
Rice Science ›› 2025, Vol. 32 ›› Issue (3): 380-399.DOI: 10.1016/j.rsci.2025.03.002
• Research Papers • Previous Articles Next Articles
Chaemyeong Lim1,#, Sae Hyun Lee1,#, Haeun Lee1, So-Yon Park2, Kiyoon Kang3, Hyeryung Yoon1, Tae-Jin Yang1, Gary Stacey2, Nam-Chon Paek1( ), Sung-Hwan Cho1(
), Sung-Hwan Cho1( )
)
Received:2024-10-25
Accepted:2024-12-31
Online:2025-05-28
Published:2025-06-16
Contact:
Nam-Chon Paek (About author:First author contact:These authors contributed equally to this work
Chaemyeong Lim, Sae Hyun Lee, Haeun Lee, So-Yon Park, Kiyoon Kang, Hyeryung Yoon, Tae-Jin Yang, Gary Stacey, Nam-Chon Paek, Sung-Hwan Cho. Global Transcriptome Analysis of Rice Seedlings in Response to Extracellular ATP[J]. Rice Science, 2025, 32(3): 380-399.
Add to citation manager EndNote|Ris|BibTeX
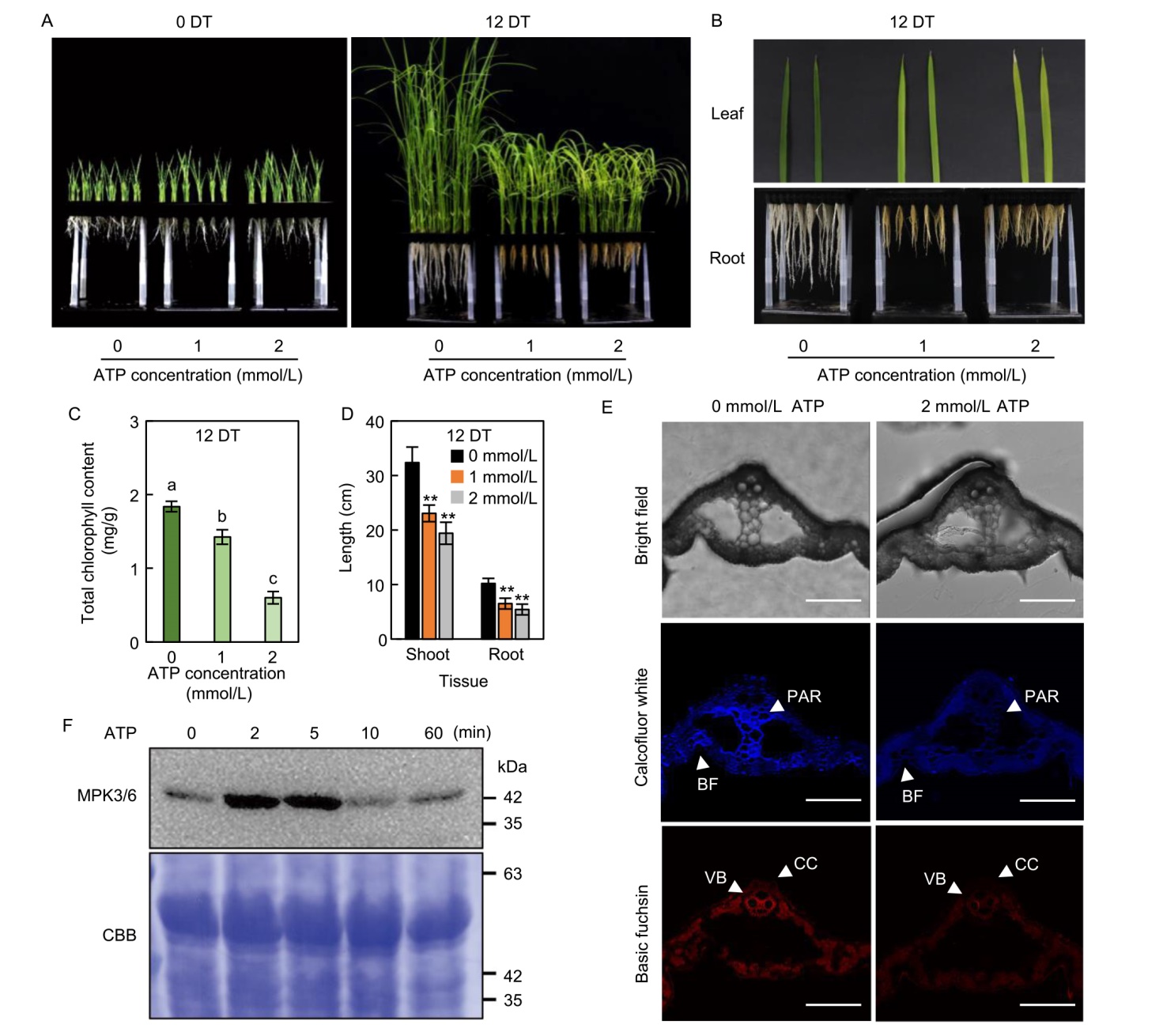
Fig. 1. Phenotypes of rice seedlings under different extracellular ATP (eATP) concentration treatments. A‒D, Whole-plant phenotype (A), leaf and root morphology (B), total chlorophyll content (C), and shoot and root lengths (D) under different ATP concentrations (0, 1, and 2 mmol/L). Data represent Mean ± SD (n = 8 in C and 10 in D). Different lowercase letters above bars indicate significant differences according to one-way analysis of variance followed by Duncan’s test (P < 0.05). Asterisks denote significant differences compared with mock control (Student’s t-test: **, P < 0.01).E, Cellulose (calcofluor white) and lignin (basic fuchsin) staining in rice leaves treated with 2 mmol/L ATP at 12 d after treatment (DT). Scale bars, 100 µm. BF, Bulliform cell; CC, Collenchyma; PAR, Parenchyma; VB, Vascular bundle.F, Time-course analysis of MPK3/6 phosphorylation in rice leaves treated with 500 µmol/L ATP. Leaves from 5-day-old wild type (WT) seedlings were sprayed with ATP, and phosphorylation was detected using anti-phospho-p44/p42 MAPK antibody. Coomassie brilliant blue (CBB) staining (bottom panel) confirmed equal protein loading. Data represent Mean ± SD from six biological replicates. WT plants (japonica cv. Dongjin) were hydroponically grown in full-strength Yoshida solution for 5 d under long-day (LD) conditions (14 h light at 30 ºC /10 h dark at 25 ºC). Seedlings were then transferred to Yoshida solution containing 0, 1, or 2 mmol/L ATP and maintained under LD conditions for 12 d. Phenotypic analyses were performed at 12 DT. All experiments were repeated three times with consistent results.
