
Rice Science ›› 2025, Vol. 32 ›› Issue (3): 400-425.DOI: 10.1016/j.rsci.2025.03.001
• Research Papers • Previous Articles Next Articles
Zeng Deyong1,2,3, Cui Jie1,2, Yin Yishu1,2, Dai Cuihong1,2, Yu Wencheng1,2,3, Zhao Haitian1,2,3, Guan Shuanghong1,2, Cheng Dayou1,2, Sun Yeqing4, Lu Weihong1,2,3( )
)
Received:2024-07-16
Accepted:2025-01-23
Online:2025-05-28
Published:2025-06-16
Contact:
Lu Weihong (Zeng Deyong, Cui Jie, Yin Yishu, Dai Cuihong, Yu Wencheng, Zhao Haitian, Guan Shuanghong, Cheng Dayou, Sun Yeqing, Lu Weihong. Generational Genetic Mechanism of Space Mutagenesis in Rice Based on Multi-Omics[J]. Rice Science, 2025, 32(3): 400-425.
Add to citation manager EndNote|Ris|BibTeX
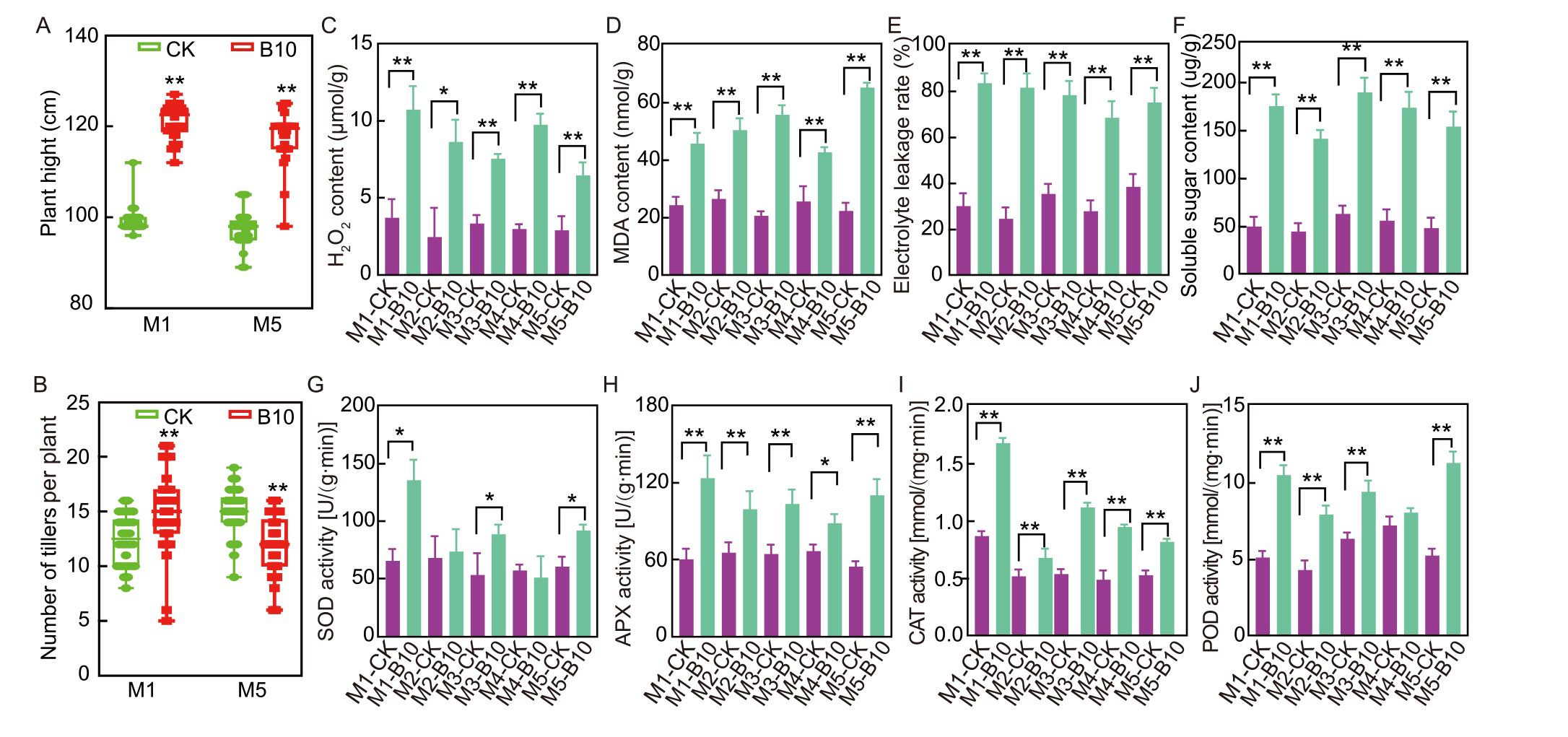
Fig. 1. Spaceflight induces phenotypic and physiological changes in different generations (M1-M5) of rice. A-J, Plant height (A), number of tillers per plant (B), H2O2 content (C), malondialdehyde (MDA) content (D), electrolyte leakage rate (E), soluble sugar content (F), superoxide dismutase (SOD) activity (G), ascorbate peroxidase (APX) activity (H), catalase (CAT) activity (I), and peroxidase (POD) activity (J) in different generation of rice. CK, Ground Dongnong 423; B10, Mutant of Dongnong 423 from SJ10 mission. In A and B, data are Mean ± SD (n = 30). In C-J, data are Mean ± SE (n = 3). * and ** indicate significant difference at the 0.05 and 0.01 levels by the Tukey’s test, respectively.
