
Rice Science ›› 2023, Vol. 30 ›› Issue (2): 113-126.DOI: 10.1016/j.rsci.2023.01.004
• Research Paper • Previous Articles Next Articles
Qiu Diyang1,2,#, Hu Rui1,3,#, Li Ji1,4, Li Ying1,2, Ding Jierong1,3, Xia Kuaifei1,6, Zhong Xuhua3, Fang Zhongming5( ), Zhang Mingyong1,6(
), Zhang Mingyong1,6( )
)
Received:2022-06-09
Accepted:2022-11-02
Online:2023-03-28
Published:2023-01-16
Contact:
Zhang Mingyong (zhangmy@scbg.ac.cn);Fang Zhongming (zmfang@gzu.edu.cn)
About author:# These authors contributed equally to this work
Qiu Diyang, Hu Rui, Li Ji, Li Ying, Ding Jierong, Xia Kuaifei, Zhong Xuhua, Fang Zhongming, Zhang Mingyong. Peptide Transporter OsNPF8.1 Contributes to Sustainable Growth under Salt and Drought Stresses, and Grain Yield under Nitrogen Deficiency in Rice[J]. Rice Science, 2023, 30(2): 113-126.
Add to citation manager EndNote|Ris|BibTeX
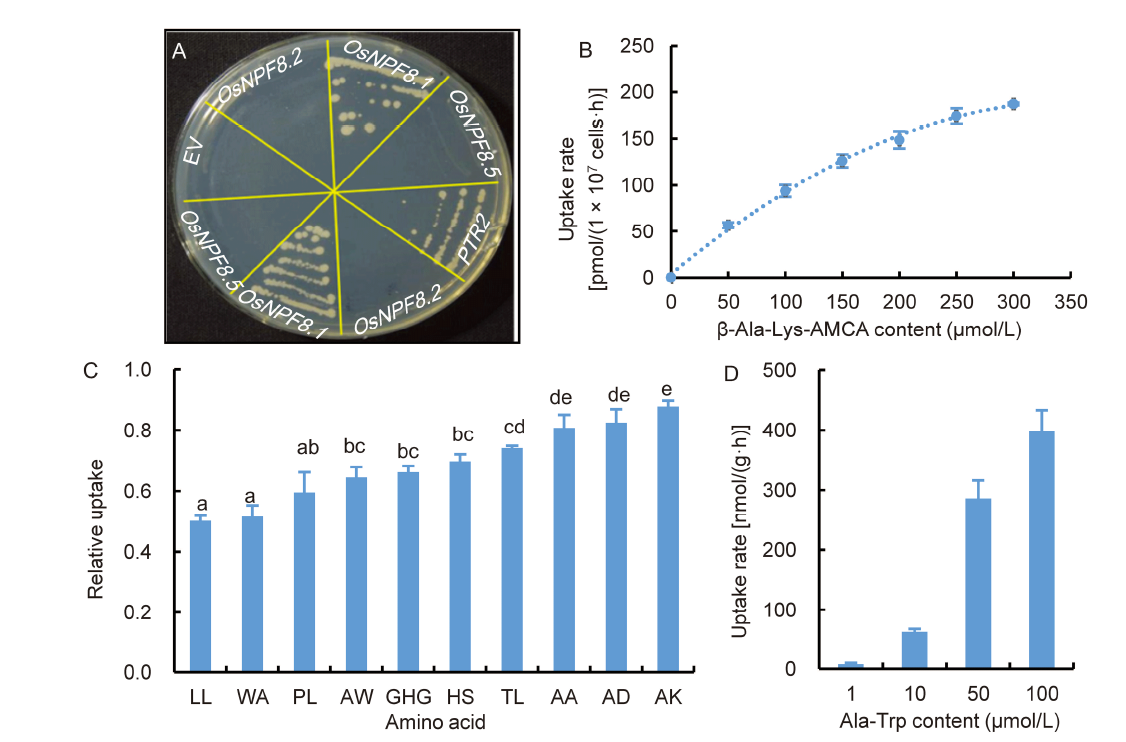
Fig. 1. OsNPF8.1 mediates di-/tri-peptide transport. A, Growth complementation of yeast ptr2 mutant. Transformants of the ptr2 yeast mutant expressing PTR2, OsNPF8.1, OsNPF8.2 and OsNPF8.5 were streaked on a solid synthetic medium with 100 μmol/L Pro-Leu as the sole source of nitrogen source. Yeast transformed with empty vector (EV) was served as a negative control. The yeast grown at 28 oC for 72 h is shown. B, Kinetics of β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (AMCA) accumulation in yeast mediated by OsNPF8.1. The uptake rates of β-Ala-Lys-AMCA by OsNPF8.1-expressing ptr2 cells at the indicated substrate concentrations are shown. The assay was carried out at 28 oC for 2 h. Values are Mean ± SD (n = 3). C, Competition assay of OsNPF8.1-mediated uptake of β-Ala-Lys-AMCA by different di-/tri-peptides. The relative uptake of β-Ala-Lys-AMCA at 50 μmol/L by OsNPF8.1-expressing ptr2 cells in the presence of various competitive substrates at 50 μmol/L is shown. The uptake of β-Ala-Lys-AMCA in absence of competitors (control) was set as 1. The assay was carried out at 28 oC for 1 h. LL, Leu-Leu; WA, Trp-Ala; PL, Pro-Leu; AW, Ala-Trp; GHG, Gly-His-Gly; HS, His-Ser; TL, Thr-Leu; AA, Ala-Ala; AD, Ala-Asp; AK, Ala-Lys. Values are Mean ± SD (n = 3), and different lowercase letters above the columns indicate significant differences by one-way ANOVA with the Tukey’s test (P < 0.05). D, 15N-labeled Ala-Trp accumulation in yeast expressing OsNPF8.1. The uptake rates of 15N-labeled Ala-Trp by OsNPF8.1-expressing ptr2 cells are shown. The uptake assay was carried out at 28 oC for 1 h. The rate of Ala-Trp uptake was estimated by the net accumulation of the 15N stable isotope in yeast cells. Values are Mean ± SD (n = 3).
