
Rice Science ›› 2025, Vol. 32 ›› Issue (5): 658-672.DOI: 10.1016/j.rsci.2025.06.001
• Research Papers • Previous Articles Next Articles
Wu Zhaozhong1,#, Zhong Zhengzheng1,#, Xu Peng1,2,#, Liu Ling1, Wang Beifang1,2, Yang Qinqin1, Wen Xiaoxia1, Ma Guifang1, Luo Mili1, Zhang Yingxin1, Liu Qun’en1, Peng Zequn1, Zhan Xiaodeng1, Cao Liyong1,2( ), Cheng Shihua1(
), Cheng Shihua1( ), Wu Weixun1(
), Wu Weixun1( )
)
Received:2025-01-27
Accepted:2025-06-20
Online:2025-09-28
Published:2025-10-11
Contact:
Wu Weixun (About author:#These authors contributed equally to this work
Wu Zhaozhong, Zhong Zhengzheng, Xu Peng, Liu Ling, Wang Beifang, Yang Qinqin, Wen Xiaoxia, Ma Guifang, Luo Mili, Zhang Yingxin, Liu Qun’en, Peng Zequn, Zhan Xiaodeng, Cao Liyong, Cheng Shihua, Wu Weixun. OsELF3.1-OsCATA-Ghd7 Pathway Regulates Rice Heading[J]. Rice Science, 2025, 32(5): 658-672.
Add to citation manager EndNote|Ris|BibTeX
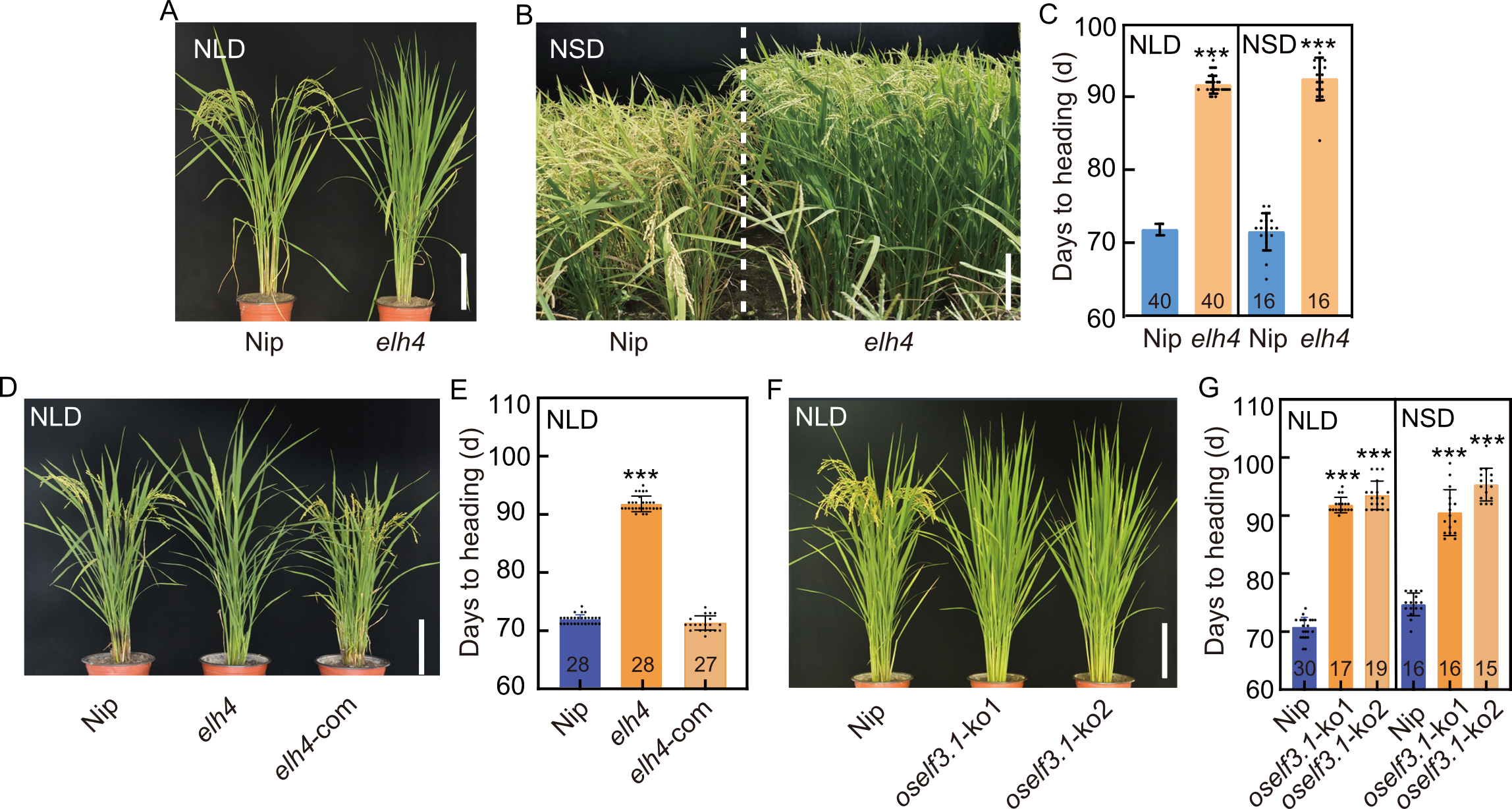
Fig. 1. Role of OsELF3.1 in rice heading. A and B, Phenotypes of Nipponbare (Nip, wild type) and elh4 mutant under natural long-day (NLD) conditions in Hangzhou, Zhejiang Province, China (A), and natural short-day (NSD) conditions in Lingshui, Hainan Province, China (B). Scale bar in A and B are 20 and 30 cm, respectively. C, Days to heading comparisons for Nip and elh4 under NLD and NSD conditions. D and E, Phenotypic analysis of Nip, elh4, and elh4 complementation (elh4-com) lines under NLD conditions (D) and corresponding heading date statistics (E). Scale bar in D is 20 cm. F and G, Phenotypic identification (F) and heading date statistics (G) for oself3.1 knockout lines (oself3.1-ko1 and oself3.1-ko2) under NLD and NSD conditions. Scale bar in F is 20 cm. In C, E, and G, data are mean ± SD (n values shown in columns). *** above bars indicate statistically significant differences at the 0.001 level by Student’s t test.