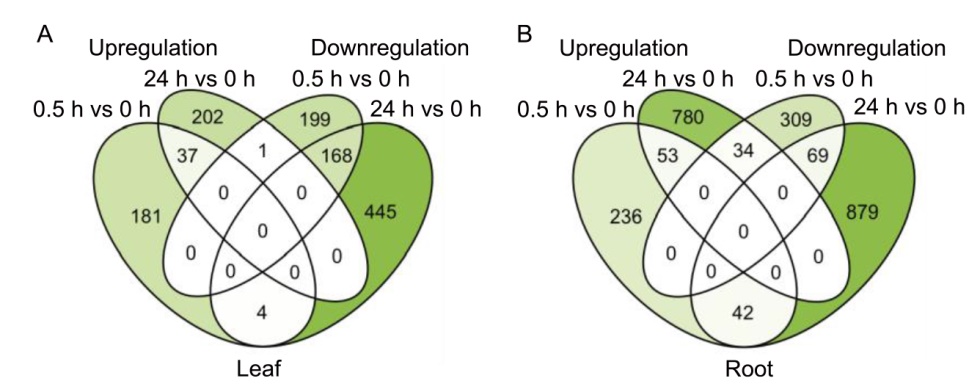
Fig. 2. Experimental designs and differentially expressed genes (DEGs) identified in rice plants exposed to eATP treatment. A and B, Venn diagrams of DEGs in leaf (A) and root (B) in different comparisons (treated vs control). Numbers of up- and downregulated genes are indicated numerically and by heatmap in each Venn diagram section. Sampling was conducted at 5 d after germination using leaves or roots of wild type seedlings grown in Yoshida solution in a growth chamber under long-day conditions (14 h of light at 30 ºC/10 h of dark at 25 ºC). Plants were treated 1 mmol/L ATP solutions at 0, 0.5, and 24 h for RNA-seq analysis.
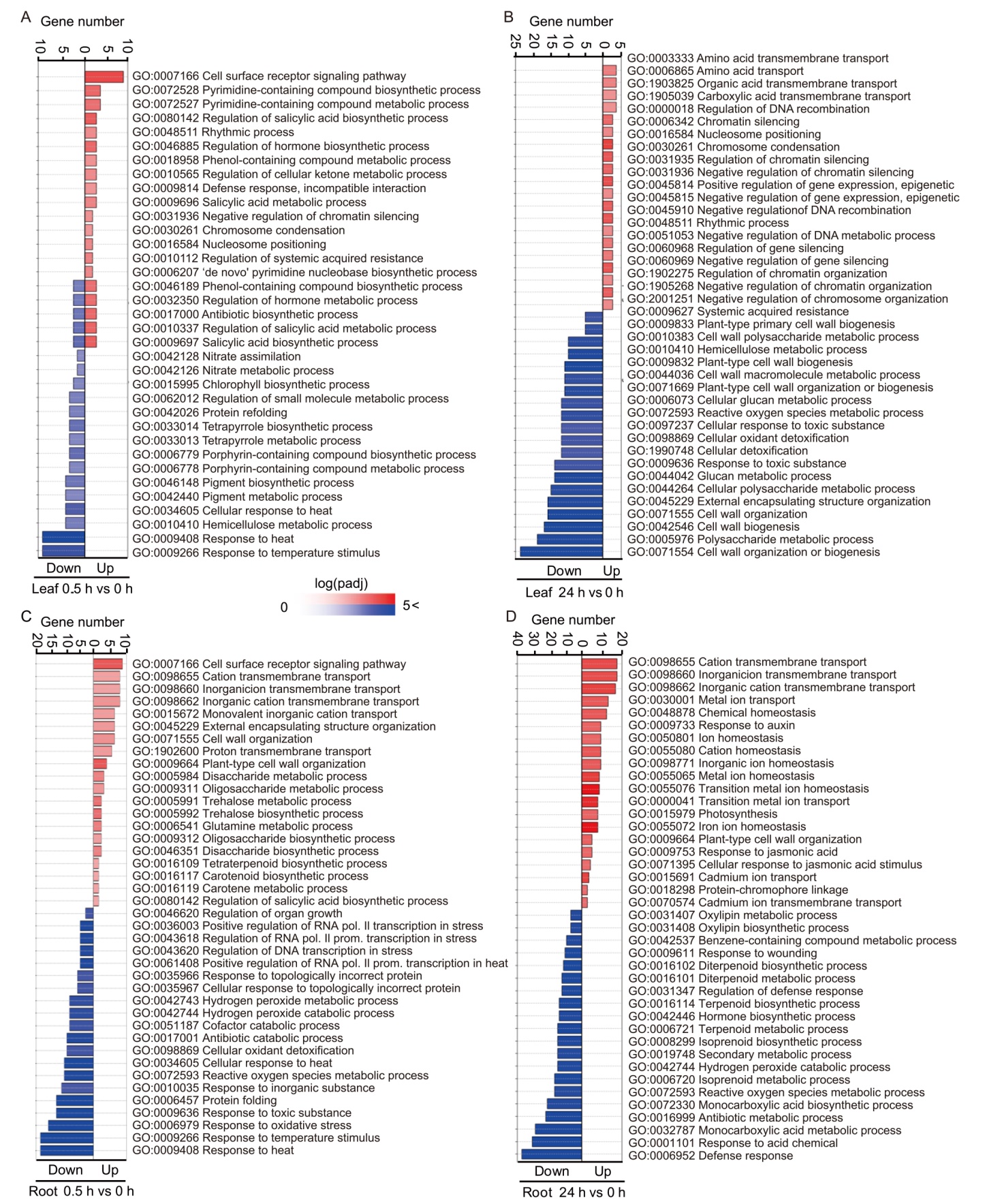
Fig. 3. Gene Ontology (GO) enrichment analysis of differentially expressed genes (DEGs) in rice leaves and roots. A and B, 0.5 h vs 0 h (A) and 24 h vs 0 h (B) of leaf comparison.C and D, 0.5 h vs 0 h (C) and 24 h vs 0 h (D) of root comparison. padj, Adjusted P-value. The gene number and the adjusted P-value for the top 20 most enriched GO in the differentially up- and downregulated genes are represented by bar size and color, respectively.
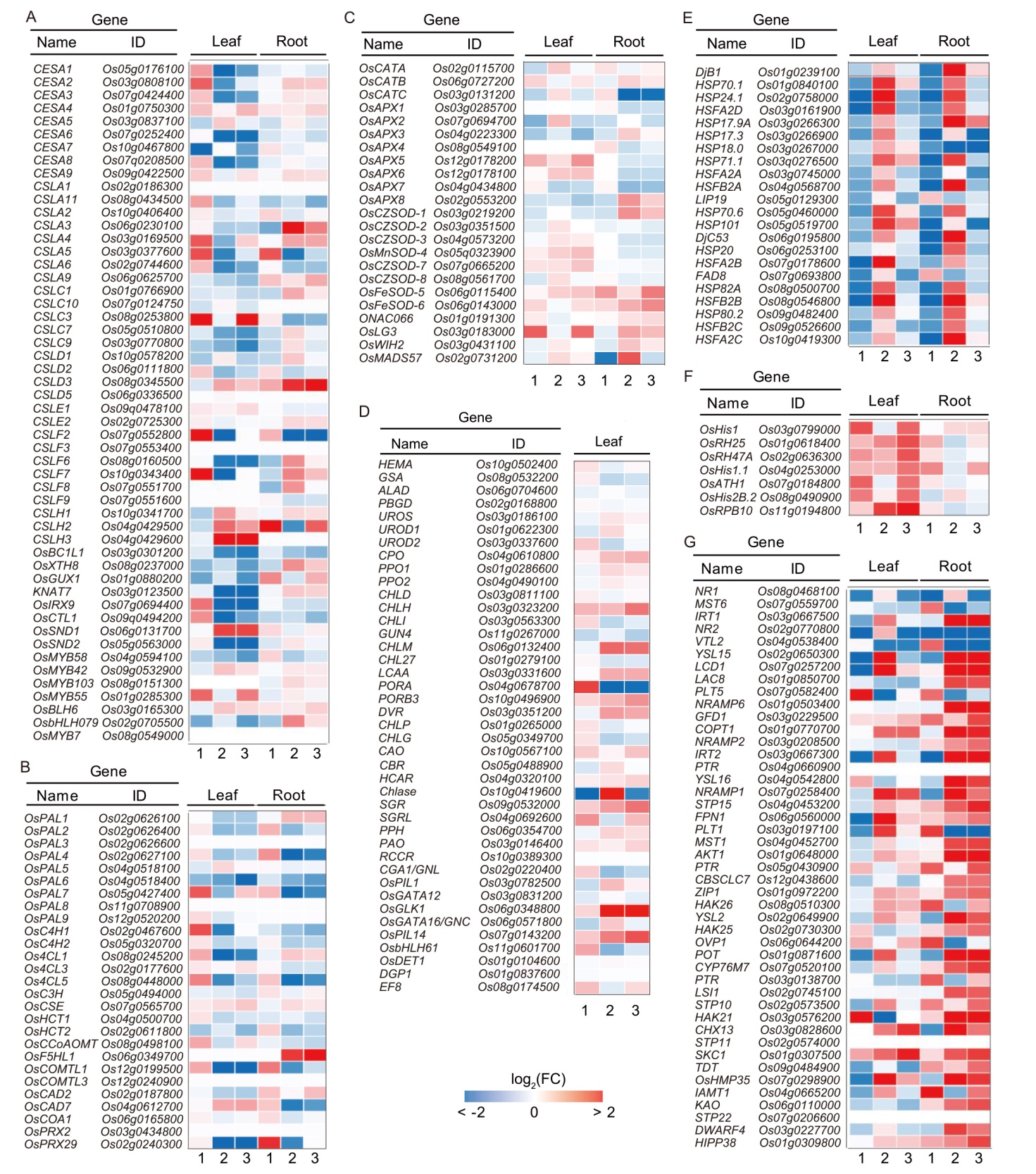