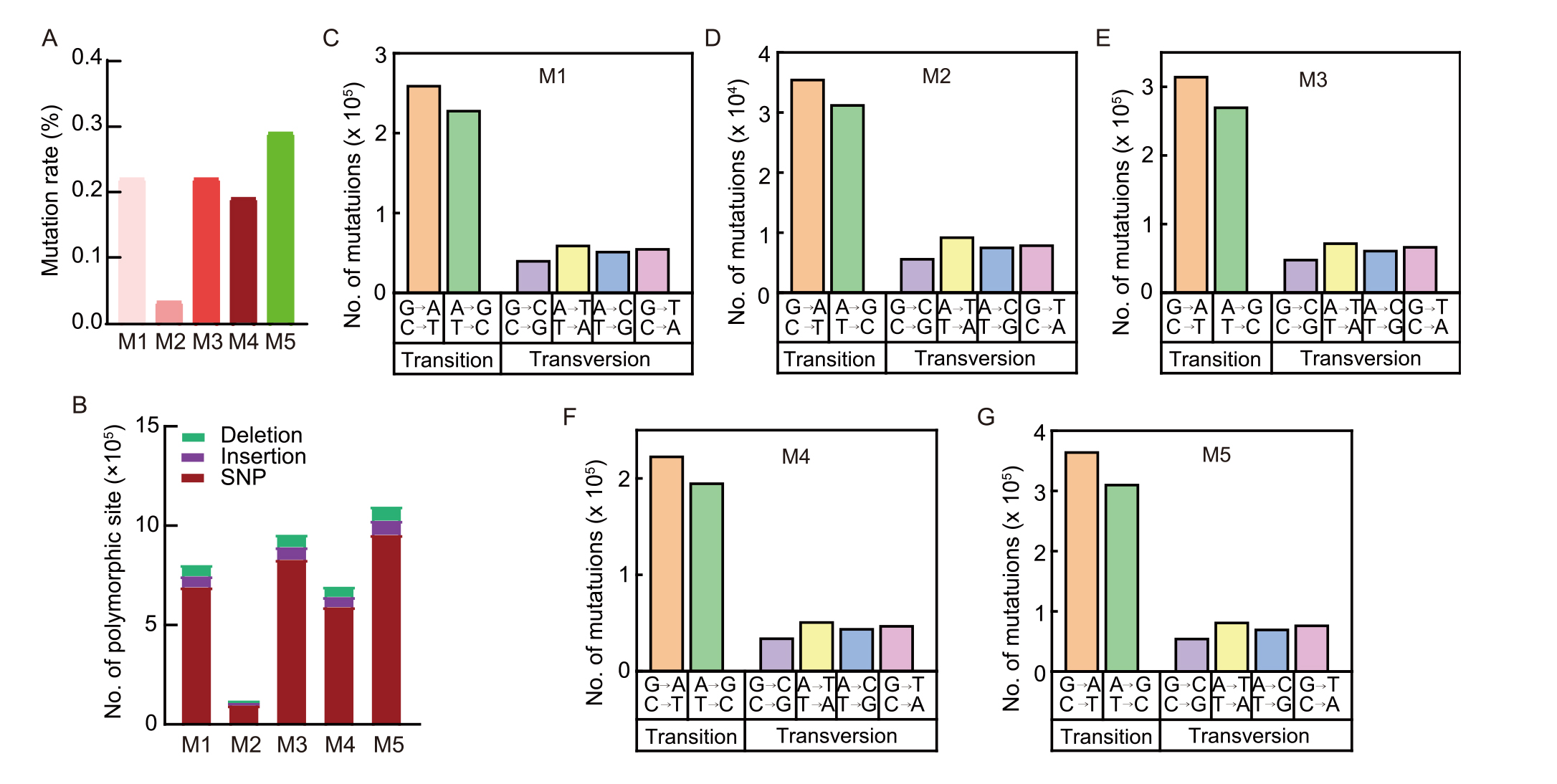
Fig. 2. Spaceflight-induced DNA polymorphisms and base substitution patterns. A, Genome mutation rate of mutant B10 in M1-M5 generations. B, Number of polymorphic loci of mutant B10 in M1-M5 generations. SNP, Single nucleotide polymorphism. C-G, Classification of base substitution mutations of mutant B10 in M1-M5 generations.