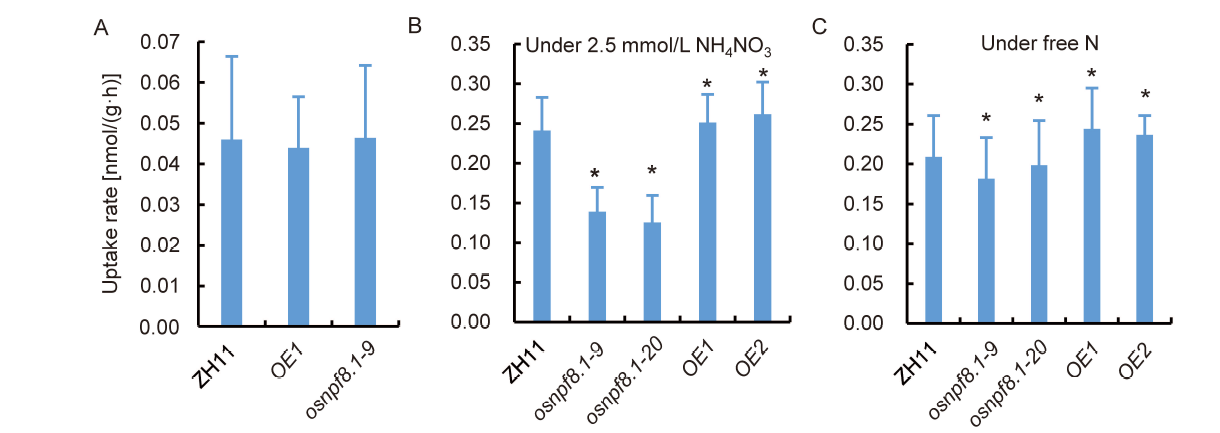
Fig. 2. OsNPF8.1 mediates uptake of β-Ala-Lys-N-7-amino-4-methylcoumarin-3-acetic acid (AMCA) in rice leaves. A, Uptake rates of β-Ala-Lys-AMCA in roots of 5 d germinating seeds under free N conditions. B and C, Leaf uptake rates of β-Ala-Lys-AMCA under normal N (B) and free N (C) treatments. OsNPF8.1 transgenic rice seedlings were grown in Yoshida solution for 2 weeks and then transferred for treatments with 2.5 mmol/L NH4NO3 (normal N) and free N for 3 d. Next, the third leaves of the rice seedlings grown in the treatment solution were used for detection of β-Ala-Lys-AMCA uptake for 4 h. ZH11, Wild type Zhonghua 11; OE, OsNPF8.1-over-expressing line; osnpf8.1, OsNPF8.1 knock-out mutant. Values are Mean ± SD, n = 12 roots in A and 18 seedling leaves in B and C for three repeats. Asterisk above the column indicates significant differences by the Student’s t-test (P < 0.05).

Fig. 3. Expression of OsNPF8.1 in wild type rice Zhonghua 11 (ZH11) plants under normal growth conditions. A-H, Activity of OsNPF8.1 promoter was revealed by β-glucuronidase (GUS) staining. GUS staining is shown in germinating seed (A); young root (E), the longitudinal (F) and vertical sections (G and H) of root from 10-day-old seedling; culm (B), the vertical section of leaf (C), and spikelet (D) at the heading stage. Scale bars are 1 mm in A, B, D and E, 100 μm in G, 10 μm in F and H, and 20 μm in C. LH, Lateral root hair; LR, Lateral root; EP, Epidermis; Ex, Exodermis; Sc, Sclerenchyma; Co, Cortex; Pe, Pericycle; LM, Late metaxylem; XY, Xylem; PL, Phloem; BS, Bundle sheath; UE, Upper epidermis. I, Expression of OsNPF8.1 in different organs. The expression of OsNPF8.1 in roots was set as 1. UBC was used as a housekeeping reference. Values are Mean ± SD (n = 6). BB, Basal part of bud outgrowth; AB, Axillary bud; LS, Leaf sheath; OL, Old leaf; NL, New leaf; Cu, Culm; OLR, Old leaf at the reproductive stage; FLR, Flag leaf; HP, Heading panicle; FP, Filling panicle.
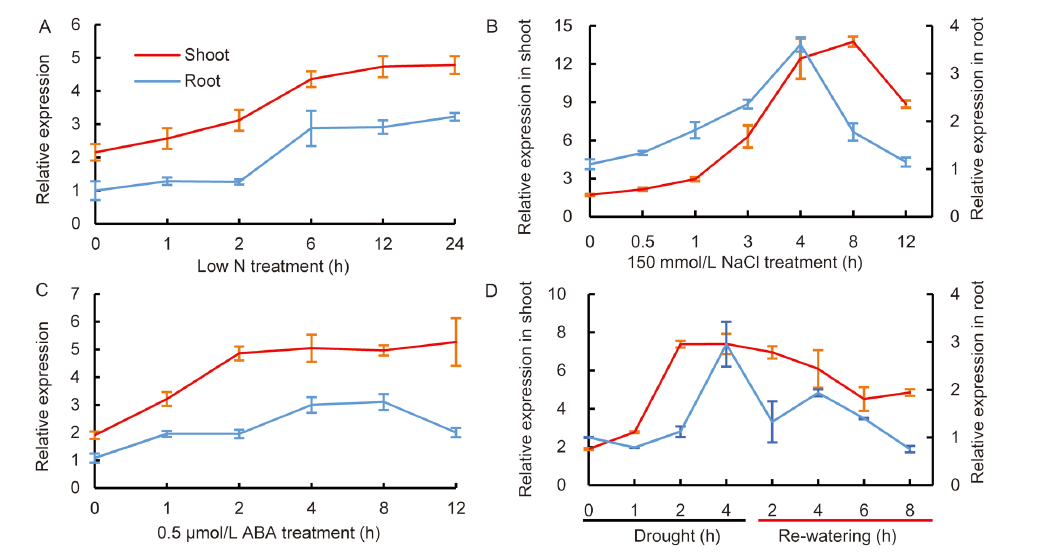
Fig. 4. Induced expression of OsNPF8.1 by low N and abiotic stresses. Results of qRT-PCR for OsNPF8.1 expression in shoots and roots of 2-week-old Zhonghua 11 (ZH11) seedlings grown in Yoshida solution with 0.25 mmol/L NH4NO3 (A), 150 mmol/L NaCl (B), 0.5 μmol/L abscisic acid (ABA) (C), or taken out of the water for 4 h and then re-watering (D). The expression of OsNPF8.1 in roots or shoots at the beginning of these treatments was set as 1. UBC was used as a housekeeping reference. Values are Mean ± SD (n = 6).