Fig. 4. Gene expression patterns of cellulose/lignin-biosynthesis, chlorophyll metabolism, reactive oxygen species (ROS) scavenging, heat response, temperature stimulation, epigenetic pathway, and inorganic ion transport and metabolism related pathways in response to eATP treatment. A‒G, Cellulose synthesis-related genes (A), lignin synthesis-related genes (B), ROS scavenging-related genes (C), chlorophyll metabolism-related genes (D), heat response and temperature stimulation related genes (E), epigenetic pathway related genes (F), and inorganic ion transport and metabolism-related genes (G). Expression fold changes (FC) are presented with a heat map. ‘1’ represents control vs 0.5 h; ‘2’ represents 0.5 h vs 24 h, and ‘3’ represents control vs 24 h.
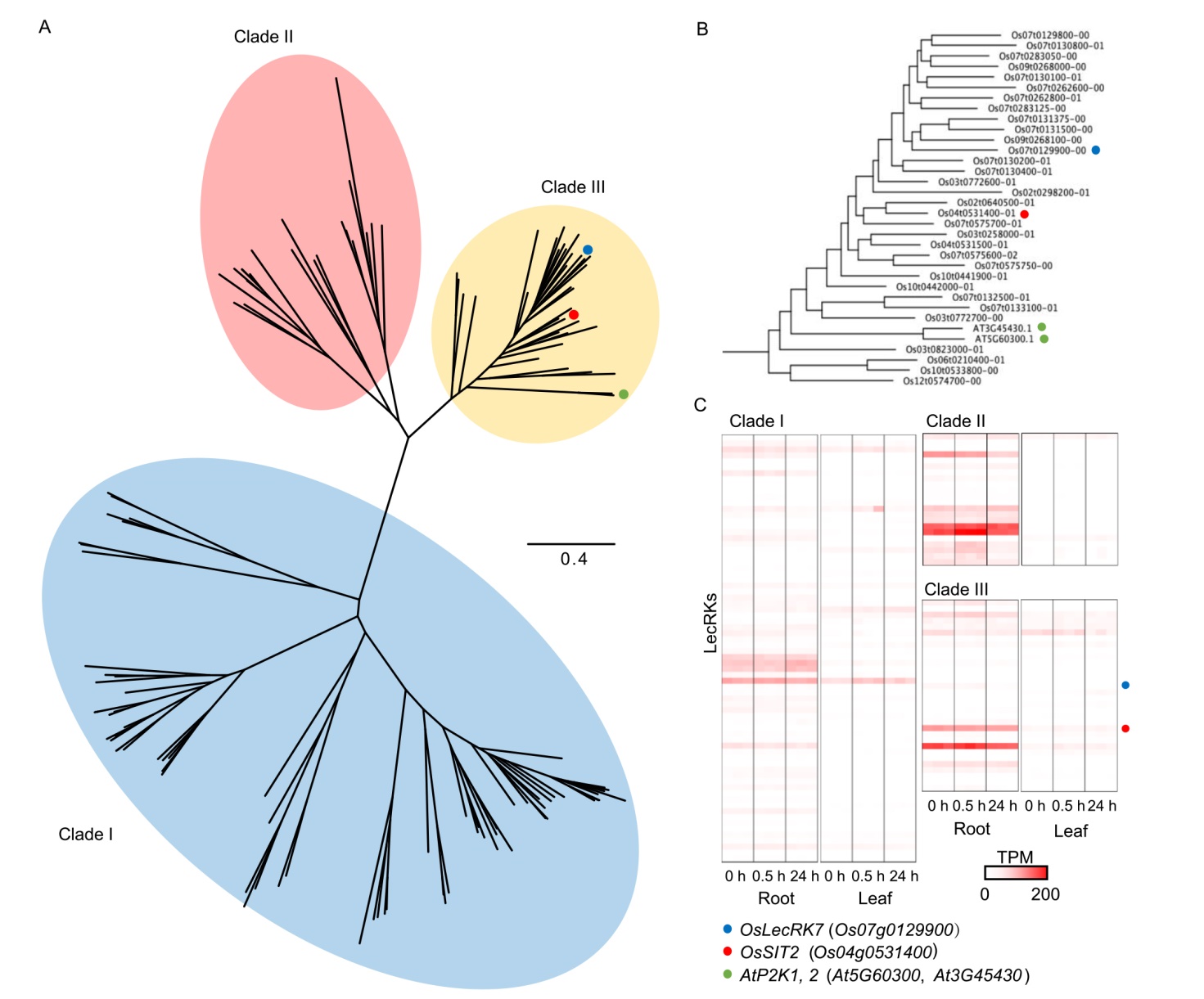
Fig. 5. Phylogenetic tree, gene expression, and domain compositions of rice LecRKs. A, The maximum likelihood phylogenetic tree with the coding sequence of LecRKs. The classified clades are shown as colored circles. Arabidopsis AtP2K1 and AtP2K2 are indicated by green dots. OsLecRK7 and OsSIT2 are indicated by blue and red dots, respectively.B, Detailed information of Clade III. C, TPM (Transcripts per million) values of LecRK genes according to ATP treatment.