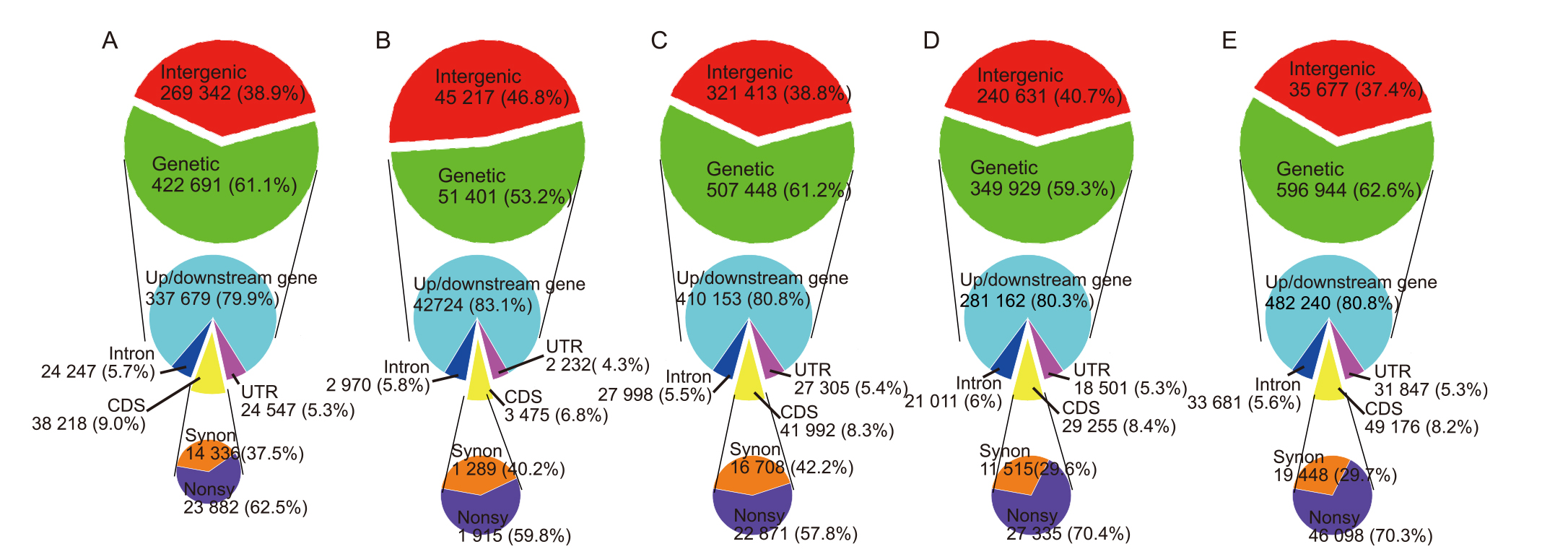
Fig. 3. Molecular characteristics of memory-induced single nucleotide polymorphisms under spaceflight stress in different generations of rice. A, M1 generation; B, M2 generation; C, M3 generation; D, M4 generation; E, M5 generation.CDS, Coding sequence; UTR, Untranslated region; Synon, Synonymous mutation; Nonsy, Nonsynonymous mutation.