Fig. 5. Low N affects growth of OsNPF8.1 transgenic rice seedlings. Photos and biomass of seedlings grown in Yoshida solution with 2.5 mmol/L NH4NO3 (A and B), 1.0 mmol/L NH4NO3 (C and D), or 0.25 mmol/L NH4NO3 (E and F) for 2 weeks. ZH11, Zhonghua 11; OE, OsNPF8.1-over-expressing line; osnpf8.1, Knock-out mutants generated using the CRISPR/Cas9 editing system. Values are Mean ± SD (n = 30). Different lowercase letters above the column indicate significant differences by one-way ANOVA with the Tukey’s test (P < 0.05). Scale bars, 3 cm.
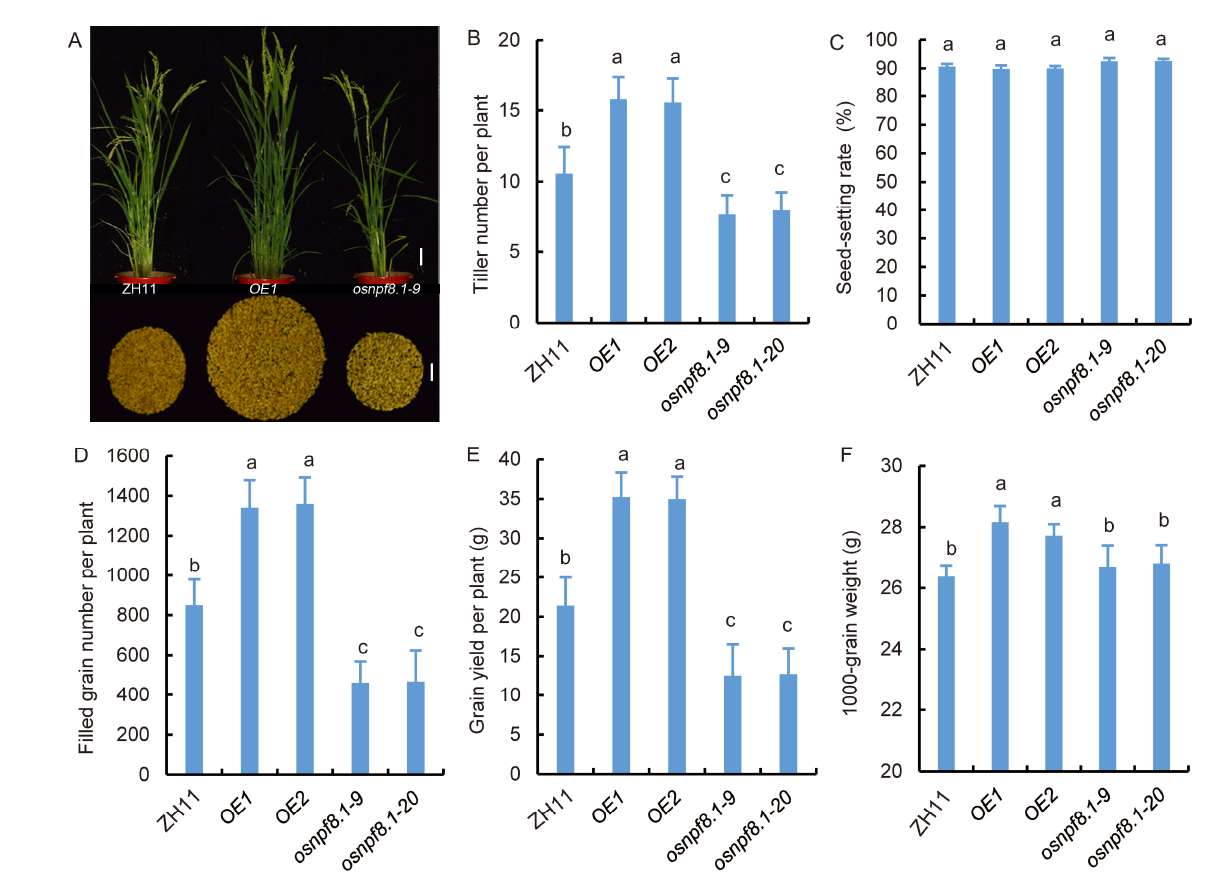
Fig. 6. Low N affects grain yield of OsNPF8.1 transgenic rice. A, Flowering plants and grains harvested from one plant. Scale bars are 5 cm for plants and 3 cm for grains. B, Number of tillers per plant. C, Seed- setting rate. D, Number of filled grains per plant. E, Grain yield per plant. F, 1000-grain weight. OsNPF8.1 transgenic rice plants were grown in a controlled paddy field without N fertilization. ZH11, Zhonghua 11; OE, OsNPF8.1-over-expressing line; osnpf8.1, OsNPF8.1 knock-out mutant. Values are Mean ± SD (n > 60). Different lowercase letters above the column indicate significant differences by one-way ANOVA with the Tukey’s test (P < 0.05).