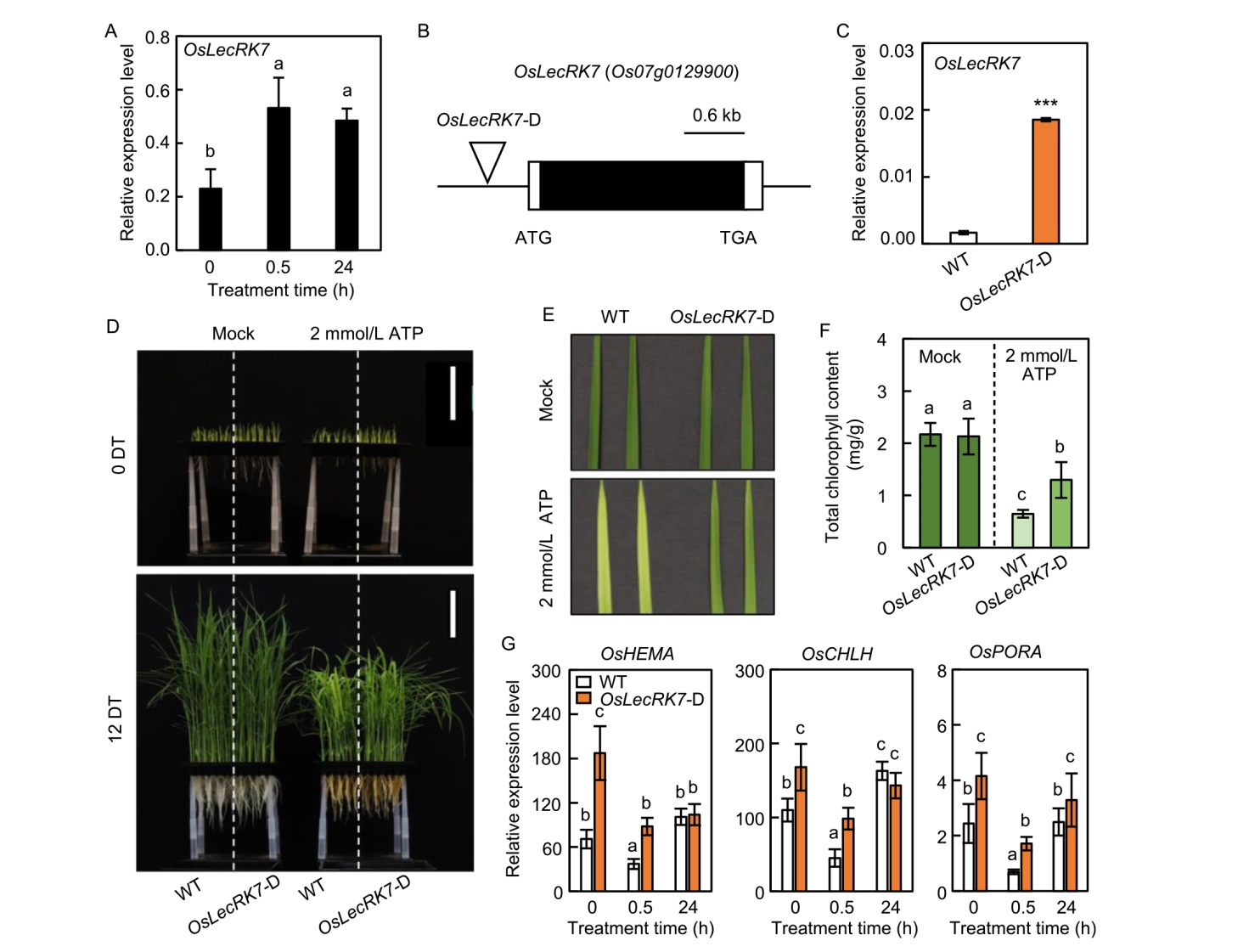
Fig. 6. OsLecRK7 is involved in eATP-mediated chlorophyll biosynthesis and cell wall metabolism. A, Relative transcript levels of OsLecRK7 in the leaves of wild type (WT) and OsLecRK7-D (enhancer-trapped T-DNA insertional activation line) plants in response to 1 mmol/L eATP. B, Schematic representation of the OsLecRK7 locus (Os07g0129900) and its T-DNA insertional activation line OsLecRK7-D. The upside-down triangle indicates the position of the T-DNA insertion (OsLecRK7-D, PFG_3A-52532). Black box represents the exon and two white boxes represent 5′-the untranslated region and 3′-untranslated region. C, Expression of OsLecRK7 was measured in the leaves of WT and OsLecRK7-D plants grown in a plant growth chamber for 10 d. Asterisks indicate statistically significant differences between WT and OsLecRK7-D plants, as determined by Student’s t-test (***, P < 0.001). D, Comparison of WT and OsLecRK7-D plants at 0 and 12 d after eATP treatment (DT).E, Leaf greenness phenotypes of 2 mmol/L ATP-treated leaves of WT and OsLecRK7-D at 12 DT. F, Changes in total chlorophyll content of WT and OsLecRK7-D at 12 DT. G, Expression of OsHEMA, OsCHLH, and OsPORA was responsive to 1 mmol/L ATP treatment. The leaves of 5-day-old WT plants were exposed to 1 mmol/L ATP for 0, 0.5, and 24 h. The relative expression levels of OsHEMA, OsCHLH, and OsPORA were normalized to OsUBQ5 (Os01g22490). Mean and standard deviation values were obtained from three biological replicates. Analysis of variance with Tukey’s multiple comparison test was used to determine statistical significance, with different lowercase letters above bars indicating statistically significant differences (P < 0.05).
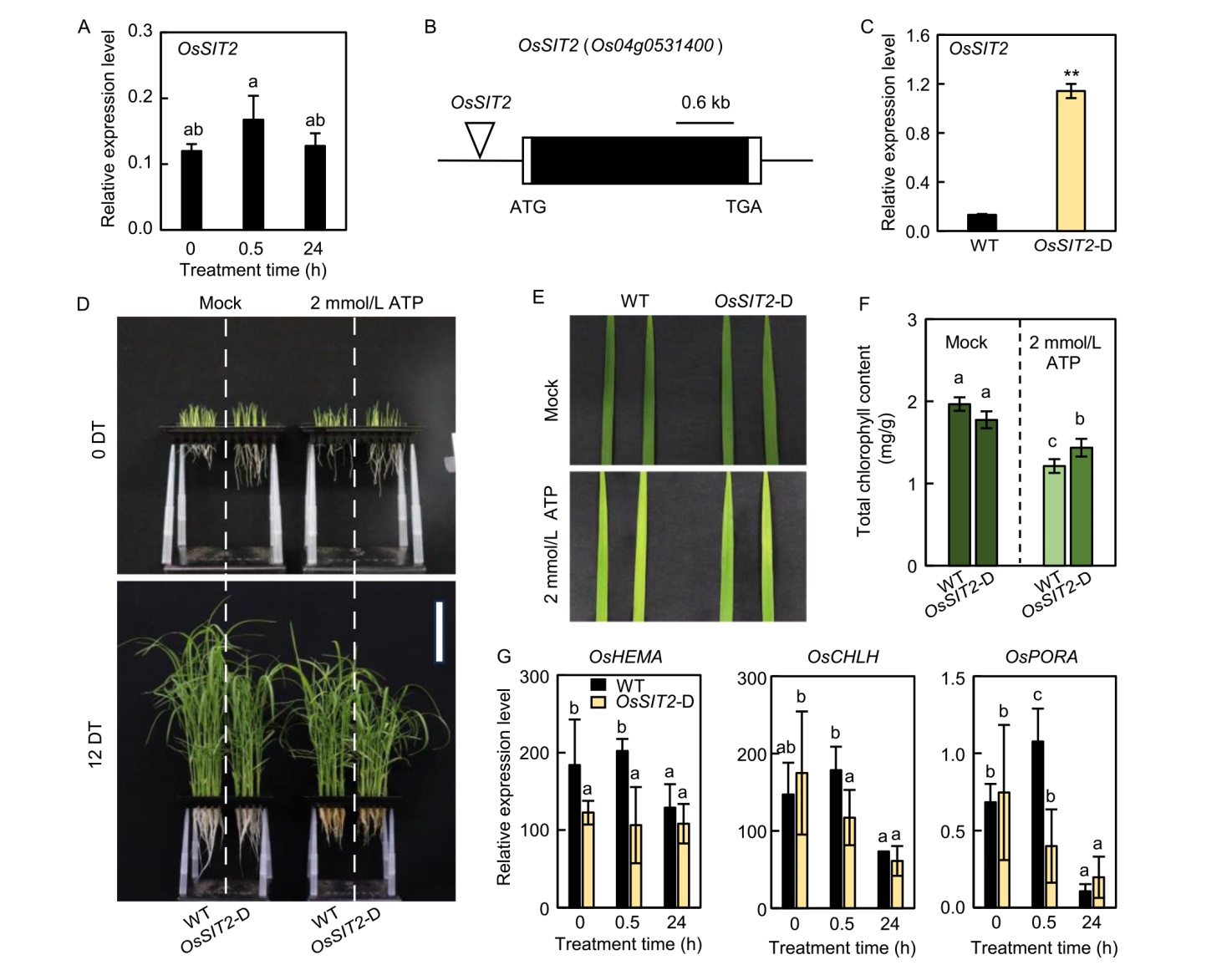