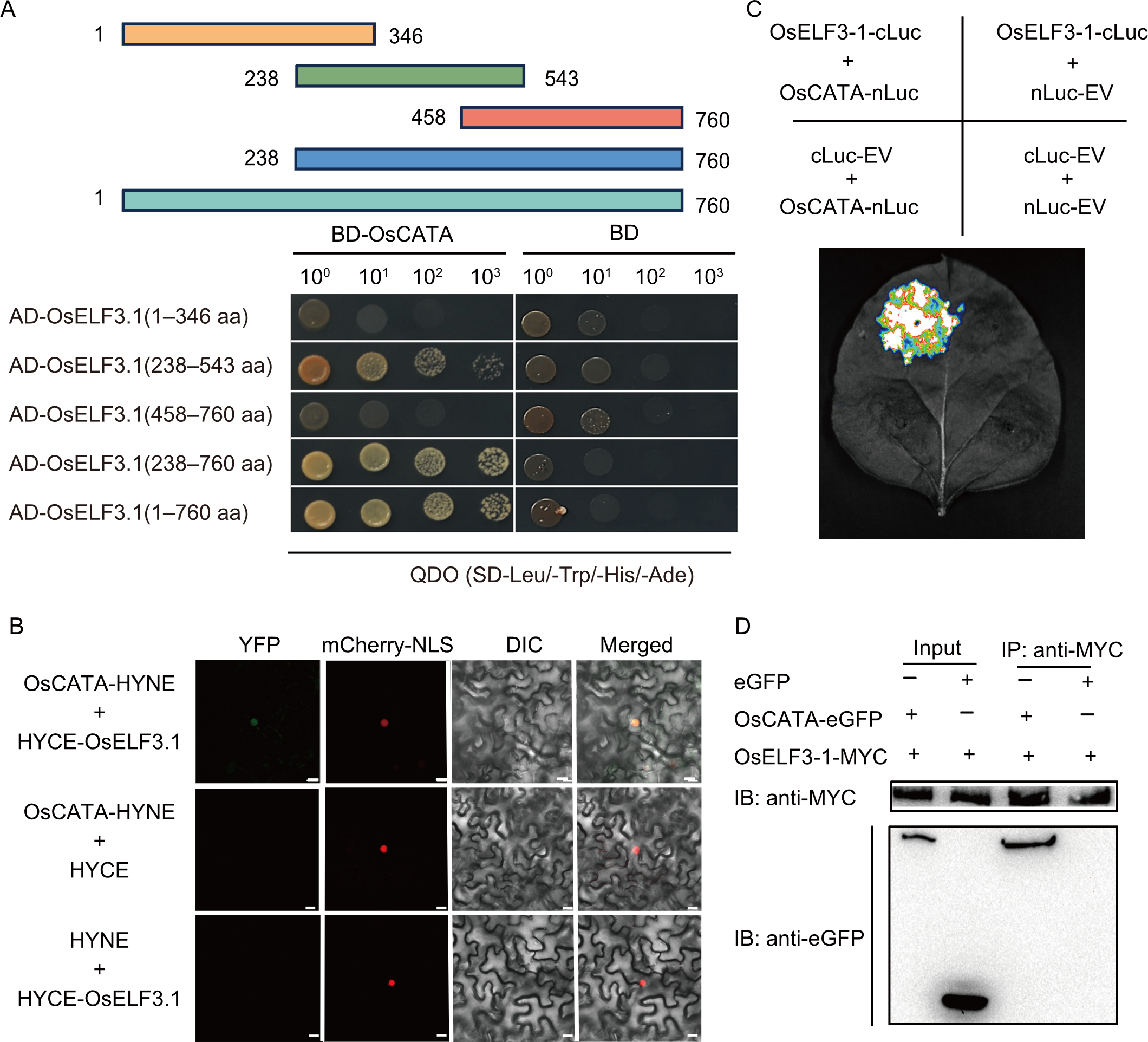
Fig. 2. Interaction between OsELF3.1 and OsCATA. A, Yeast two-hybrid (Y2H) assays reveal interactions between OsELF3.1 and OsCATA. OsELF3.1 and its truncated fragments served as prey against bait constructs, with co-transformed Y2H Gold yeast cells assessed on QDO (SD-Leu/-Trp/-His/-Ade) media. AD, GAL4 activation domain; BD, GAL4 DNA-binding domain. B, Bimolecular fluorescence complementation (BiFC) assays validate the interaction. Constructs were agroinfiltrated into Nicotiana benthamiana, and fluorescence signals were detected 4 d post-infiltration using a confocal microscope (Zeiss 700). mCherry-NLS served as a nuclear marker. Scale bars, 20 µm. YFP, Yellow fluorescent protein; DIC, Differential interference contrast. C, Luminescence complementation imaging (LCI) assays confirm the interaction of OsELF3.1 with OsCATA. Constructs were transiently expressed in N. benthamiana, with nLuc and cLuc as negative controls. Luminescence was visualized using a low-light, cooled charge-coupled device (CCD) camera. cLuc, C-terminal fragment of luciferase; nLuc, N-terminal fragment of luciferase; EV, Empty vector. D, Co-immunoprecipitation (Co-IP) assays demonstrate interaction in rice protoplasts co-transfected with OsELF3.1 and OsCATA. Immunoprecipitation was performed with anti-MYC sepharose beads, and proteins were detected using anti-GFP and anti-MYC antibodies.

Fig. 3. Phenotypes of oscata knockout and oself3.1 oscata double mutants. A, CRISPR-induced mutations in oscata. Protospacer adjacent motif (PAM) sequences are shown in orange, with mutation sites in red tangerine. B and C, Phenotypes of Nipponbare (Nip, wild type) and oscata knockout mutants (oscata-ko) under natural long-day (NLD) conditions at the heading stage of Nip (B) and days to heading (C) of Nip and oscata-ko mutants under NLD and natural short-day (NSD) conditions. Scale bar in B is 20 cm. D, Phenotypes were assessed of Nip and oscata-ko mutants at 60 d post-heading of Nip under NLD conditions. Scale bar, 20 cm. E, CRISPR-induced mutations in oself3.1 oscata double mutants (oself3.1 oscata-ko1 and oself3.1 oscata-ko2). PAM sequences are shown in orange, with mutation sites in red. F and G, Phenotypes (F) and days to heading (G) of Nip and oslelf3.1 oscata double mutants (oself3.1 oscata-ko1 and oself3.1 oscata-ko2) under NLD conditions. Scal bar in F is 20 cm. H and I, Phenotypes were assessed in Nip, oslelf3.1 mutants (oslelf3.1-ko1 and oslelf3.1-ko2, H), and oslelf3.1 oscata double mutants (oself3.1 oscata-ko1 and oself3.1 oscata-ko2, I) at 60 d post-heading under NLD conditions. Scale bars, 20 cm. In C and G, data are mean ± SD (n values are shown in columns). ***, Significant differences at the 0.001 levels by Student’s t test.