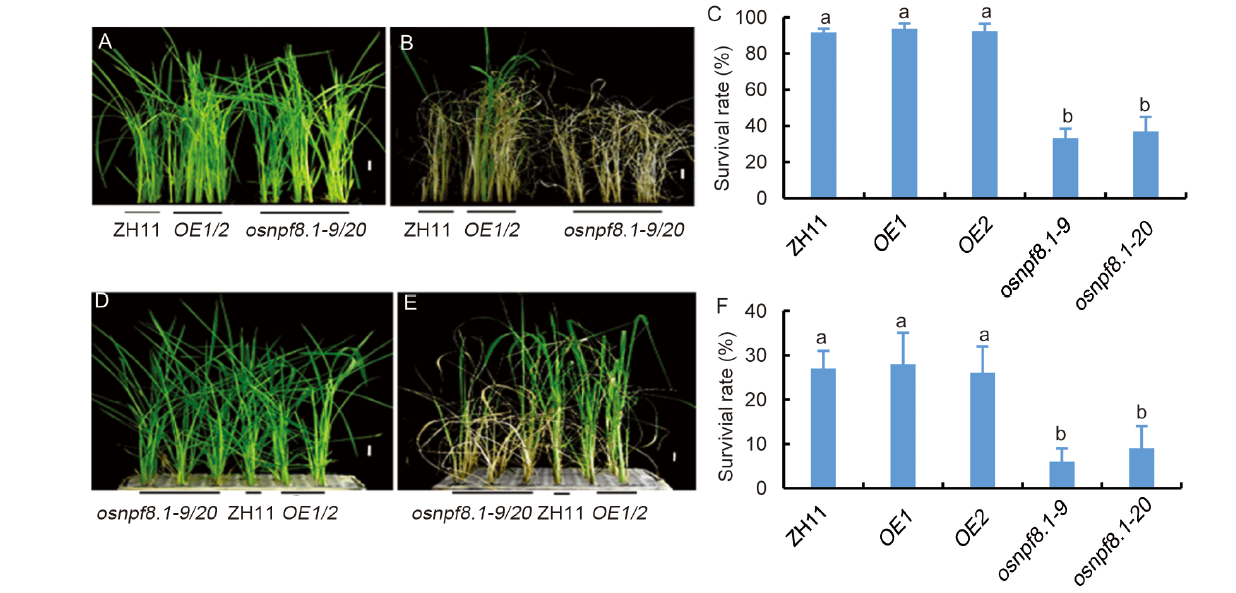
Fig. 7. Knock-out of OsNPF8.1 decreases tolerance of rice to drought and NaCl stresses. A-C, Photos and survival rates of OsNPF8.1 transgenic rice plants after drought treatment. Two-week-old seedlings (A) were taken out of Yoshida solution for 12 h, and then transferred into Yoshida solution for 1 week (B) to calculate the survival rate (C). D-F, Photos and survival rates of OsNPF8.1 transgenic rice plants after NaCl treatment. Two-week-old seedlings (D) were grown in Yoshida solution supplied with 150 mmol/L NaCl for 7 d (E) to calculate the survival rate (F). ZH11, Zhonghua 11; OE, OsNPF8.1-over-expressing line; osnpf8.1, OsNPF8.1 knock-out mutant. Values are Mean ± SD (n > 30). Different lowercase letters above the column indicate significant differences by one-way ANOVA with Tukey’s test (P < 0.05). Scale bars are 3 cm.
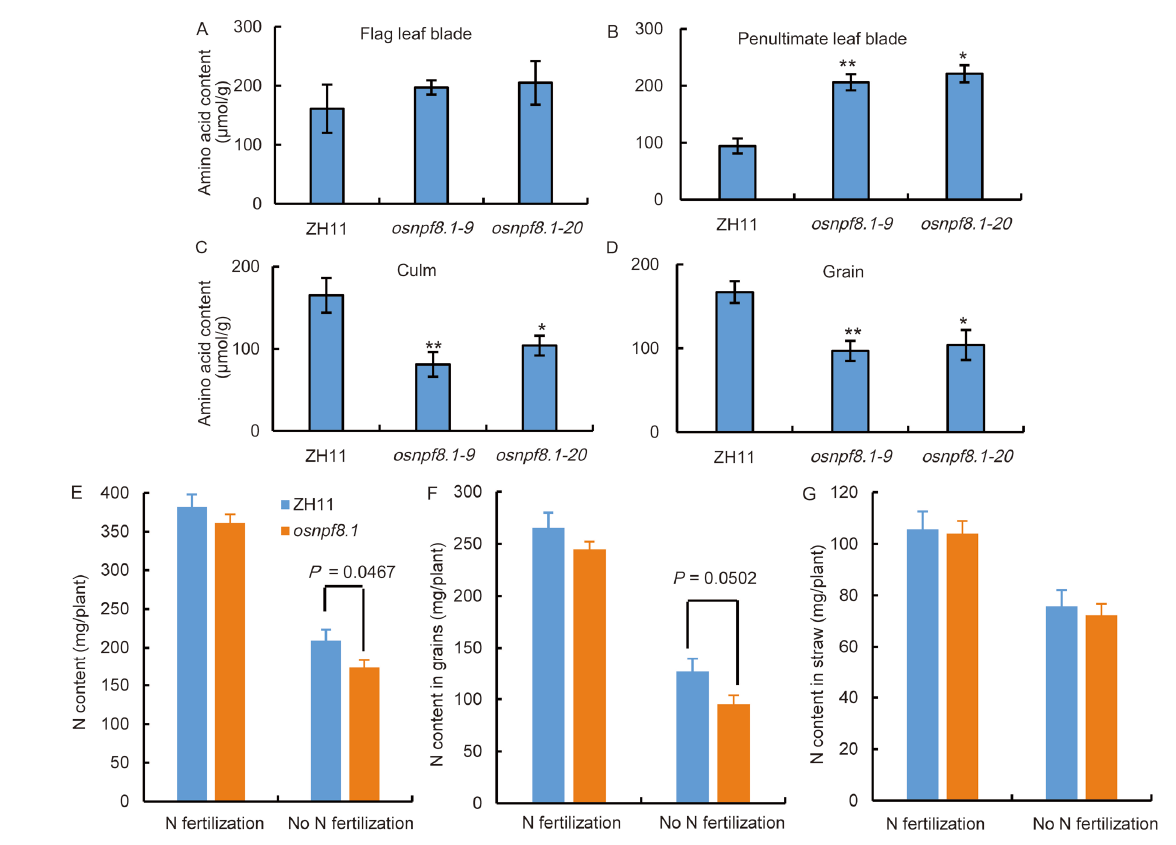
Fig. 8. Free amino acid contents (A-D) and N contents (E-G) in various organs of osnpf8.1 mutants and ZH11 plants. A-D, Flag leaf blades (A), penultimate leaf blades (B), culms (C), and grains (D) from filling plants (at 30 d after flowering) under N deficiency were sampled. The contents of free amino acids in these organs were determined through a ninhydrin assay. E-G, Total N content of whole plant (E), grains (F), straw of a plant (G) with normal N fertilization and without N fertilization. ZH11, Zhonghua 11; osnpf8.1, OsNPF8.1 knock-out mutant. Error bars indicate the SD (n = 6 plants for three repeats). * and ** denote significant differences from ZH11 at the 0.05 and 0.01 levels, respectively.