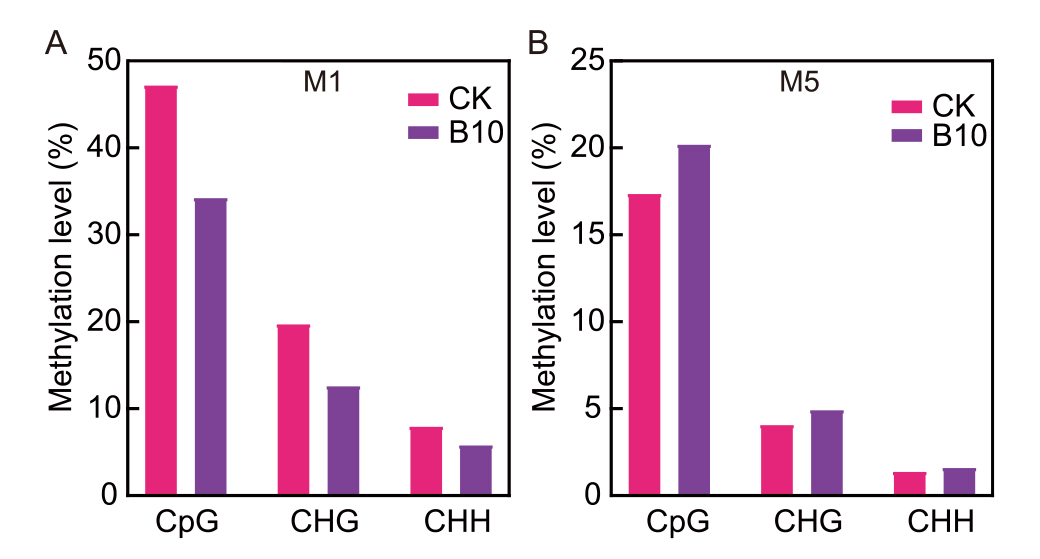
Fig. 4. Spaceflight induces methylation patterns in M1 (A) and M5 (B) generations of rice. CK, Ground Dongnong 423; B10, Mutant of Dongnong 423 from SJ10 mission.
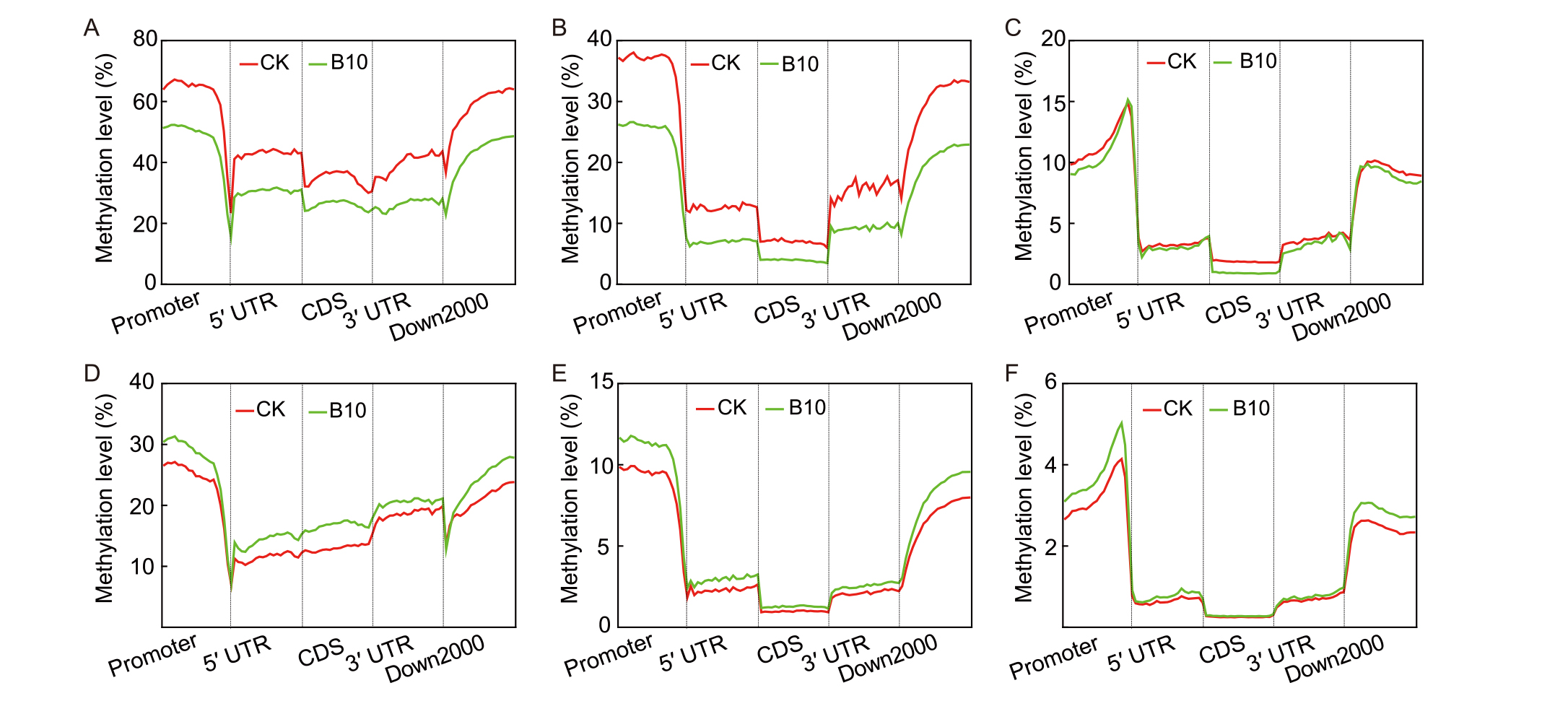
Fig. 5. Line graph of genome methylation of M1 and M5 generation rice in ground control and spaceflight groups. A-C, CpG methylation (A), CHG methylation (B), and CHH methylation (C) in the M1 generation.D-F, CpG methylation (D), CHG methylation (E), and CHH methylation (F) in the M5 generation.5′ UTR, 5′-Untranslated