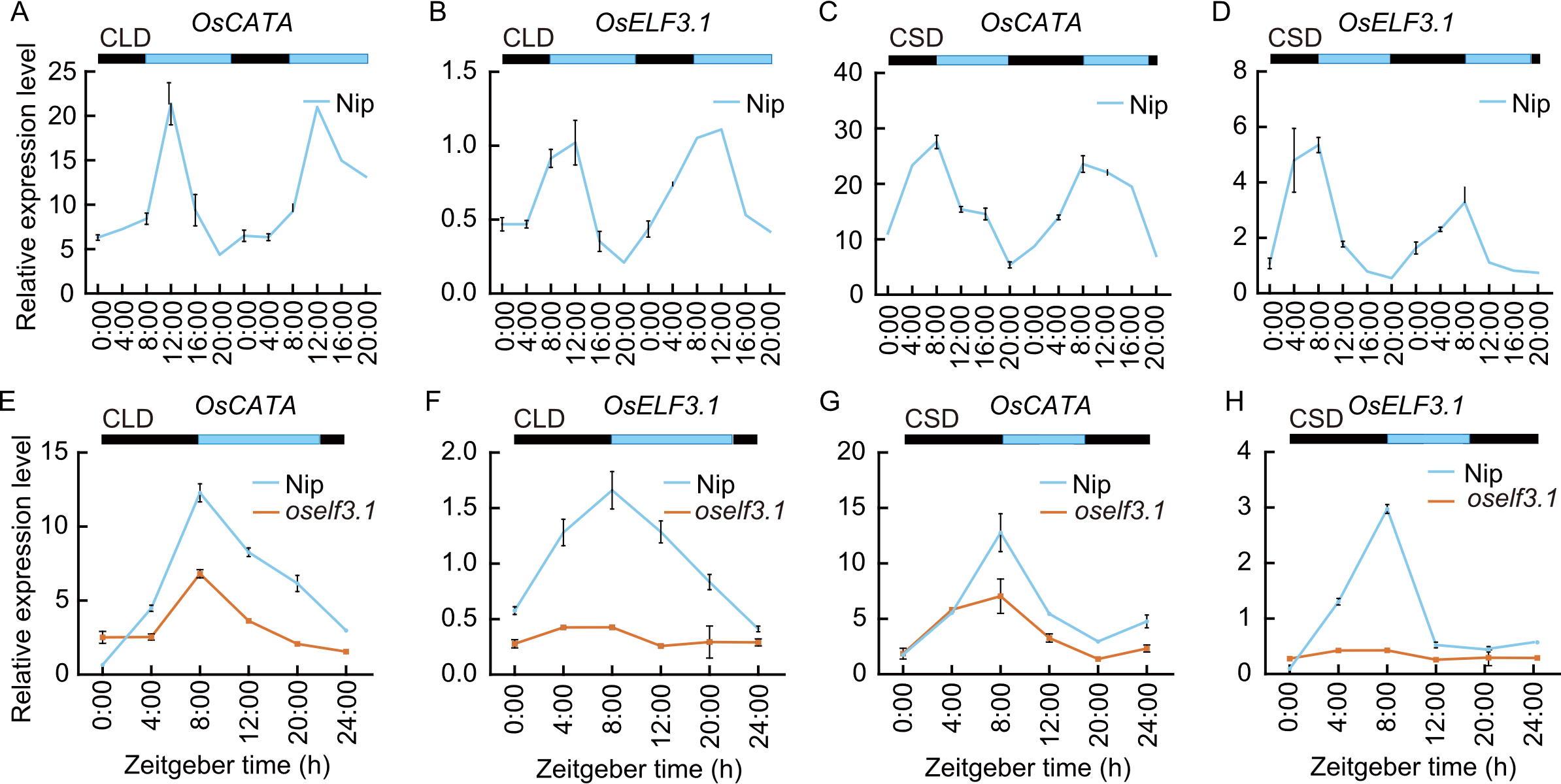
Fig. 4. Diurnal expression patterns of OsCATA and OsELF3.1. A-D, qRT-PCR analysis of OsCATA and OsELF3.1 in leaves of Nipponbare (Nip) plants under controlled long-day (CLD, A and B) and controlled short-day (CSD, C and D) conditions. E-H, Expression profiles of OsCATA and OsELF3.1 in leaves of Nip and oself3.1 plants under CLD (E and F) and CSD (G and H). Leaf samples were collected at 50 d after sowing in CLD (A, B, E, and F) and 40 d after sowing in CSD (C, D, G, and H) conditions. Transcript levels were normalized to ubiquitin (Os03g0234350), with data as mean ± SD (n = 3). Zeitgeber time (ZT) defines light onset (ZT8). Light and dark periods are represented by blue and black bars, respectively.
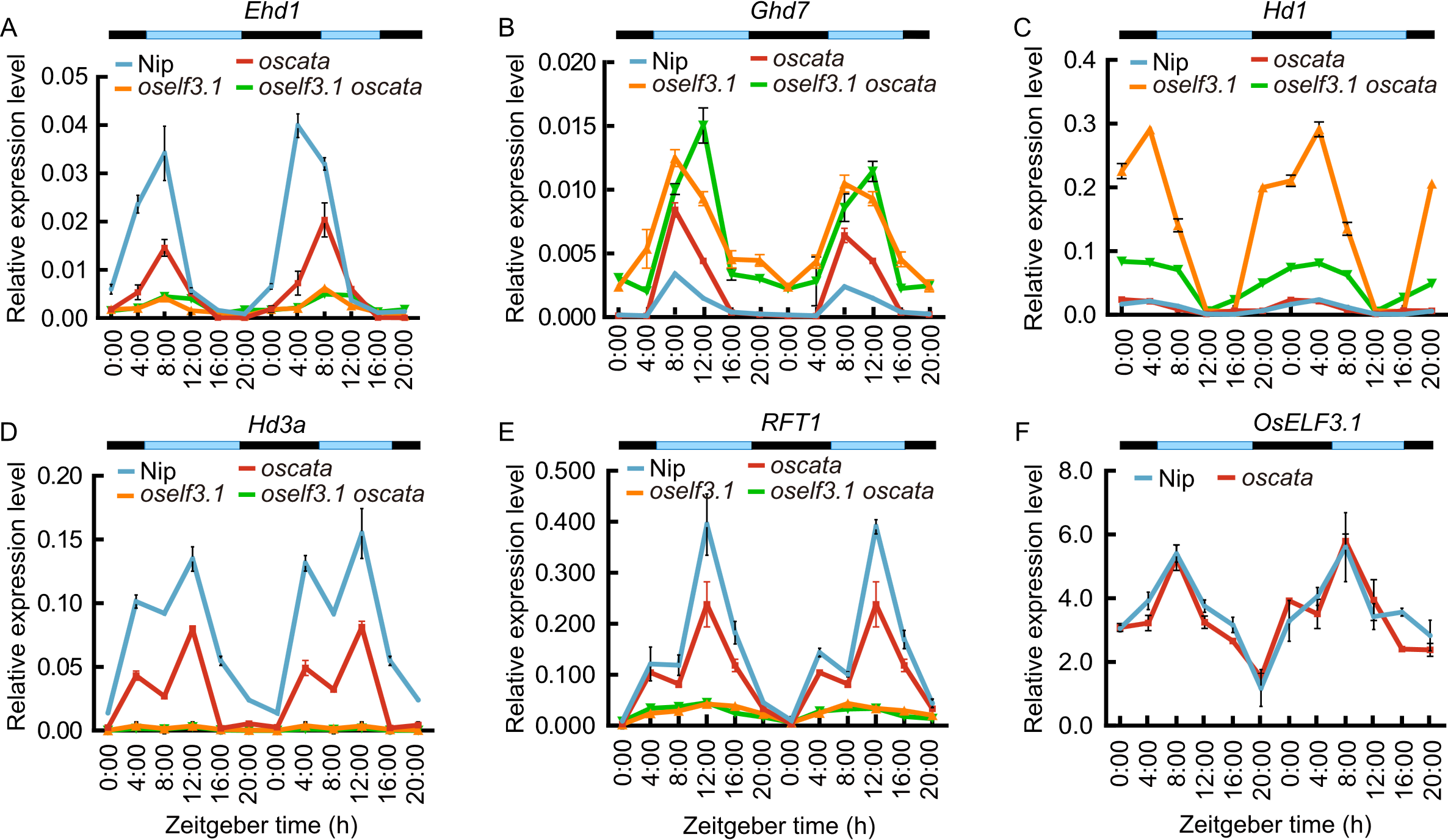
Fig. 5. Expression levels of genes related to heading date. qRT-PCR analyses of Ehd1 (A), Ghd7 (B), Hd1 (C), Hd3a (D), and RFT1 (E) in leaves of Nipponbare (Nip), oself3.1, oscata, and oself3.1 oscata double mutants, and OsELF3.1 (F) in Nip and oscata plants at 50 d after sowing under nature long-day conditions in Hangzhou filed. Data were normalized to ubiquitin (Os03g0234350) and represent mean ± SD (n = 3). Blue and black bars indicate light and dark periods, respectively.