| [1] | Babst B A, Gao F, Acosta-Gamboa L M, Karve A, Schueller M J, Lorence A. 2019. Three NPF genes in Arabidopsis are necessary for normal nitrogen cycling under low nitrogen stress. Plant Physiol Biochem, 143: 1-10. |
| [2] | Bates L S, Waldren R P, Teare I D. 1973. Rapid determination of free proline for water-stress studies. Plant Soil, 39(1): 205-207. |
| [3] |
Bourbouloux A, Shahi P, Chakladar A, Delrot S, Bachhawat A K. 2000. Hgt1p, a high affinity glutathione transporter from the yeast Saccharomyces cerevisiae. J Biol Chem, 275(18): 13259-13265.
PMID |
| [4] | Cai H J, Kauffman S, Naider F, Becker J M. 2006. Genomewide screen reveals a wide regulatory network for di/tripeptide utilization in Saccharomyces cerevisiae. Genetics, 172(3): 1459-1476. |
| [5] | Chen C Z, Lv X F, Li J Y, Yi H Y, Gong J M. 2012. Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol, 159(4): 1582-1590. |
| [6] | Chen K E, Chen H Y, Tseng C S, Tsay Y F. 2020. Improving nitrogen use efficiency by manipulating nitrate remobilization in plants. Nat Plants, 6(9): 1126-1135. |
| [7] | Chiang C S, Stacey G, Tsay Y F. 2004. Mechanisms and functional properties of two peptide transporters, AtPTR2 and fPTR2. J Biol Chem, 279(29): 30150-30157. |
| [8] | Choi M G, Kim E J, Song J Y, Choi S B, Cho S W, Park C S, Kang C S, Park Y I. 2020. Peptide transporter2 (PTR2) enhances water uptake during early seed germination in Arabidopsis thaliana. Plant Mol Biol, 102(6): 615-624. |
| [9] |
Corratgé-Faillie C, Lacombe B. 2017. Substrate (un)specificity of Arabidopsis NRT1/PTR FAMILY (NPF) proteins. J Exp Bot, 68(12): 3107-3113.
PMID |
| [10] |
Dieck S T, Heuer H, Ehrchen J, Otto C, Bauer K. 1999. The peptide transporter PepT2 is expressed in rat brain and mediates the accumulation of the fluorescent dipeptide derivative beta-Ala- Lys-Nɛ-AMCA in astrocytes. Glia, 25(1): 10-20.
PMID |
| [11] |
Dietrich D, Hammes U, Thor K, Suter-Grotemeyer M, Flückiger R, Slusarenko A J, Ward J M, Rentsch D. 2004. AtPTR1, a plasma membrane peptide transporter expressed during seed germination and in vascular tissue of Arabidopsis. Plant J, 40(4): 488-499.
PMID |
| [12] | Fan S C, Lin C S, Hsu P K, Lin S H, Tsay Y F. 2009. The Arabidopsis nitrate transporter NRT1.7, expressed in phloem, is responsible for source-to-sink remobilization of nitrate. Plant Cell, 21(9): 2750-2761. |
| [13] |
Fan T, Yang W, Zeng X, Xu X L, Xu Y L, Fan X R, Luo M, Tian C G, Xia K F, Zhang M Y. 2020. A rice autophagy gene OsATG8b is involved in nitrogen remobilization and control of grain quality. Front Plant Sci, 11: 588.
PMID |
| [14] | Fan X R, Xie D, Chen J G, Lu H Y, Xu Y L, Ma C, Xu G H. 2014. Over-expression of OsPTR6 in rice increased plant growth at different nitrogen supplies but decreased nitrogen use efficiency at high ammonium supply. Plant Sci, 227: 1-11. |
| [15] | Fang Z M, Xia K F, Yang X, Grotemeyer M S, Meier S, Rentsch D, Xu X L, Zhang M Y. 2013. Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol J, 11(4): 446-458. |
| [16] | Fang Z M, Bai G X, Huang W T, Wang Z X, Wang X L, Zhang M Y. 2017. The rice peptide transporter OsNPF7.3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front Plant Sci, 8: 1338. |
| [17] | Fei Y J, Ganapathy V, Leibach F H. 1998. Molecular and structural features of the proton-coupled oligopeptide transporter superfamily. Prog Nucleic Acid Res Mol Biol, 58: 239-261. |
| [18] | Granell A, Cercos M, Carbonell J. 1998. Plant cysteine proteinases in germination and senescence. In: Barrett A J, Rawlings N D, Woessner J F. Handbook of Proteolytic Enzymes. San Diego, London, UK: Academic Press: 578-583. |
| [19] | Guan Y, Liu D F, Qiu J, Liu Z J, He Y N, Fang Z J, Huang X H, Gong J M. 2022. The nitrate transporter OsNPF7.9 mediates nitrate allocation and the divergent nitrate use efficiency between indica and japonica rice. Plant Physiol, 189(1): 215-229. |
| [20] |
Hiei Y, Komari T, Kubo T. 1997. Transformation of rice mediated by Agrobacterium tumefaciens. Plant Mol Biol, 35: 205-218.