region; CDS, Coding sequence; 3′ UTR, 3′-Untranslated region; Down2000, 2000 kb downstream of the gene.
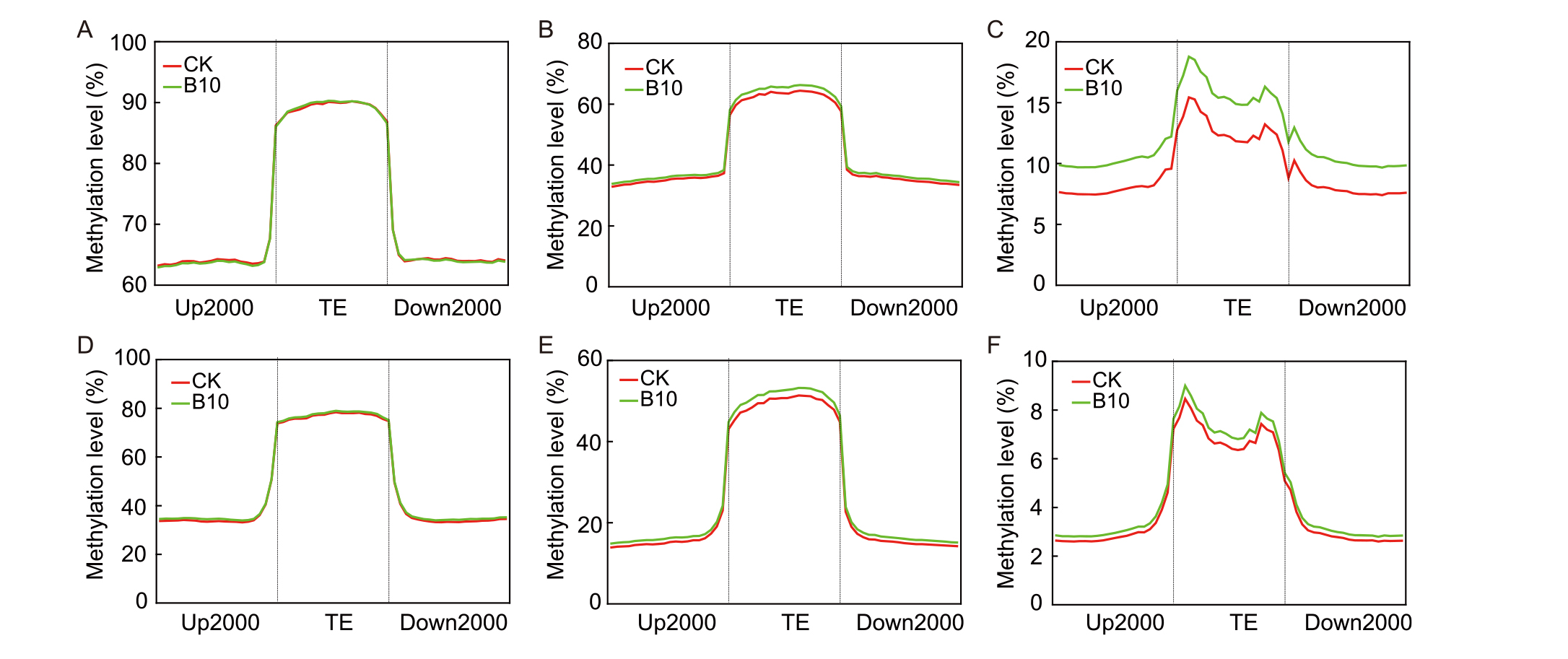
Fig. 6. Methylation patterns of transposable element (TE) in different generations of rice induced by spaceflight. A-C, CpG methylation (A), CHG methylation (B), and CHH methylation (C) in the M1 generation.D-F, CpG methylation (D), CHG methylation (E), and CHH methylation (F) in the M5 generation.Up2000, 2000-kb upstream of the gene; TE, Transposable element; Down 2000, 2000-kb downstream of the gene.