Fig. 7. OsSIT2 is involved in chlorophyll biosynthesis mediated by eATP. A, Relative transcript levels of OsSIT2 in the leaves of wild type (WT) and OsSIT2-D (enhancer-trapped T-DNA insertional activation line) plants in response to 1 mmol/L eATP.B, Schematic representation of the OsSIT2 locus (Os04g0531400) and its T-DNA insertional activation line OsSIT2-D. The upside-down triangle indicates the position of the T-DNA insertion (OsSIT2-D, PFG_3A-10378). The black box represents the exon and the two white boxes represent the 5′-untranslated region and 3′-untranslated region. C, Expression of OsSIT2 was measured in the leaves of WT and OsSIT2 plants grown in a plant growth chamber for 10 d. Asterisks indicate statistically significant differences between WT and OsSIT2-D plants, as determined by Student’s t-test (**, P < 0.01). D, Comparison of WT and OsSIT2-D plants at 0 and 12 d after eATP treatment (DT). E, Leaf greenness phenotypes of 2 mmol/L ATP-treated leaves of WT and OsSIT2-D at 12 DT. F, Changes in total chlorophyll content of WT and OsSIT2-D at 12 DT. G, Expression of OsHEMA, OsCHLH, and OsPORA was responsive to 2 mmol/L ATP treatment. The leaves of 5-day-old WT plants were exposed to 1 mmol/L ATP for 0, 0.5, and 24 h. The relative expression levels of OsHEMA, OsCHLH, and OsPORA were normalized to the OsUBQ5 (Os01g22490). Mean and standard deviation values were obtained from three biological replicates. Analysis of variance with Tukey’s multiple comparison test was used to determine statistical significance, with different lowercase letters above bars indicating statistically significant differences (P < 0.05).
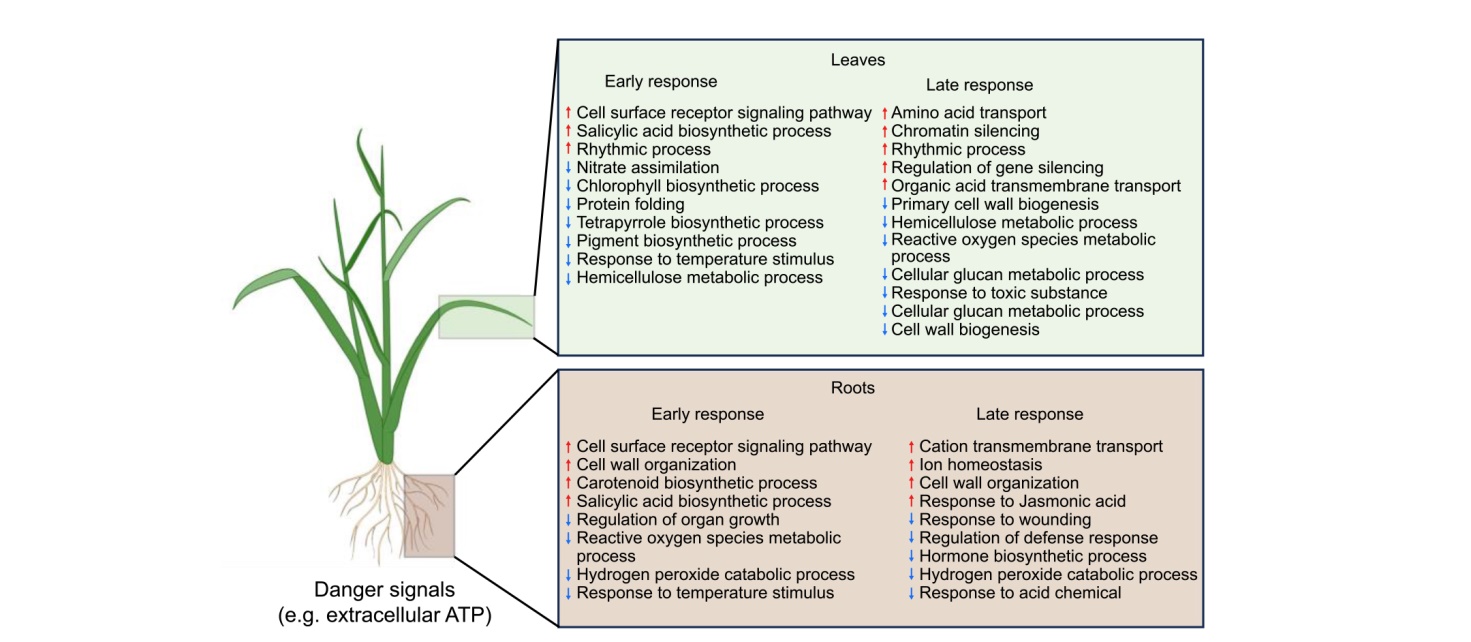
Fig. 8. A schematic representation of proposed effects of leaf and root on molecular processes in eATP-treated rice plants. Red and blue arrows indicate upregulated and downregulated genetic pathways in response to eATP, respectively.