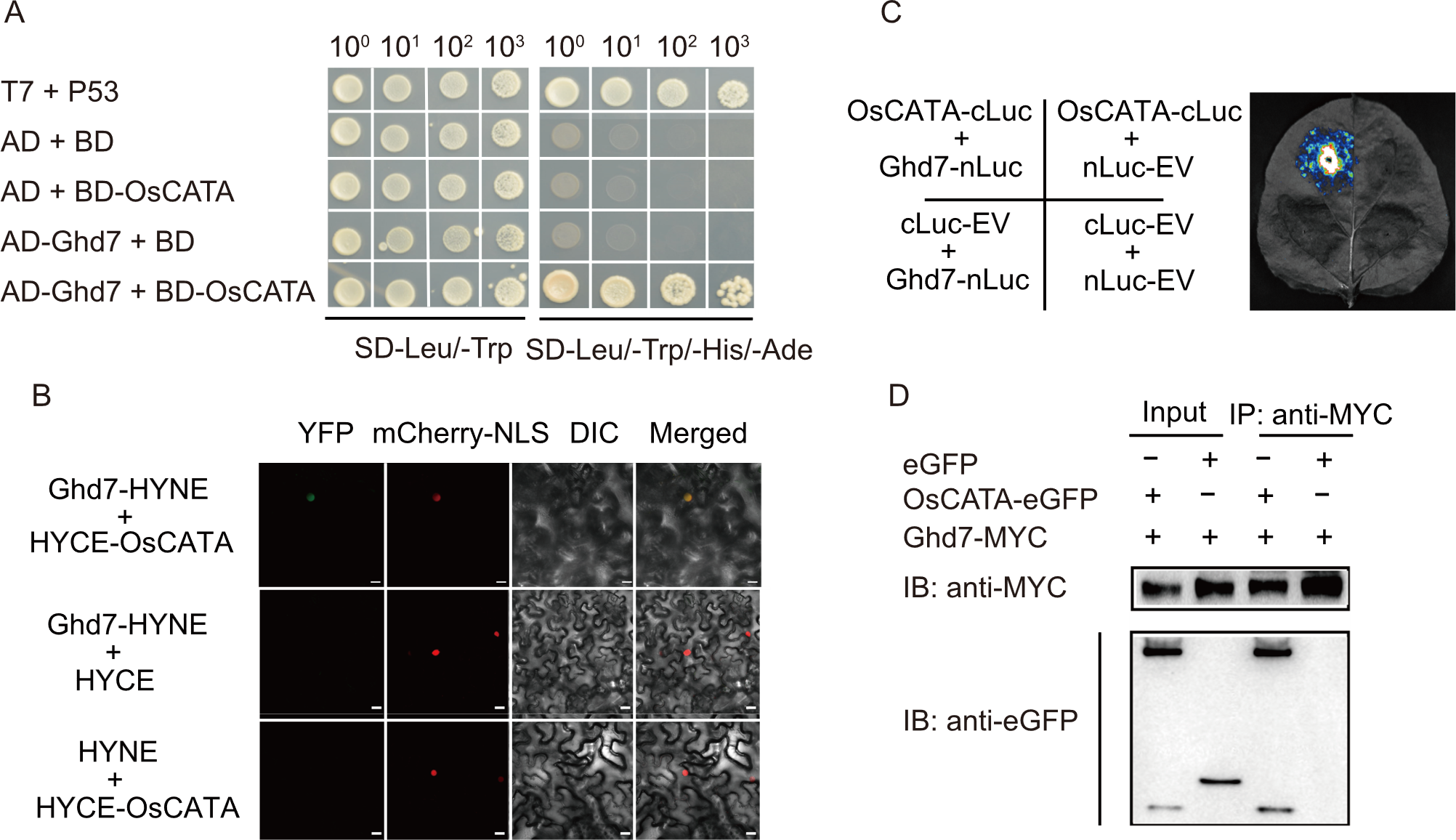
Fig. 6. OsCATA interacts with Ghd7. A, Yeast two-hybrid (Y2H) assays confirm interactions between OsCATA and Ghd7 using co-transformed Y2HGold yeast cells on DDO (SD-Leu/-Trp) and QDO (SD-Leu/-Trp/-His/-Ade) media. T7 and P53 serve as a positive interaction control pair; AD, GAL4 activation domain; BD, GAL4 DNA-binding domain. B, Bimolecular fluorescence complementation (BiFC) assays demonstrate OsCATA-Ghd7 interaction in rice protoplasts. Fluorescence signals were observed using a confocal microscope (Zeiss 980) 20-24 h after infiltration. mCherry-NLS was used as a nuclear marker. YFP, Yellow fluorescent protein; NLS, Nuclear localization signal; DIC, Differential interference contrast; HYNE, N-terminal half of YFP; HYCE, C-terminal half of YFP. Scale bars, 20 µm. C, Luminescence complementation imaging (LCI) assays validate the interaction between OsCATA and Ghd7 in Nicotiana benthamiana. Negative controls included nLuc and cLuc. Luminescence was captured using a Charge-Coupled Device camera at 3 d post-infiltration. cLuc, C-terminal fragment of luciferase; nLuc, N-terminal fragment of luciferase; EV, Empty vector. D, Co-immunoprecipitation (Co-IP) assays reveal interaction in protoplasts co-transfected with OsCATA-MYC and Ghd7-eGFP, detected with anti-MYC and anti-GFP antibodies. GFP, Green fluorescent protein; MYC, MYC epitope tag; IB, Immunoblot; IP, Immunoprecipitation.