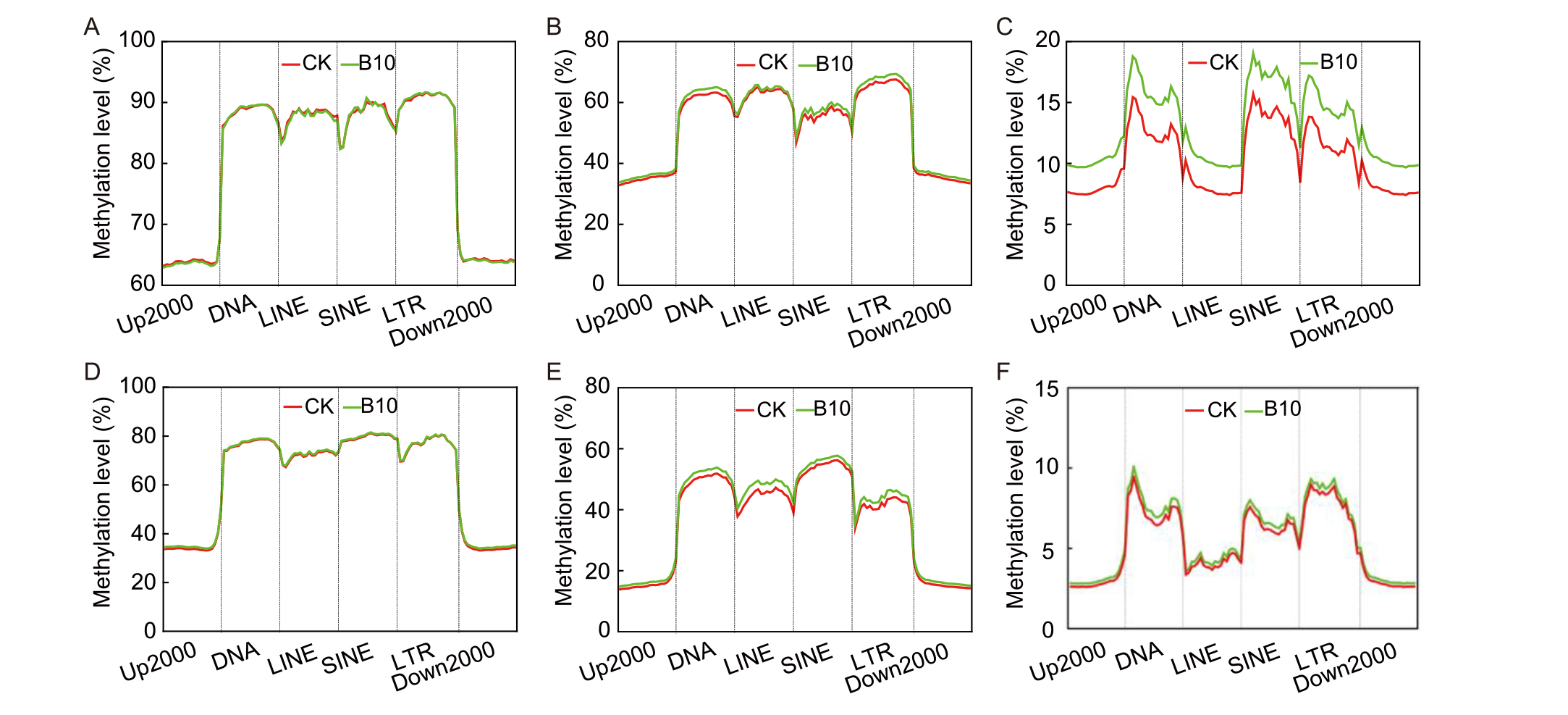
Fig. 7. Spaceflight-induced methylation change patterns of different classes of transposable elements in rice. A-C, CpG methylation (A), CHG methylation (B), and CHH methylation (C) in the M1 generation. D-E, CpG methylation (D), CHG methylation (E), and CHH methylation (F) environment in the M5 generation.Up2000, 2000-kb upstream of the gene; DNA, DNA transposon; LINE, Long interspersed nuclear element; SINE, Short interspersed nuclear element; LTR, Long terminal repeat; Down2000, 2000-kb downstream of the gene.