| [1] | Adachi H, Nakano T, Miyagawa N, et al. 2015. WRKY transcription factors phosphorylated by MAPK regulate a plant immune NADPH oxidase in Nicotiana benthamiana. Plant Cell, 27(9): 2645-2663. |
| [2] | Bellard C, Bertelsmeier C, Leadley P, et al. 2012. Impacts of climate change on the future of biodiversity. Ecol Lett, 15(4): 365-377. |
| [3] | Benedetti M, Pontiggia D, Raggi S, et al. 2015. Plant immunity triggered by engineered in vivo release of oligogalacturonides, damage-associated molecular patterns. Proc Natl Acad Sci USA, 112(17): 5533-5538. |
| [4] | Bigeard J, Colcombet J, Hirt H. 2015. Signaling mechanisms in pattern-triggered immunity (PTI). Mol Plant, 8(4): 521-539. |
| [5] | Cao Y R, Tanaka K, Nguyen C T, et al. 2014. Extracellular ATP is a central signaling molecule in plant stress responses. Curr Opin Plant Biol, 20: 82-87. |
| [6] | Chen D Q, Cao Y R, Li H, et al. 2017. Extracellular ATP elicits DORN1-mediated RBOHD phosphorylation to regulate stomatal aperture. Nat Commun, 8(1): 2265. |
| [7] | Chen D Q, Hao F S, Mu H Q, et al. 2021. S-acylation of P2K 1 mediates extracellular ATP-induced immune signaling in Arabidopsis. Nat Commun, 12(1): 2750. |
| [8] | Chen H, Fang R Q, Deng R F, et al. 2021. The OsmiRNA166b-OsHox32 pair regulates mechanical strength of rice plants by modulating cell wall biosynthesis. Plant Biotechnol J, 19(7): 1468-1480. |
| [9] | Chivasa S, Ndimba B K, Simon W J, et al. 2005. Extracellular ATP functions as an endogenous external metabolite regulating plant cell viability. Plant Cell, 17(11): 3019-3034. |
| [10] | Cho S H, Tóth K, Kim D, et al. 2022. Activation of the plant mevalonate pathway by extracellular ATP. Nat Commun, 13(1): 450. |
| [11] | Choi J, Tanaka K, Cao Y R, et al. 2014. Identification of a plant receptor for extracellular ATP. Science, 343: 290-294. |
| [12] | Clark G, Torres J, Finlayson S, et al. 2010. Apyrase (nucleoside triphosphate-diphosphohydrolase) and extracellular nucleotides regulate cotton fiber elongation in cultured ovules. Plant Physiol, 152(2): 1073-1083. |
| [13] | Deng S R, Sun J, Zhao R, et al. 2015. Populus euphratica APYRASE2 enhances cold tolerance by modulating vesicular trafficking and extracellular ATP in Arabidopsis plants. Plant Physiol, 169(1): 530-548. |
| [14] | Duong H N, Cho S H, Wang L M, et al. 2022. Cyclic nucleotide-gated ion channel 6 is involved in extracellular ATP signaling and plant immunity. Plant J, 109(6): 1386-1396. |
| [15] | Emenecker R J, Strader L C. 2020. Auxin-abscisic acid interactions in plant growth and development. Biomolecules, 10(2): 281. |
| [16] | Feng H Q, Guan D D, Bai J Y, et al. 2015. Extracellular ATP: A potential regulator of plant cell death. Mol Plant Pathol, 16(6): 633-639. |
| [17] | Frost C J, Mescher M C, Carlson J E, et al. 2008. Plant defense priming against herbivores: Getting ready for a different battle. Plant Physiol, 146(3): 818-824. |
| [18] | Gong J Q, Wang Z Y, Guo Z J, et al. 2023. DORN1 and GORK regulate stomatal closure in Arabidopsis mediated by volatile organic compound ethyl vinyl ketone. Int J Biol Macromol, 231: 123503. |
| [19] | Gust A A, Pruitt R, Nürnberger T. 2017. Sensing danger: Key to activating plant immunity. Trends Plant Sci, 22(9): 779-791. |
| [20] | Hamann E, Blevins C, Franks S J, et al. 2021. Climate change alters plant-herbivore interactions. New Phytol, 229(4): 1894-1910. |
| [21] | Hao L H, Wang W X, Chen C, et al. 2012. Extracellular ATP promotes stomatal opening of Arabidopsis thaliana through heterotrimeric G protein α subunit and reactive oxygen species. Mol Plant, 5(4): 852-864. |
| [22] | He Y G, Guan H M, Li B, et al. 2023. Transcriptome analysis reveals the dynamic and rapid transcriptional reprogramming involved in heat stress and identification of heat response genes in rice. Int J Mol Sci, 24(19): 14802. |
| [23] | Hou S G, Liu Z Y, Shen H X, et al. 2019. Damage-associated molecular pattern-triggered immunity in plants. Front Plant Sci, 10: 646. |
| [24] | Huang J L, Qin F, Zang G C, et al. 2013. Mutation of OsDET1 increases chlorophyll content in rice. Plant Sci, 210: 241-249. |
| [25] | Hudson D, Guevara D R, Hand A J, et al. 2013. Rice cytokinin GATA Transcription Factor1 regulates chloroplast development and plant architecture. Plant Physiol, 162(1): 132-144. |
| [26] | Inoue H, Kobayashi T, Nozoye T, et al. 2009. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem, 284(6): 3470-3479. |
| [27] | Ishimaru Y, Masuda H, Bashir K, et al. 2010. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J, 62(3): 379-390. |
| [28] | Jang Y H, Park J R, Kim E G, et al. 2022. OsbHLHq11, the basic helix-loop-helix transcription factor, involved in regulation of chlorophyll content in rice. Biology-Basel, 11(7): 1000. |
| [29] | Jeandet P, Formela-Luboińska M, Labudda M, et al. 2022. The role of sugars in plant responses to stress and their regulatory function during development. Int J Mol Sci, 23(9): 5161. |
| [30] | Jeong D H, An S, Kang H G, et al. 2002. T-DNA insertional mutagenesis for activation tagging in rice. Plant Physiol, 130(4): 1636-1644. |
| [31] | Jewell J B, Tanaka K. 2019. Transcriptomic perspective on extracellular ATP signaling: A few curious trifles. Plant Signal Behav, 14(11): 1659079. |
| [32] | Jewell J B, Sowders J M, He R F, et al. 2019. Extracellular ATP shapes a defense-related transcriptome both independently and along with other defense signaling pathways. Plant Physiol, 179(3): 1144-1158. |
| [33] | Jewell J B, Berim A, Tripathi D, et al. 2022. Activation of indolic glucosinolate pathway by extracellular ATP in Arabidopsis. Plant Physiol, 190(3): 1574-1578. |
| [34] | Kawahara Y, de la Bastide M, Hamilton J P, et al. 2013. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice, 6(1): 4. |
| [35] | Khakh B S, Burnstock G. 2009. The double life of ATP. Sci Am, 301(6): 84-90/92. |
| [36] | Khush G S. 2005. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol, 59(1): 1-6. |
| [37] | Kim D, Paggi J M, Park C, et al. 2019. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat Biotechnol, 37(8): 907-915. |
| [38] | Kim D, Chen D Q, Ahsan N, et al. 2023. The Raf-like MAPKKK INTEGRIN-LINKED KINASE 5 regulates purinergic receptor-mediated innate immunity in Arabidopsis. Plant Cell, 35(5): 1572-1592. |
| [39] | Kim S Y, Sivaguru M, Stacey G. 2006. Extracellular ATP in plants: Visualization, localization, and analysis of physiological significance in growth and signaling. Plant Physiol, 142(3): 984-992. |
| [40] | Kuai P, Lou Y G. 2024. Advances in molecular interactions between rice and insect herbivores. Crop Health, 2(1): 6. |
| [41] | Lew R R, Dearnaley J D W. 2000. Extracellular nucleotide effects on the electrical properties of growing Arabidopsis thaliana root hairs. Plant Sci, 153(1): 1-6. |
