen Y, Zhou Y M, Jan J, Liang Y C, Li T Q. 2017. The apoplasmic pathway via the root apex and lateral roots contributes to Cd hyperaccumulation in the hyperaccumulator Sedum alfredii. J Exp Bot, 68(3): 739-751. |
| [31] | Uraguchi S, Mori S, Kuramata M, Kawasaki A, Arao T, Ishikawa S. 2009. Root-to-shoot Cd translocation via the xylem is the major process determining shoot and grain cadmium accumulation in rice. J Exp Bot, 60(9): 2677-2688. |
| [32] | Uraguchi S, Fujiwara T. 2012. Cadmium transport and tolerance in rice: Perspectives for reducing grain cadmium accumulation. Rice, 5(1): 5. |
| [33] | Uraguchi S, Fujiwara T. 2013. Rice breaks ground for cadmium- free cereals. Curr Opin Plant Biol, 16(3): 328-334. |
| [34] | Wang F J, Zhang Y T, Guo Q X, Tan H F, Han J H, Lin H R, Wei H W, Xu G W, Zhu C. 2018. Effects of exogenous 5-aminolevulinic acid and 24-epibrassinolide on Cd accumulation in rice from Cd-contaminated soil. Rice Sci, 25(6): 320-329. |
| [35] | White P J. 2001. The pathways of calcium movement to the xylem. J Exp Bot, 52: 891-899. |
| [36] | Wilkins D A. 1987. The measurement of tolerance to edaphic factors by means of root growth. New Phytol, 80: 623-633. |
| [37] | Williams P N, Lei M, Sun G, Huang Q, Lu Y, Deacon C, Meharg A A, Zhu Y G. 2009. Occurrence and partitioning of cadmium, arsenic and lead in mine impacted paddy rice: Hunan, China. Environ Sci Technol, 43(3): 637-642. |
| [38] | Wu C, Ye Z H, Shu W S, Zhu Y G, Wong M H. 2011. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J Exp Bot, 62(8): 2889-2898. |
| [39] | Yamaguchi N, Mori S, Baba K, Kaburagi-Yada S, Arao T, Kitajima N, Hokura A, Terada Y. 2011. Cadmium distribution in the root tissues of solanaceous plants with contrasting root-to-shoot Cd translocation efficiencies. Environ Exp Bot, 71(2): 198-206. |
| [40] | Ye J, Yan C L, Liu J C, Lu H L, Liu T, Song Z F. 2012. Effects of silicon on the distribution of cadmium compartmentation in root tips of Kandelia obovata (S., L.) Yong. Environ Pollut, 162: 369-373. |
| [41] | Zhuang P, Lu H, Li Z, Zou B, McBride M B. 2014. Multiple exposure and effects assessment of heavy metals in the population near mining area in South China. PLoS One, 9(4): e94484. |
| [1] | Prathap V, Suresh KUMAR, Nand Lal MEENA, Chirag MAHESHWARI, Monika DALAL, Aruna TYAGI. Phosphorus Starvation Tolerance in Rice Through a Combined Physiological, Biochemical and Proteome Analysis [J]. Rice Science, 2023, 30(6): 8-. |
| [2] | Serena REGGI, Elisabetta ONELLI, Alessandra MOSCATELLI, Nadia STROPPA, Matteo Dell’ANNO, Kiril PERFANOV, Luciana ROSSI. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Rice Engineered Lines [J]. Rice Science, 2023, 30(6): 6-. |
| [3] | Sundus ZAFAR, XU Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 4-. |
| [4] | Kankunlanach KHAMPUANG, Nanthana CHAIWONG, Atilla YAZICI, Baris DEMIRER, Ismail CAKMAK, Chanakan PROM-U-THAI. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 9-. |
| [5] | FAN Fengfeng, CAI Meng, LUO Xiong, LIU Manman, YUAN Huanran, CHENG Mingxing, Ayaz AHMAD, LI Nengwu, LI Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Rice Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 14-. |
| [6] | LIN Shaodan, YAO Yue, LI Jiayi, LI Xiaobin, MA Jie, WENG Haiyong, CHENG Zuxin, YE Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 10-. |
| [7] | Md. Forshed DEWAN, Md. AHIDUZZAMAN, Md. Nahidul ISLAM, Habibul Bari SHOZIB. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and South-East Asia: A Review [J]. Rice Science, 2023, 30(6): 5-. |
| [8] | Raja CHAKRABORTY, Pratap KALITA, Saikat SEN. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Chakhao Poireiton (A Pigmented Black Rice) in High-Fat High-Sugar induced Rats [J]. Rice Science, 2023, 30(6): 11-. |
| [9] | LI Qianlong, FENG Qi, WANG Heqin, KANG Yunhai, ZHANG Conghe, DU Ming, ZHANG Yunhu, WANG Hui, CHEN Jinjie, HAN Bin, FANG Yu, WANG Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 7-. |
| [10] | JI Dongling, XIAO Wenhui, SUN Zhiwei, LIU Lijun, GU Junfei, ZHANG Hao, Tom Matthew HARRISON, LIU Ke, WANG Zhiqin, WANG Weilu, YANG Jianchang. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 12-. |
| [11] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [12] | Monica Ruffini Castiglione, Stefania Bottega, Carlo Sorce, Carmelina SpanÒ. Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa [J]. Rice Science, 2023, 30(5): 449-458. |
| [13] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| [14] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [15] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||