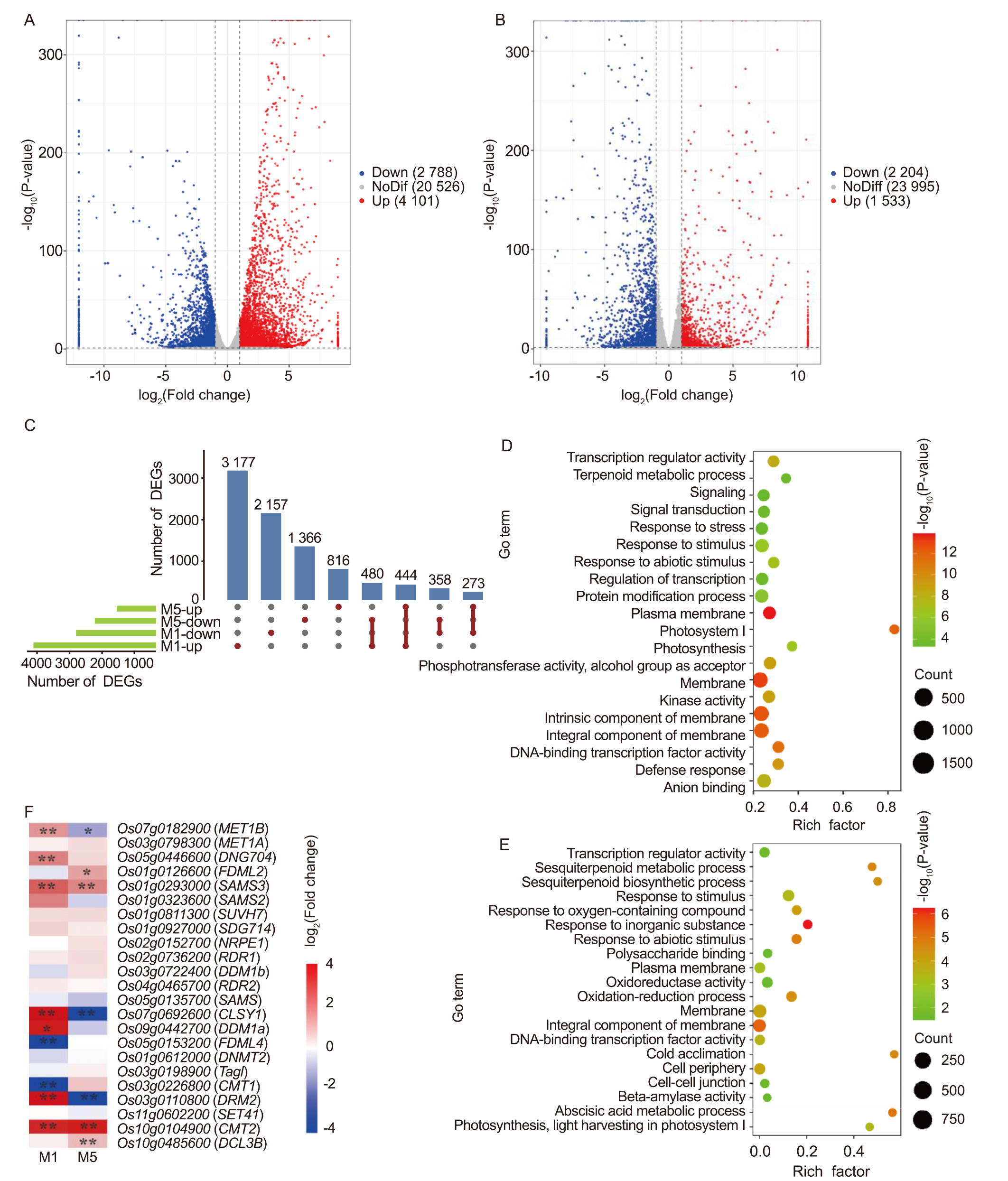
Fig. 8. Spaceflight stress memory induces differentially expressed genes (DEGs) in different rice generations. A and B, Volcano plots of DEGs in M1 (A) and M5 (B) generations. Down, Downregulated DEGs; Up, Upregulated DEGs; NoDif, Genes with no significant differential expression. C, UpSet plot showing unique and shared up- or downregulated DEGs between M1 and M5 generations.D and E, Gene Ontology (GO) analysis of DEGs in M1 (D) and M5 (E) generations. F, Expression changes of genes associated with DNA methylation or demethylation. * and ** represent significant differences at the 0.05 and 0.01 levels, respectively.