PMID |
| [21] |
Higgins C F, Payne J W. 1978. Peptide transport by germinating barley embryos: Uptake of physiological di- and oligopeptides. Planta, 138(3): 211-215.
PMID |
| [22] |
Higgins C F, Payne J W. 1981. The peptide pools of germinating barley grains: Relation to hydrolysis and transport of storage proteins. Plant Physiol, 67(4): 785-792.
PMID |
| [23] | Hu B, Wang W, Ou S J, Tang J Y, Li H, Che R H, Zhang Z H, Chai X Y, Wang H R, Wang Y Q, Liang C Z, Liu L C, Piao Z Z, Deng Q Y, Deng K, Xu C, Liang Y, Zhang L H, Li L G, Chu C C. 2015. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat Genet, 47(7): 834-838. |
| [24] |
Island M D, Perry J R, Naider F, Becker J M. 1991. Isolation and characterization of S. cerevisiae mutants deficient in amino acid-inducible peptide transport. Curr Genet, 20(6): 457-463.
PMID |
| [25] |
Ito K, Hikida A, Kawai S, Lan V T T, Motoyama T, Kitagawa S, Yoshikawa Y, Kato R, Kawarasaki Y. 2013. Analysing the substrate multispecificity of a proton-coupled oligopeptide transporter using a dipeptide library. Nat Commun, 4: 2502.
PMID |
| [26] | Jain M, Nijhawan A, Tyagi A K, Khurana J P. 2006. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem Biophys Res Commun, 345(2): 646-651. |
| [27] |
Jamai A, Chollet J F, Delrot S. 1994. Proton-peptide co-transport in broad bean leaf tissues. Plant Physiol, 106(3): 1023-1031.
PMID |
| [28] | Jensen J M, Simonsen F C, Mastali A, Hald H, Lillebro I, Diness F, Olsen L, Mirza O. 2012. Biophysical characterization of the proton- coupled oligopeptide transporter YjdL. Peptides, 38(1): 89-93. |
| [29] | Jones L J, Haugland R P, Singer V L. 2003. Development and characterization of the NanoOrange® protein quantitation assay: A fluorescence-based assay of proteins in solution. BioTechniques, 34(4): 850-861. |
| [30] |
Kamachi K, Yamaya T, Mae T, Ojima K. 1991. A role for glutamine synthetase in the remobilization of leaf nitrogen during natural senescence in rice leaves. Plant Physiol, 96(2): 411-417.
PMID |
| [31] | Karim S, Holmström K O, Mandal A, Dahl P, Hohmann S, Brader G, Palva E T, Pirhonen M. 2007. AtPTR3, a wound-induced peptide transporter needed for defence against virulent bacterial pathogens in Arabidopsis. Planta, 225(6): 1431-1445. |
| [32] |
Komarova N Y, Thor K, Gubler A, Meier S, Dietrich D, Weichert A, Suter Grotemeyer M, Tegeder M, Rentsch D. 2008. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol, 148(2): 856-869.
PMID |
| [33] |
Léran S, Varala K, Boyer J C, Chiurazzi M, Crawford N, Daniel- Vedele F, David L, Dickstein R, Fernandez E, Forde B, Gassmann W, Geiger D, Gojon A, Gong J M, Halkier B A, Harris J M, Hedrich R, Limami A M, Rentsch D, Seo M, Tsay Y F, Zhang M Y, Coruzzi G, Lacombe B. 2014. A unified nomenclature of nitrate transporter 1/peptide transporter family members in plants. Trends Plant Sci, 19(1): 5-9.
PMID |
| [34] | Li F Q, Chung T, Pennington J G, Federico M L, Kaeppler H F, Kaeppler S M, Otegui M S, Vierstra R D. 2015. Autophagic recycling plays a central role in maize nitrogen remobilization. Plant Cell, 27(5): 1389-1408. |
| [35] |
Lin C M, Koh S, Stacey G, Yu S M, Lin T Y, Tsay Y F. 2000. Cloning and functional characterization of a constitutively expressed nitrate transporter gene, OsNRT1, from rice. Plant Physiol, 122(2): 379-388.
PMID |
| [36] | Ma X L, Zhang Q Y, Zhu Q L, Liu W, Chen Y, Qiu R, Wang B, Yang Z F, Li H Y, Lin Y R, Xie Y Y, Shen R X, Chen S F, Wang Z, Chen Y L, Guo J X, Chen L T, Zhao X C, Dong Z C, Liu Y G. 2015. A robust CRISPR/Cas9 system for convenient, high- efficiency multiplex genome editing in monocot and dicot plants. Mol Plant, 8(8): 1274-1284. |
| [37] | Mae T, Ohira K. 1981. The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol, 22(6): 1067-1074. |
| [38] | Mae T, Ohira K. 1984. The relationship between proteolytic activity and loss of soluble protein in rice leaves from anthesis through senescence. Soil Sci Plant Nutr, 30(3): 427-434. |
| [39] | Mae T, Hoshino T. 1985. Proteinase activities and loss of nitrogen in the senescing leaves of field-grown rice (Oryza sativa L.). Soil Sci Plant Nutr, 31(4): 589-600. |
| [40] |
Makino A. 2011. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol, 155(1): 125-129.
PMID |
| [41] |
Mao C J, Lu S C, Lv B, Zhang B, Shen J B, He J M, Luo L Q, Xi D D, Chen X, Ming F. 2017. A rice NAC transcription factor promotes leaf senescence via ABA biosynthesis. Plant Physiol, 174(3): 1747-1763.