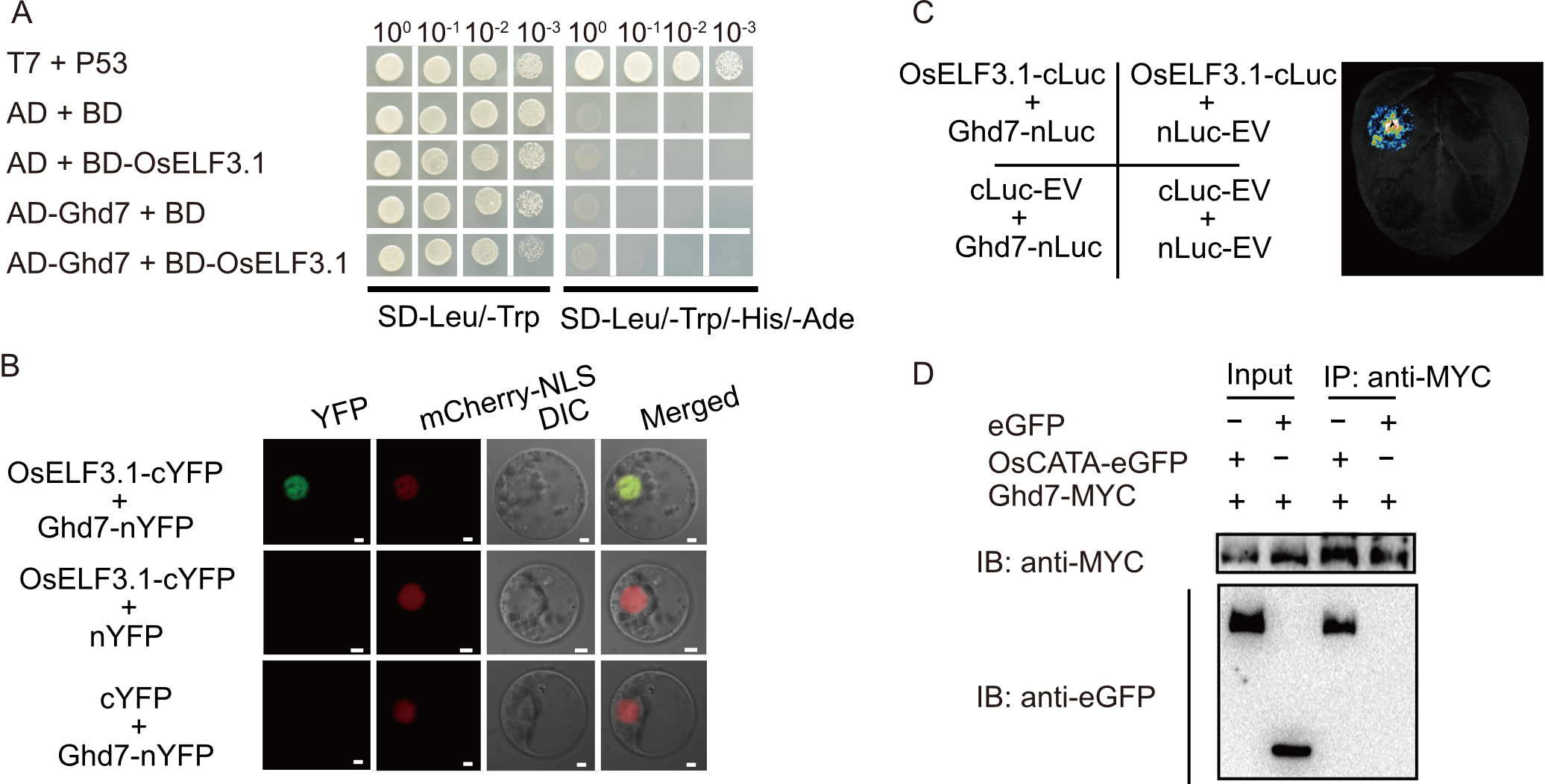
Fig. 7. OsELF3.1 interacts with Ghd7. A, Yeast two-hybrid (Y2H) assays confirm OsELF3.1-Ghd7 interaction on DDO (SD-Leu/-Trp) and QDO (SD-Leu/-Trp/-His/-Ade) media. T7 and P53 serve as a positive interaction control pair; AD, GAL4 activation domain; BD, GAL4 DNA-binding domain. B, Bimolecular fluorescence complementation (BiFC) assays illustrate OsELF3.1-Ghd7 interaction in rice protoplasts, with fluorescence detected 20-24 h after infiltration using a confocal microscope (Zeiss 980). mCherry-NLS was used as a nuclear marker. cYFP, C-terminal fragment of yellow fluorescent protein; nYFP, N-terminal fragment of yellow fluorescent protein; Scale bars, 20 µm. C, Luminescence complementation imaging (LCI) assays verify the interaction of OsELF3.1 and Ghd7 in Nicotiana benthamiana. Luminescence was captured using a Charge-Coupled Device camera. cLuc, C-terminal fragment of luciferase; nLuc, N-terminal fragment of luciferase; EV, Empty vector. D, Co-immunoprecipitation (Co-IP) assays confirm the interaction in rice protoplasts co-transfected with OsELF3.1-MYC and Ghd7-eGFP. The proteins from crude lysates (upper, input) and the immunoprecipitated proteins (lower) were detected with anti-GFP and anti-MYC antibodies. GFP, Green fluorescent protein; MYC, MYC epitope tag; IB, Immunoblot; IP, Immunoprecipitation.