| [42] | Li C H, Wang G, Zhao J L, et al. 2014. The receptor-like kinase SIT1 mediates salt sensitivity by activating MAPK3/6 and regulating ethylene homeostasis in rice. Plant Cell, 26(6): 2538-2553. |
| [43] | Li Z Y, Mo W P, Jia L Q, et al. 2019. Rice FLUORESCENT1 is involved in the regulation of chlorophyll. Plant Cell Physiol, 60(10): 2307-2318. |
| [44] | Liao Y, Smyth G K, Shi W. 2014. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics, 30(7): 923-930. |
| [45] | Lim C, Kim Y, Shim Y, et al. 2024. Rice OsGATA16 is a positive regulator for chlorophyll biosynthesis and chloroplast development. Plant J, 117(2): 599-615. |
| [46] | Liu X L, Tian Y L, Chi W C, et al. 2022. Alternative splicing of OsGS1;1 affects nitrogen-use efficiency, grain development, and amylose content in rice. Plant J, 110(6): 1751-1762. |
| [47] | Livak K J, Schmittgen T D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔC(T) method. Methods, 25(4): 402-408. |
| [48] | Lu G W, Casaretto J A, Ying S, et al. 2017. Overexpression of OsGATA12 regulates chlorophyll content, delays plant senescence and improves rice yield under high density planting. Plant Mol Biol, 94(1/2): 215-227. |
| [49] | Ma H G, Gao Y J, Wang Y G, et al. 2022. Regulatory mechanisms of mitogen-activated protein kinase cascades in plants: More than sequential phosphorylation. Int J Mol Sci, 23(7): 3572. |
| [50] | Morrissey J, Guerinot M L. 2009. Iron uptake and transport in plants: The good, the bad, and the ionome. Chem Rev, 109(10): 4553-4567. |
| [51] | Murugesan S, Chelliah S. 1986. Yield loss and economic injury by rice leaf-folder. Indian J Agric Sci, 56(4): 282-285. |
| [52] | Myers R J, Fichman Y, Stacey G, et al. 2022. Extracellular ATP plays an important role in systemic wound response activation. Plant Physiol, 189(3): 1314-1325. |
| [53] | Nakamura H, Muramatsu M, Hakata M, et al. 2009. Ectopic overexpression of the transcription factor OsGLK1 induces chloroplast development in non-green rice cells. Plant Cell Physiol, 50(11): 1933-1949. |
| [54] | Nguyen L T, Schmidt H A, von Haeseler A, et al. 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol, 32(1): 268-274. |
| [55] | Padmavathi C, Katti G, Padmakumari A P, et al. 2013. The effect of leaffolder Cnaphalocrocis medinalis (guenee) [Lepidoptera: Pyralidae] injury on the plant physiology and yield loss in rice. J Appl Entomol, 137(4): 249-256. |
| [56] | Pham A Q, Cho S H, Nguyen C T, et al. 2020. Arabidopsis lectin receptor kinase P2K2 is a second plant receptor for extracellular ATP and contributes to innate immunity. Plant Physiol, 183(3): 1364-1375. |
| [57] | Porra R J, Thompson W A, Kriedemann P E. 1989. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta Bioenerg, 975(3): 384-394. |
| [58] | Quevillon E, Silventoinen V, Pillai S, et al. 2005. InterProScan: Protein domains identifier. Nucleic Acids Res, 33: W116-W120. |
| [59] | Robinson M D, McCarthy D J, Smyth G K. 2010. edgeR: A bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics, 26(1): 139-140. |
| [60] | Roux S J, Song C, Jeter C. 2006. Regulation of plant growth and development by extracellular nucleotides. In: Baluška F, Mancuso S, Volkmann D. Communication in Plants: Neuronal Aspects of Plant Life Berlin, Heidelberg: Springer Berlin Heidelberg: 221-234. |
| [61] | Ruan J J, Zhou Y X, Zhou M L, et al. 2019. Jasmonic acid signaling pathway in plants. Int J Mol Sci, 20(10): 2479. |
| [62] | Sakuraba Y, Kim E Y, Han S H, et al. 2017. Rice Phytochrome-Interacting Factor-Like1 (OsPIL1) is involved in the promotion of chlorophyll biosynthesis through feed-forward regulatory loops. J Exp Bot, 68(15): 4103-4114. |
| [63] | Savary S, Willocquet L, Pethybridge S J, et al. 2019. The global burden of pathogens and pests on major food crops. Nat Ecol Evol, 3(3): 430-439. |
| [64] | Snoeck S, Guayazán-Palacios N, Steinbrenner A D. 2022. Molecular tug-of-war: Plant immune recognition of herbivory. Plant Cell, 34(5): 1497-1513. |
| [65] | Song C J, Steinebrunner I, Wang X Z, et al. 2006. Extracellular ATP induces the accumulation of superoxide via NADPH oxidases in Arabidopsis. Plant Physiol, 140(4): 1222-1232. |
| [66] | Sowders J M, Tanaka K. 2023. A histochemical reporter system to study extracellular ATP response in plants. Front Plant Sci, 14: 1183335. |
| [67] | Sowders J M, Jewell J B, Tripathi D, et al. 2023. The intrinsically disordered C-terminus of purinoceptor P2K1 fine-tunes plant responses to extracellular ATP. FEBS Lett, 597(16): 2059-2071. |
| [68] | Steinebrunner I, Wu J, Sun Y, et al. 2003. Disruption of apyrases inhibits pollen germination in Arabidopsis. Plant Physiol, 131(4): 1638-1647. |
| [69] | Takahashi R, Ishimaru Y, Nakanishi H, et al. 2011. Role of the iron transporter OsNRAMP1 in cadmium uptake and accumulation in rice. Plant Signal Behav, 6(11): 1813-1816. |
| [70] | Tanaka K, Heil M. 2021. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu Rev Phytopathol, 59: 53-75. |
| [71] | Tanaka K, Swanson S J, Gilroy S, et al. 2010. Extracellular nucleotides elicit cytosolic free calcium oscillations in Arabidopsis. Plant Physiol, 154(2): 705-719. |
| [72] | Tanaka K, Choi J, Cao Y R, et al. 2014. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci, 5: 446. |
| [73] | Tang W Q, Brady S R, Sun Y, et al. 2003. Extracellular ATP inhibits root gravitropism at concentrations that inhibit polar auxin transport. Plant Physiol, 131(1): 147-154. |
| [74] | Terrile M C, Tonón C V, Iglesias M J, et al. 2010. Extracellular ATP and nitric oxide signaling pathways regulate redox-dependent responses associated to root hair growth in etiolated Arabidopsis seedlings. Plant Signal Behav, 5(6): 698-701. |
| [75] | Thomas C, Sun Y, Naus K, et al. 1999. Apyrase functions in plant phosphate nutrition and mobilizes phosphate from extracellular ATP. Plant Physiol, 119(2): 543-552. |
| [76] | Tonón C, Cecilia Terrile M, José Iglesias M, et al. 2010. Extracellular ATP, nitric oxide and superoxide act coordinately to regulate hypocotyl growth in etiolated Arabidopsis seedlings. J Plant Physiol, 167(7): 540-546. |
| [77] | Tripathi D, Zhang T, Koo A J, et al. 2018. Extracellular ATP acts on jasmonate signaling to reinforce plant defense. Plant Physiol, 176(1): 511-523. |
| [78] | Udvardy J, Farkas G L. 1973. ATP stimulates the formation of nucleases in excised Avena leaves. Zeitschrift für Pflanzenphysiologie, 69(5): 394-401. |
| [79] | Ullah A, Akbar A, Yang X Y. 2019. Jasmonic acid (JA)-mediated signaling in leaf senescence. In: Sarwat M, Tuteja N. Senescence Signalling and Control in Plants. Amsterdam, the Netherland: Elsevier: 111-123. |