PMID |
| [42] | Masclaux C, Quillere I, Gallais A, Hirel B. 2001. The challenge of remobilisation in plant nitrogen economy: A survey of physio- agronomic and molecular approaches. Ann Appl Biol, 138(1): 69-81. |
| [43] | Masclaux-Daubresse C, Reisdorf-Cren M, Orsel M. 2008. Leaf nitrogen remobilisation for plant development and grain filling. Plant Biol, 10: 23-36. |
| [44] | Masclaux-Daubresse C, Daniel-Vedele F, Dechorgnat J, Chardon F, Gaufichon L, Suzuki A. 2010. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann Bot, 105(7): 1141-1157. |
| [45] |
Melcher K. 2000. A modular set of prokaryotic and eukaryotic expression vectors. Anal Biochem, 277(1): 109-120.
PMID |
| [46] |
Miranda M, Borisjuk L, Tewes A, Dietrich D, Rentsch D, Weber H, Wobus U. 2003. Peptide and amino acid transporters are differentially regulated during seed development and germination in Faba bean. Plant Physiol, 132(4): 1950-1960.
PMID |
| [47] |
Moore S. 1968. Amino acid analysis: Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction. J Biol Chem, 243(23): 6281-6283.
PMID |
| [48] |
Okumoto S, Pilot G. 2011. Amino acid export in plants: A missing link in nitrogen cycling. Mol Plant, 4(3): 453-463.
PMID |
| [49] | Ouyang J, Cai Z Y, Xia K F, Wang Y Q, Duan J, Zhang M Y. 2010. Identification and analysis of eight peptide transporter homologs in rice. Plant Sci, 179(4): 374-382. |
| [50] |
Paungfoo-Lonhienne C, Schenk P M, Lonhienne T G A, Brackin R, Meier S, Rentsch D, Schmidt S. 2009. Nitrogen affects cluster root formation and expression of putative peptide transporters. J Exp Bot, 60(9): 2665-2676.
PMID |
| [51] | Rakotoson T, Dusserre R, Letourmy P, Frouin J, Ratsimiala I S, Rakotoarisoa N V, Cao T, Brocke K V, Ramanantsoanirina A, Ahmadi N, Raboin L. 2021. Genome-wide association study of nitrogen use efficiency and agronomic traits in upland rice. Rice Sci, 28(4): 379-390. |
| [52] |
Rentsch D, Schmidt S, Tegeder M. 2007. Transporters for uptake and allocation of organic nitrogen compounds in plants. FEBS Lett, 581(12): 2281-2289.
PMID |
| [53] |
Roberts I N, Caputo C, Criado M V, Funk C. 2012. Senescence- associated proteases in plants. Physiol Plant, 145(1): 130-139.
PMID |
| [54] |
Salmenkallio M, Sopanen T. 1989. Amino acid and peptide uptake in the Scutella of germinating grains of barley, wheat, rice, and maize. Plant Physiol, 89(4): 1285-1291.
PMID |
| [55] | Shimizu T, Kanno Y, Suzuki H, Watanabe S, Seo M. 2021. Arabidopsis NPF4.6 and NPF5.1 control leaf stomatal aperture by regulating abscisic acid transport. Genes, 12(6): 885. |
| [56] |
Solcan N, Kwok J, Fowler P W, Cameron A D, Drew D, Iwata S, Newstead S. 2012. Alternating access mechanism in the POT family of oligopeptide transporters. EMBO J, 31(16): 3411-3421.
PMID |
| [57] |
Song W, Koh S, Czako M, Marton L, Drenkard E, Becker J M, Stacey G. 1997. Antisense expression of the peptide transport gene AtPTR2-B delays flowering and arrests seed development in transgenic Arabidopsis plants. Plant Physiol, 114(3): 927-935.
PMID |
| [58] |
Sopanen T. 1979. Development of peptide transport activity in barley Scutellum during germination. Plant Physiol, 64(4): 570-574.
PMID |
| [59] |
Stacey G, Koh S, Granger C, Becker J M. 2002. Peptide transport in plants. Trends Plant Sci, 7(6): 257-263.
PMID |
| [60] |
Steiner H Y, Song W, Zhang L, Naider F, Becker J M, Stacey G. 1994. An Arabidopsis peptide transporter is a member of a new class of membrane transport proteins. Plant Cell, 6(9): 1289-1299.
PMID |
| [61] |
Tang Z, Chen Y, Chen F, Ji Y C, Zhao F J. 2017. OsPTR7 (OsNPF8.1), a putative peptide transporter in rice, is involved in dimethylarsenate accumulation in rice grain. Plant Cell Physiol, 58(5): 904-913.
PMID |
| [62] | Vittozzi Y, Nadzieja M, Rogato A, Radutoiu S, Valkov V T, Chiurazzi M. 2021. The Lotus japonicus NPF3.1 is a nodule- induced gene that plays a positive role in nodule functioning. Front Plant Sci, 12: 688187. |
| [63] |
Wang Q, Huang Y G, Ren Z J, Zhang X X, Ren J, Su J Q, Zhang C, Tian J, Yu Y J, Gao G F, Li L G, Kong Z S. 2020. Transfer cells mediate nitrate uptake to control root nodule symbiosis. Nat Plants, 6(7): 800-808.
PMID |
| [64] | Watanabe S, Takahashi N, Kanno Y, Suzuki H, Aoi Y, Takeda- Kamiya N, Toyooka K, Kasahara H, Hayashi K I, Umeda M, Seo M. 2020. The Arabidopsis NRT1/PTR FAMILY protein NPF7.3/NRT1.5 is an indole-3-butyric acid transporter involved in root gravitropism. Proc Natl Acad Sci USA, 117(49): 31500-31509. |
| [65] |
West C E, Waterworth W M, Stephens S M, Smith C P, Bray C M. 1998. Cloning and functional characterisation of a peptide transporter expressed in the scutellum of barley grain during the early stages of germination. Plant J, 15(2): 221-229.