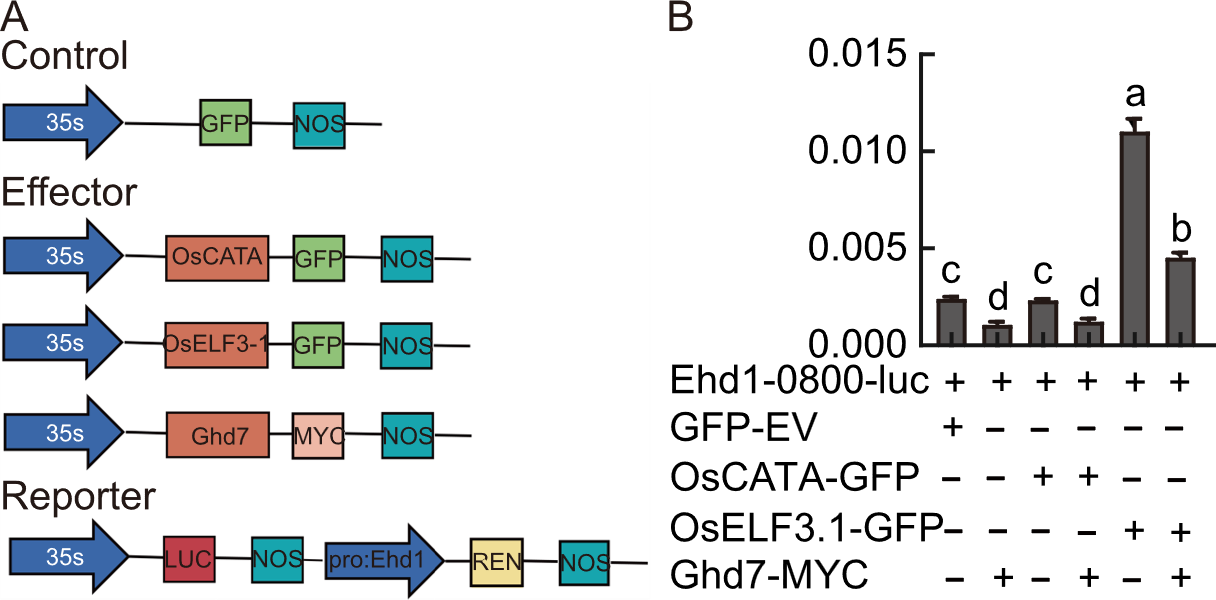
Fig. 8. Transcriptional activity assays. A, Illustration of constructs used in transcriptional activity assays. B, Relative LUC activity was measured in rice protoplasts with combinations of OsCATA, OsELF3.1, Ghd7, and the Ehd1 reporter. LUC and REN served as internal controls. Data are mean ± SD (n = 3). Different lowercase letters above bars indicate significant differences (P < 0.05) based on Duncan’s multiple range test. GFP, Green fluorescent protein; NOS, Nopaline synthase terminator; MYC, MYC epitope tag; LUC, Luciferase; REN, Renilla; EV, Empty vector.
| [1] | Abe A, Kosugi S, Yoshida K, et al. 2012. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat Biotechnol, 30(2): 174-178. |
| [2] | Cai Z Z, Zhang Y D, Tang W Q, et al. 2022. LUX ARRHYTHMO interacts with ELF3a and ELF4a to coordinate vegetative growth and photoperiodic flowering in rice. Front Plant Sci, 13: 853042. |
| [3] | Cheng Q, Gan Z R, Wang Y P, et al. 2020. The soybean gene J contributes to salt stress tolerance by up-regulating salt-responsive genes. Front Plant Sci, 11: 272. |
| [4] | Doi K, Izawa T, Fuse T, et al. 2004. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Genes Dev, 18(8): 926-936. |
| [5] | Du Y Y, Wang P C, Chen J, et al. 2008. Comprehensive functional analysis of the catalase gene family in Arabidopsis thaliana. J Integr Plant Biol, 50(10): 1318-1326. |
| [6] | Fang X L, Han Y P, Liu M S, et al. 2021. Modulation of evening complex activity enables north-to-south adaptation of soybean. Sci China Life Sci, 64(2): 179-195. |
| [7] | Fu C, Yang X O, Chen X, et al. 2009. OsEF3, a homologous gene of Arabidopsis ELF3, has pleiotropic effects in rice. Plant Biol, 11(5), 751-757. |
| [8] | Fujiwara S, Oda A, Yoshida R, et al. 2008. Circadian clock proteins LHY and CCA1 regulate SVP protein accumulation to control flowering in Arabidopsis. Plant Cell, 20(11): 2960-2971. |
| [9] | Gao M J, He Y, Yin X, et al. 2021. Ca2+ sensor-mediated ROS scavenging suppresses rice immunity and is exploited by a fungal effector. Cell, 184(21): 5391-5404. |
| [10] | Guo T, Chen K, Dong N Q, et al. 2018. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell, 30(4): 871-888. |
| [11] | Hu B, Jiang Z M, Wang W, et al. 2019. Nitrate-NRT1.1B-SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat Plants, 5(4): 401-413. |
| [12] | Hu Y, Song S, Weng X Y, et al. 2021. The heading-date gene Ghd7 inhibits seed germination by modulating the balance between abscisic acid and gibberellins. Crop J, 9(2): 297-304. |
| [13] | Izawa T. 2007. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot, 58(12): 3091-3097. |
| [14] | Joo J, Lee Y H, Song S I. 2014. Rice CatA, CatB, and CatC are involved in environmental stress response, root growth, and photorespiration, respectively. J Plant Biol, 57( 6): 375-382. |
| [15] | Jung J H, Barbosa A D, Hutin S, et al. 2020. A prion-like domain in ELF3 functions as a thermosensor in Arabidopsis. Nature, 585: 256-260. |
| [16] | Komiya R, Yokoi S, Shimamoto K. 2009. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development, 136(20): 3443-3450. |
| [17] | Lai A G, Doherty C J, Mueller-Roeber B, et al. 2012. CIRCADIAN CLOCK-ASSOCIATED 1 regulates ROS homeostasis and oxidative stress responses. Proc Natl Acad Sci USA, 109(42): 17129-17134. |
| [18] | Liu X L, Covington M F, Fankhauser C, et al. 2001. ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell, 13(6): 1293-1304. |
| [19] | Liu Z, Cao M A, Kuča K, et al. 2024. Cloning of CAT genes in Satsuma mandarin and their expression characteristics in response to environmental stress and arbuscular mycorrhizal fungi. Plant Cell Rep, 43(5): 123. |
| [20] | Luan W J, Chen H Z, Fu Y P, et al. 2009. The effect of the crosstalk between photoperiod and temperature on the heading-date in rice. PLoS One, 4(6): e5891. |
| [21] | Matsubara K, Ogiso-Tanaka E, Hori K, et al. 2012. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol, 53(4): 709-716. |
| [22] | McClung C R. 1997. Regulation of catalases in Arabidopsis. Free Radic Biol Med, 23(3): 489-496. |
| [23] | Mhamdi A, Queval G, Chaouch S, et al. 2010. Catalase function in plants: A focus on Arabidopsis mutants as stress-mimic models. J Exp Bot, 61(15): 4197-4220. |
| [24] | Mhamdi A, Noctor G, Baker A. 2012. Plant catalases: Peroxisomal redox guardians. Arch Biochem Biophys, 525(2): 181-194. |
| [25] | Michael T P, McClung C R. 2002. Phase-specific circadian clock regulatory elements in Arabidopsis. Plant Physiol, 130(2): 627-638. |
| [26] | Mouradov A, Cremer F, Coupland G. 2002. Control of flowering time: Interacting pathways as a basis for diversity. Plant Cell, 14: S111-S130. |
| [27] | Nemoto Y, Nonoue Y, Yano M, Izawa T. 2016. Hd1, a CONSTANS ortholog in rice, functions as an Ehd1 repressor through interaction with monocot‐specific CCT‐domain protein Ghd7. Plant J, 86(3): 221-233. |
| [28] | Ning Y S, Shi X T, Wang R Y, et al. 2015. OsELF3-2, an ortholog of Arabidopsis ELF3, interacts with the E3 ligase APIP6 and negatively regulates immunity against Magnaporthe oryzae in rice. Mol Plant, 8(11): 1679-1682. |
| [29] | Nusinow D A, Helfer A, Hamilton E E, et al. 2011. The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature, 475(7356): 398-402. |