| [80] | Ursache R, Andersen T G, Marhavý P, et al. 2018. A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J, 93(2): 399-412. |
| [81] | Vaid N, Pandey P K, Tuteja N. 2012. Genome-wide analysis of lectin receptor-like kinase family from Arabidopsis and rice. Plant Mol Biol, 80(4/5): 365-388. |
| [82] | Vitoriano C B, Calixto C P G. 2021. Reading between the lines: RNA-seq data mining reveals the alternative message of the rice leaf transcriptome in response to heat stress. Plants, 10(8): 1647. |
| [83] | Wang L M, Ning Y Z, Sun J, et al. 2022. Arabidopsis thaliana CYCLIC NUCLEOTIDE-GATED CHANNEL2 mediates extracellular ATP signal transduction in root epidermis. New Phytol, 234(2): 412-421. |
| [84] | Weerasinghe R R, Swanson S J, Okada S F, et al. 2009. Touch induces ATP release in Arabidopsis roots that is modulated by the heterotrimeric G-protein complex. FEBS Lett, 583(15): 2521-2526. |
| [85] | Wu J, Steinebrunner I, Sun Y, et al. 2007. Apyrases (nucleoside triphosphate-diphosphohydrolases) play a key role in growth control in Arabidopsis. Plant Physiol, 144(2): 961-975. |
| [86] | Wu Y S, Qin B Z, Feng K L, et al. 2018. Extracellular ATP promoted pollen germination and tube growth of Nicotiana tabacum through promoting K+ and Ca2+ absorption. Plant Reprod, 31(4): 399-410. |
| [87] | Xiang X, Liu S H, Li H J, et al. 2023. Defense strategies of rice in response to the attack of the herbivorous insect, Chilo suppressalis. Int J Mol Sci, 24(18): 14361. |
| [88] | Yang X Y, Wang B C, Farris B, et al. 2015. Modulation of root skewing in Arabidopsis by apyrases and extracellular ATP. Plant Cell Physiol, 56(11): 2197-2206. |
| [89] | Yoshida S, Fomo D A, Cock J H, et al. 1976. Routine procedure for growing rice plants in culture solution. In: Laboratory Manual for Physiological Studies of Rice. Los Banos, Laguna, the Philippines: International Rice Research Institute: 57-63. |
| [90] | Yu G C, Wang L G, Han Y Y, et al. 2012. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS, 16(5): 284-287. |
| [91] | Yu X D, Xu Y R, Yan S P. 2021. Salicylic acid and ethylene coordinately promote leaf senescence. J Integr Plant Biol, 63(5): 823-827. |
| [92] | Zhang C, Zhang J X, Tang Y J, et al. 2021. DEEP GREEN PANICLE1 suppresses GOLDEN2-LIKE activity to reduce chlorophyll synthesis in rice glumes. Plant Physiol, 185(2): 469-477. |
| [93] | Zhao L M, Wang H J, Martins P D, et al. 2023. The Arabidopsis thaliana onset of leaf death 12 mutation in the lectin receptor kinase P2K2 results in an autoimmune phenotype. BMC Plant Biol, 23(1): 294. |
| [94] | Zhu R J, Dong X X, Hao W W, et al. 2017. Heterotrimeric G protein-regulated Ca2+ influx and PIN2 asymmetric distribution are involved in Arabidopsis thaliana roots’ avoidance response to extracellular ATP. Front Plant Sci, 8: 1522. |
| [1] | Chen Su, Ma Feilong, Chen Jiaoyang, Qi Man, Wei Qianshu, Tao Zhihuan, Sun Bo. Function of R2R3-Type Myeloblastosis Transcription Factors in Plants [J]. Rice Science, 2025, 32(3): 307-321. |
| [2] | Yang Yajun, Lu Yanhui, Tian Junce, Zheng Xusong, Guo Jiawen, Liu Xiaowei, Lü Zhongxian, Xu Hongxing. Sustainable Management Strategies for Rice Leaffolder, Cnaphalocrocis medinalis (Guenée): Progress and Prospects [J]. Rice Science, 2025, 32(3): 322-338. |
| [3] | Xie Yuhao, Xie Wenya, Zhao Jianhua, Xue Xiang, Cao Wenlei, Shi Xiaopin, Wang Zhou, Wang Yiwen, Wang Guangda, Feng Zhiming, Hu Keming, Chen Xijun, Chen Zongxiang, Zuo Shimin. OsERF7 Negatively Regulates Resistance to Sheath Blight Disease by Inhibiting Phytoalexin Biosynthesis [J]. Rice Science, 2025, 32(3): 367-379. |
| [4] | Zeng Deyong, Cui Jie, Yin Yishu, Dai Cuihong, Yu Wencheng, Zhao Haitian, Guan Shuanghong, Cheng Dayou, Sun Yeqing, Lu Weihong. Generational Genetic Mechanism of Space Mutagenesis in Rice Based on Multi-Omics [J]. Rice Science, 2025, 32(3): 400-425. |
| [5] | Sanchika Snehi, Ravi Kiran Kt, Sanket Rathi, Sameer Upadhyay, Suneetha Kota, Satish Kumar Sanwal, Lokeshkumar Bm, Arun Balasubramaniam, Nitish Ranjan Prakash, Pawan Kumar Singh. Discerning Genes to Deliver Varieties: Enhancing Vegetative- and Reproductive-Stage Flooding Tolerance in Rice [J]. Rice Science, 2025, 32(2): 160-176. |
| [6] | Nie Lixiao, Guo Xiayu, Wang Weiqin, Qi Yucheng, Ai Zhiyong, He Aibin. Regulation of Regeneration Rate to Enhance Ratoon Rice Production [J]. Rice Science, 2025, 32(2): 177-192. |
| [7] | Wang Shuman, Zhang Linqi, Gao Ruiren, Wei Guangbo, Dong Weiguo, Xu Jiming, Wang Zhiye. Establishing Programmable CRISPR/Cas13b-Mediated Knockdown System in Rice [J]. Rice Science, 2025, 32(2): 217-227. |
| [8] | Uthpal Krishna Roy, Babita Pal, Soumen Bhattacharjee. A Novel Approach for Screening Salinity-Tolerant Rice Germplasm by Exploring Redox-Regulated Cytological Fingerprint [J]. Rice Science, 2025, 32(2): 228-242. |
| [9] | He Zhenrui, Zhao Wenhua, Cheng Baoping, Yang Mei, Yang Yingqing, Zhu Yiming, Zhou Erxun. Molecular and Biological Characterization of Novel Mitovirus Infecting Phytopathogenic Fungus Ustilaginoidea virens [J]. Rice Science, 2025, 32(2): 243-258. |
| [10] | He Chen, Ruan Yunze, Jia Zhongjun. A Meta-Analysis of 30 Years in China and Micro-District Experiments Shows Organic Fertilizer Quantification Combined with Chemical Fertilizer Reduction Enhances Rice Yield on Saline-Alkali Land [J]. Rice Science, 2025, 32(2): 259-272. |
| [11] | Wang Mingyue, Zhao Weibo, Feng Xiaoya, Chen Yi, Li Junhao, Fu Jinmei, Yan Yingchun, Chu Zhaohui, Huang Wenchao. Disruption of Energy Metabolism and Reactive Oxygen Species Homeostasis in Honglian Type-Cytoplasmic Male Sterility (HL-CMS) Rice Pollen [J]. Rice Science, 2025, 32(1): 81-93. |
| [12] | Intan Farahanah, Shariza Sahudin, Hannis Fadzillah Mohsin, Siti Alwani Ariffin, Liyana Dhamirah Aminuddin. Understanding Investigational Perspective of Antioxidant and Antibacterial Properties of Rice [J]. Rice Science, 2025, 32(1): 15-31. |
| [13] | Jeberlin Prabina Bright, Hemant S. Maheshwari, Sugitha Thangappan, Kahkashan Perveen, Najat A. Bukhari, Debasis Mitra, Riyaz Sayyed, Andrea Mastinu. Biofilmed-PGPR: Next-Generation Bioinoculant for Plant Growth Promotion in Rice under Changing Climate [J]. Rice Science, 2025, 32(1): 94-106. |
| [14] | Wang Haoran, Chen Guoqing, Feng Guozhong. Expanding Viral Diversity in Rice Fields by Next-Generation Sequencing [J]. Rice Science, 2025, 32(1): 44-51. |
| [15] | Surangkana Chimthai, Sulaiman Cheabu, Wanchana Aesomnuk, Siriphat Ruengphayak, Siwaret Arikit, Apichart Vanavichit, Chanate Malumpong. Breeding for Heat Tolerant Aromatic Rice Varieties and Identification of Novel QTL Regions Associated with Heat Tolerance During Reproductive Phase by QTL-Seq [J]. Rice Science, 2025, 32(1): 67-80. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||