PMID |
| [66] |
Winspear M J, Preston K R, Rastogi V, Oaks A. 1984. Comparisons of peptide hydrolase activities in cereals. Plant Physiol, 75(2): 480-482.
PMID |
| [67] |
Wu H, Xiang J, Zhang Y P, Zhang Y K, Peng S B, Chen H Z, Zhu D F. 2018. Effects of post-anthesis nitrogen uptake and translocation on photosynthetic production and rice yield. Sci Rep, 8(1): 12891.
PMID |
| [68] | Yoshida S, Forno D, Cock J, Gomez K. 1976. Laboratory Manual for Physiological Studies of Rice. Manila, the Philippines: International Rice Research Institute. |
| [69] | Yu J L, Zhen X X, Li X, Li N, Xu F. 2019. Increased autophagy of rice can increase yield and nitrogen use efficiency (NUE). Front Plant Sci, 10: 584. |
| [70] | Zhao X B, Huang J Y, Yu H H, Wang L, Xie W B. 2010. Genomic survey, characterization and expression profile analysis of the peptide transporter family in rice (Oryza sativa L.). BMC Plant Biol, 10: 92. |
| [1] | Prathap V, Suresh KUMAR, Nand Lal MEENA, Chirag MAHESHWARI, Monika DALAL, Aruna TYAGI. Phosphorus Starvation Tolerance in Rice Through a Combined Physiological, Biochemical and Proteome Analysis [J]. Rice Science, 2023, 30(6): 8-. |
| [2] | Serena REGGI, Elisabetta ONELLI, Alessandra MOSCATELLI, Nadia STROPPA, Matteo Dell’ANNO, Kiril PERFANOV, Luciana ROSSI. Seed-Specific Expression of Apolipoprotein A-IMilano Dimer in Rice Engineered Lines [J]. Rice Science, 2023, 30(6): 6-. |
| [3] | Sundus ZAFAR, XU Jianlong. Recent Advances to Enhance Nutritional Quality of Rice [J]. Rice Science, 2023, 30(6): 4-. |
| [4] | Kankunlanach KHAMPUANG, Nanthana CHAIWONG, Atilla YAZICI, Baris DEMIRER, Ismail CAKMAK, Chanakan PROM-U-THAI. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 9-. |
| [5] | FAN Fengfeng, CAI Meng, LUO Xiong, LIU Manman, YUAN Huanran, CHENG Mingxing, Ayaz AHMAD, LI Nengwu, LI Shaoqing. Novel QTLs from Wild Rice Oryza longistaminata Confer Rice Strong Tolerance to High Temperature at Seedling Stage [J]. Rice Science, 2023, 30(6): 14-. |
| [6] | LIN Shaodan, YAO Yue, LI Jiayi, LI Xiaobin, MA Jie, WENG Haiyong, CHENG Zuxin, YE Dapeng. Application of UAV-Based Imaging and Deep Learning in Assessment of Rice Blast Resistance [J]. Rice Science, 2023, 30(6): 10-. |
| [7] | Md. Forshed DEWAN, Md. AHIDUZZAMAN, Md. Nahidul ISLAM, Habibul Bari SHOZIB. Potential Benefits of Bioactive Compounds of Traditional Rice Grown in South and South-East Asia: A Review [J]. Rice Science, 2023, 30(6): 5-. |
| [8] | Raja CHAKRABORTY, Pratap KALITA, Saikat SEN. Phenolic Profile, Antioxidant, Antihyperlipidemic and Cardiac Risk Preventive Effect of Chakhao Poireiton (A Pigmented Black Rice) in High-Fat High-Sugar induced Rats [J]. Rice Science, 2023, 30(6): 11-. |
| [9] | LI Qianlong, FENG Qi, WANG Heqin, KANG Yunhai, ZHANG Conghe, DU Ming, ZHANG Yunhu, WANG Hui, CHEN Jinjie, HAN Bin, FANG Yu, WANG Ahong. Genome-Wide Dissection of Quan 9311A Breeding Process and Application Advantages [J]. Rice Science, 2023, 30(6): 7-. |
| [10] | JI Dongling, XIAO Wenhui, SUN Zhiwei, LIU Lijun, GU Junfei, ZHANG Hao, Tom Matthew HARRISON, LIU Ke, WANG Zhiqin, WANG Weilu, YANG Jianchang. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 12-. |
| [11] | Nazaratul Ashifa Abdullah Salim, Norlida Mat Daud, Julieta Griboff, Abdul Rahim Harun. Elemental Assessments in Paddy Soil for Geographical Traceability of Rice from Peninsular Malaysia [J]. Rice Science, 2023, 30(5): 486-498. |
| [12] | Monica Ruffini Castiglione, Stefania Bottega, Carlo Sorce, Carmelina SpanÒ. Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa [J]. Rice Science, 2023, 30(5): 449-458. |
| [13] | Tan Jingyi, Zhang Xiaobo, Shang Huihui, Li Panpan, Wang Zhonghao, Liao Xinwei, Xu Xia, Yang Shihua, Gong Junyi, Wu Jianli. ORYZA SATIVA SPOTTED-LEAF 41 (OsSPL41) Negatively Regulates Plant Immunity in Rice [J]. Rice Science, 2023, 30(5): 426-436. |
| [14] | Ammara Latif, Sun Ying, Pu Cuixia, Noman Ali. Rice Curled Its Leaves Either Adaxially or Abaxially to Combat Drought Stress [J]. Rice Science, 2023, 30(5): 405-416. |
| [15] | Liu Qiao, Qiu Linlin, Hua Yangguang, Li Jing, Pang Bo, Zhai Yufeng, Wang Dekai. LHD3 Encoding a J-Domain Protein Controls Heading Date in Rice [J]. Rice Science, 2023, 30(5): 437-448. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||