| [30] | Riseh R S, Fathi F, Vatankhah M, et al. 2024. Catalase-associated immune responses in plant-microbe interactions: A review. Int J Biol Macromol, 280: 135859. |
| [31] | Ronald J, Su C, Wang L, et al. 2022. Cellular localization of Arabidopsis EARLY FLOWERING3 is responsive to light quality. Plant Physiol, 190(2): 1024-1036. |
| [32] | Sakuraba Y, Jeong J, Kang M Y, et al. 2014. Phytochrome-interacting transcription factors PIF4 and PIF5 induce leaf senescence in Arabidopsis. Nat Commun, 5: 4636. |
| [33] | Sakuraba Y, Han S H, Yang H J, et al. 2016. Mutation of Rice Early Flowering3.1 (OsELF3.1) delays leaf senescence in rice. Plant Mol Biol, 92(1/2): 223-234. |
| [34] | Su T, Wang P P, Li H J, et al. 2018. The Arabidopsis catalase triple mutant reveals important roles of catalases and peroxisome-derived signaling in plant development. J Integr Plant Biol, 60(7): 591-607. |
| [35] | Sun K L, Huang M H, Zong W B, et al. 2022. Hd1, Ghd7, and DTH8 synergistically determine the rice heading date and yield-related agronomic traits. J Genet Genomics, 49(5): 437-447. |
| [36] | Sun K L, Zong W B, Xiao D D, et al. 2023. Effects of the core heading date genes Hd1, Ghd7, DTH8 and PRR37 on yield-related traits in rice. Theor Appl Genet, 136(11): 227. |
| [37] | Takahashi Y, Shimamoto K. 2011. Hd1), an ortholog of Arabidopsis CONSTANS, is a possible target of human selection during domestication to diversify flowering times of cultivated rice. Genes Genet Syst, 86(3): 175-182. |
| [38] | Tamaki S, Tsuji H, Matsumoto A, et al. 2015. FT-like proteins induce transposon silencing in the shoot apex during floral induction in rice. Proc Natl Acad Sci USA, 112(8): E901-E910. |
| [39] | Wang B F, Xue P, Zhang Y X, et al. 2024. OsCPK12 phosphorylates OsCATA and OsCATC to regulate H2O2 homeostasis and improve oxidative stress tolerance in rice. Plant Commun, 5(3): 100780. |
| [40] | Wang C, Shen L, Fu Y P, et al. 2015. A simple CRISPR/Cas9 system for multiplex genome editing in rice. J Genet Genomics, 42(12): 703-706. |
| [41] | Wang Q, Su Q M, Nian J Q, et al. 2021. The Ghd7 transcription factor represses ARE1 expression to enhance nitrogen utilization and grain yield in rice. Mol Plant, 14(6): 1012-1023. |
| [42] | Wang X L, He Y Q, Wei H, et al. 2021. A clock regulatory module is required for salt tolerance and control of heading date in rice. Plant Cell Environ, 44(10): 3283-3301. |
| [43] | Weng X Y, Wang L, Wang J, et al. 2014. Grain number, plant height, and heading date7 is a central regulator of growth, development, and stress response. Plant Physiol, 164( 2): 735-747. |
| [44] | Xia A A, Zheng L M, Wang Z, et al. 2023. The RHW1-ZCN4 regulatory pathway confers natural variation of husk leaf width in maize. New Phytol, 239(6): 2367-2381. |
| [45] | Xu P, Zhang Y X, Wen X X, et al. 2023. The clock component OsLUX regulates rice heading through recruiting OsELF3-1 and OsELF4s to repress Hd1 and Ghd7. J Adv Res, 48: 17-31. |
| [46] | Xu X, Shi X T, You X M, et al. 2024. A pair of E3 ubiquitin ligases control immunity and flowering by targeting different ELF3 proteins in rice. Dev Cell, 59(20): 2731-2744.e4. |
| [47] | Xue W Y, Xing Y Z, Weng X Y, et al. 2008. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet, 40(6): 761-767. |
| [48] | Yang Y, Peng Q, Chen G X, et al. 2013. OsELF3 is involved in circadian clock regulation for promoting flowering under long-day conditions in rice. Mol Plant, 6(1): 202-215. |
| [49] | Yi H, Shi H, Mao W, et al. 2024. E3 ubiquitin ligase IPI1 controls rice immunity and flowering via both E3 ligase-dependent and -independent pathways. Dev Cell, 59(20): 2719-2730. |
| [50] | You X M, Zhang F, Liu Z, et al. 2022. Rice catalase OsCATC is degraded by E3 ligase APIP6 to negatively regulate immunity. Plant Physiol, 190(2): 1095-1099. |
| [51] | Yu J W, Rubio V, Lee N Y, et al. 2008. COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell, 32(5): 617-630. |
| [52] | Zagotta M T, Hicks K A, Jacobs C I, et al. 1996. The Arabidopsis ELF3 gene regulates vegetative photomorphogenesis and the photoperiodic induction of flowering. Plant J, 10(4): 691-702. |
| [53] | Zhang Z S, Xu Y Y, Xie Z W, et al. 2016. Association-dissociation of glycolate oxidase with catalase in rice: A potential switch to modulate intracellular H2O2 levels. Mol Plant, 9(5): 737-748. |
| [54] | Zhang Z Y, Hu W, Shen G J, et al. 2017. Alternative functions of Hd1 in repressing or promoting heading are determined by Ghd7 status under long-day conditions. Sci Rep, 7(1): 5388. |
| [55] | Zhao J M, Huang X, Ouyang X H, et al. 2012. OsELF3-1, an ortholog of Arabidopsis EARLY FLOWERING 3, regulates rice circadian rhythm and photoperiodic flowering. PLoS One, 7(8): e43705. |
| [56] | Zhao Y P, Zhao B B, Xie Y R, et al. 2023. The evening complex promotes maize flowering and adaptation to temperate regions. Plant Cell, 35(1): 369-389. |
| [57] | Zhong H H, McClung C R. 1996. The circadian clock gates expression of two Arabidopsis catalase genes to distinct and opposite circadian phases. Mol Gen Genet, 251(2): 196-203. |
| [58] | Zhou S R, Zhu S S, Cui S, et al. 2021. Transcriptional and post-transcriptional regulation of heading date in rice. New Phytol, 230(3): 943-956. |
| [59] | Zhou Y B, Liu C, Tang D Y, et al. 2018. The receptor-like cytoplasmic kinase STRK1 phosphorylates and activates CatC, thereby regulating H2O2 homeostasis and improving salt tolerance in rice. Plant Cell, 30(5): 1100-1118. |
| [60] | Zhu C M, Peng Q, Fu D B, et al. 2018. The E3 ubiquitin ligase HAF1 modulates circadian accumulation of EARLY FLOWERING3 to control heading date in rice under long-day conditions. Plant Cell, 30(10): 2352-2367. |
| [61] | Zong W B, Ren D, Huang M H, et al. 2021. Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol, 229(3): 1635-1649. |
| [62] | Zou J J, Li X D, Ratnasekera D, et al. 2015. Arabidopsis CALCIUM-DEPENDENT PROTEIN KINASE8 and CATALASE3 function in abscisic acid-mediated signaling and H2O2 homeostasis in stomatal guard cells under drought stress. Plant Cell, 27(5): 1445-1460. |
| [1] | Daisy Wilson, Valeria Gonzalez, Hamidreza Sharifan. Evaluating Efficacy of ZnO and MgO Nanoparticles on Post-Harvested Rice to Enhance Food Security Against Agroterrorism [J]. Rice Science, 2025, 32(5): 717-726. |
| [2] | Mareyam Mukhtar, Amresh Kumar, Ashfak S. Mujawar, Bhuvnesh Sareen, Suhas G. Karkute, Rohini Sreevathsa, Amitha Mithra Sevanthi, Amolkumar U. Solanke. Genome-Wide Identification of Dopamine β-Monooxygenase N-Terminal Gene Family in Rice and Its Role in Response to Blast Disease and Abiotic Stress [J]. Rice Science, 2025, 32(5): 685-703. |
| [3] | Li Haifeng, Fan Jiayi. Functions of Rice E3 Ubiquitin Ligases in Response to Environmental Stress and in Regulating Grain Size [J]. Rice Science, 2025, 32(5): 649-657. |
| [4] | Pan Pan, Guo Wenlong, Li Hengbo, Shao Yifan, Guo Zhihao, Jin Ye, Cheng Yanrong, Yu Guoping, Fu Zhenshi, Hu Lin, Zheng Xiaoming, Zhou Guomin, Zhang Jianhua. Accelerating Wild Rice Disease-Resistant Germplasm Exploration: Artificial Intelligence (AI)-Powered Wild Rice Blast Disease Level Evaluation and Disease-Resistance Identification [J]. Rice Science, 2025, 32(5): 727-746. |
| [5] | Sabarinathan Selvaraj, Parameswaran Chidambaranathan, Goutam Kumar Dash, Priyadarsini Sanghamitra, Kishor Pundlik Jeughale, Cayalvizhi Balasubramaniasai, Devraj Lenka, Basavantraya Navadagi Devanna, Seenichamy Rathinam Prabhukarthikeyan, Sanghamitra Samantaray, Amaresh Kumar Nayak. Long-Range Admixture Linkage Disequilibrium and Allelic Responses of Sub1 and TPP7 under Consecutive Stress in Rice Validated Through Mendelian Randomization [J]. Rice Science, 2025, 32(5): 704-716. |
| [6] | Yong Jin Choi, Sun-Hwa Ha. Metabolic Engineering in Rice for Functional Metabolite Production [J]. Rice Science, 2025, 32(4): 475-498. |
| [7] | Dinuka Nuwan Tharaka, Nadeeka D. Tissera, Gayan Priyadarshana, Damayanthi Dahanayake. A Comprehensive Review of Hierarchical Porous Carbon Synthesis from Rice Husk [J]. Rice Science, 2025, 32(4): 499-511. |
| [8] | Li Xinyan, Weng Lüshui, Xiao Youlun, Li Jinjiang, Deng Lihua, Liu Qing, Kang Weiwei, Duan Yaping, Yang Daji, Xiao Guoying. Characteristic Analysis of Penta-Resistance Restorer Line for Hybrid Rice [J]. Rice Science, 2025, 32(4): 512-524. |
| [9] | Zhou Lin, Jiang Hong, Huang Long, Li Ziang, Yao Zhonghao, Li Linhan, Ji Kangwei, Li Yijie, Tang Haijuan, Cheng Jinping, Bao Yongmei, Huang Ji, Zhang Hongsheng, Chen Sunlu. Genome-Wide Association Study of Brown Rice Weight Identifies an RNA-Binding Protein Antagonistically Regulating Grain Weight and Panicle Number [J]. Rice Science, 2025, 32(4): 525-536. |
| [10] | Ratan Kumar Ganapati, Chen Kai, Zhao Xiuqin, Zheng Tianqing, Zhang Fan, Zhai Laiyuan, Xu Jianlong. Genome-Wide Association Study and Haplotype Analysis Jointly Identify New Candidate Genes for Alkaline Tolerance at Seedling Stage in Rice [J]. Rice Science, 2025, 32(4): 537-548. |
| [11] | Hou Yuxuan, Zhu Jie, Lu Chenglong, Fan Libo, Liang Mengqi, Zhang Xiaobo, Cheng Benyi, Xu Xia, Gong Junyi. A Recombinase-Aided Amplification-Lateral Flow Dipstick Detection Technique for Early On-Site Diagnosis of Bacterial Blight Caused by Xanthomonas oryzae pv. oryzae in Rice [J]. Rice Science, 2025, 32(4): 575-584. |
| [12] | Chen Su, Ma Feilong, Chen Jiaoyang, Qi Man, Wei Qianshu, Tao Zhihuan, Sun Bo. Function of R2R3-Type Myeloblastosis Transcription Factors in Plants [J]. Rice Science, 2025, 32(3): 307-321. |
| [13] | Yang Yajun, Lu Yanhui, Tian Junce, Zheng Xusong, Guo Jiawen, Liu Xiaowei, Lü Zhongxian, Xu Hongxing. Sustainable Management Strategies for Rice Leaffolder, Cnaphalocrocis medinalis (Guenée): Progress and Prospects [J]. Rice Science, 2025, 32(3): 322-338. |
| [14] | Xie Yuhao, Xie Wenya, Zhao Jianhua, Xue Xiang, Cao Wenlei, Shi Xiaopin, Wang Zhou, Wang Yiwen, Wang Guangda, Feng Zhiming, Hu Keming, Chen Xijun, Chen Zongxiang, Zuo Shimin. OsERF7 Negatively Regulates Resistance to Sheath Blight Disease by Inhibiting Phytoalexin Biosynthesis [J]. Rice Science, 2025, 32(3): 367-379. |
| [15] | Chaemyeong Lim, Sae Hyun Lee, Haeun Lee, So-Yon Park, Kiyoon Kang, Hyeryung Yoon, Tae-Jin Yang, Gary Stacey, Nam-Chon Paek, Sung-Hwan Cho. Global Transcriptome Analysis of Rice Seedlings in Response to Extracellular ATP [J]. Rice Science, 2025, 32(3): 380-399. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||