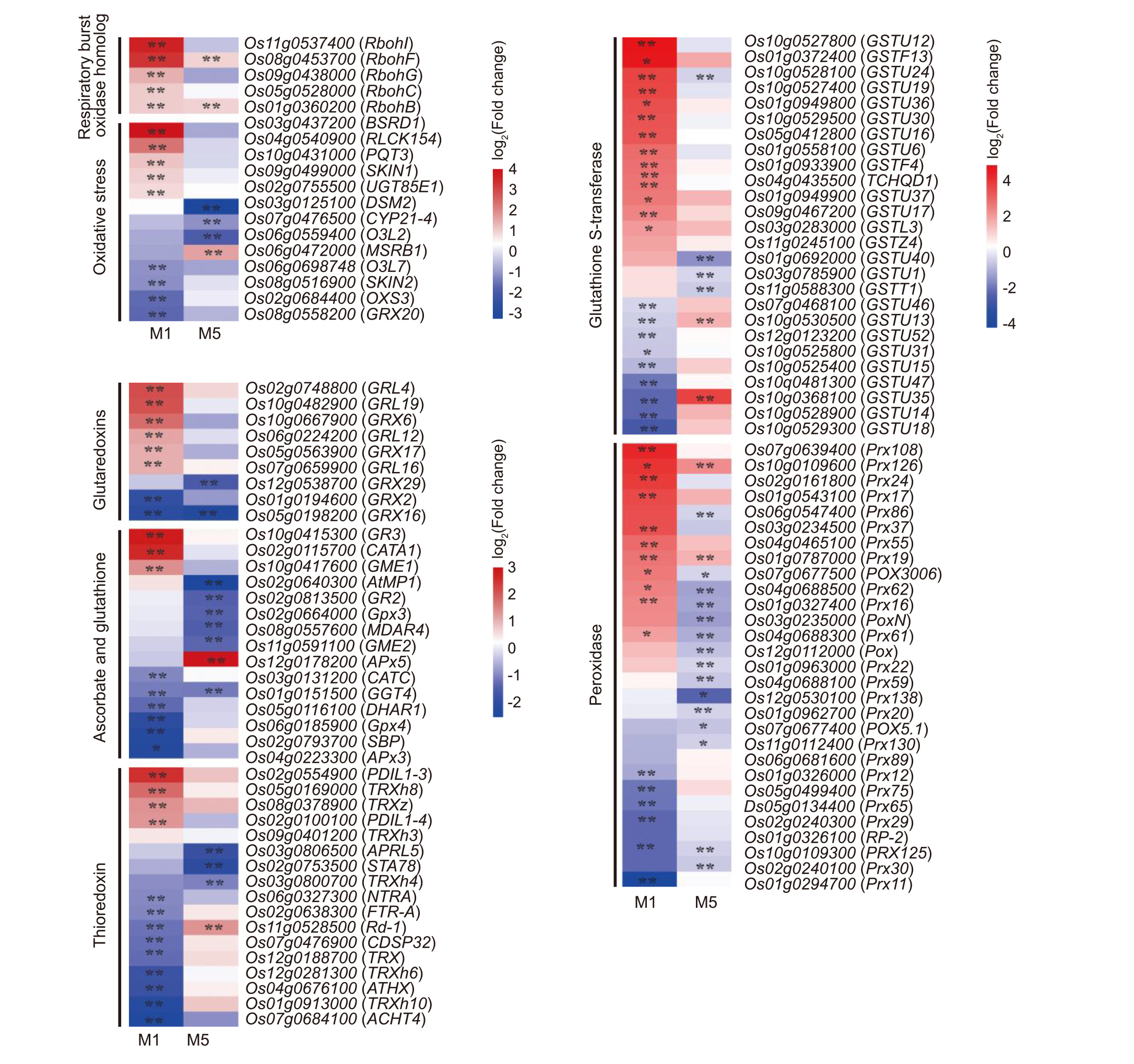
Fig. 9. Spaceflight stress memory induces differential expression of reactive oxygen species metabolism-related genes in different generations of rice. * and ** represent significant differences at the 0.05 and 0.01 levels, respectively.
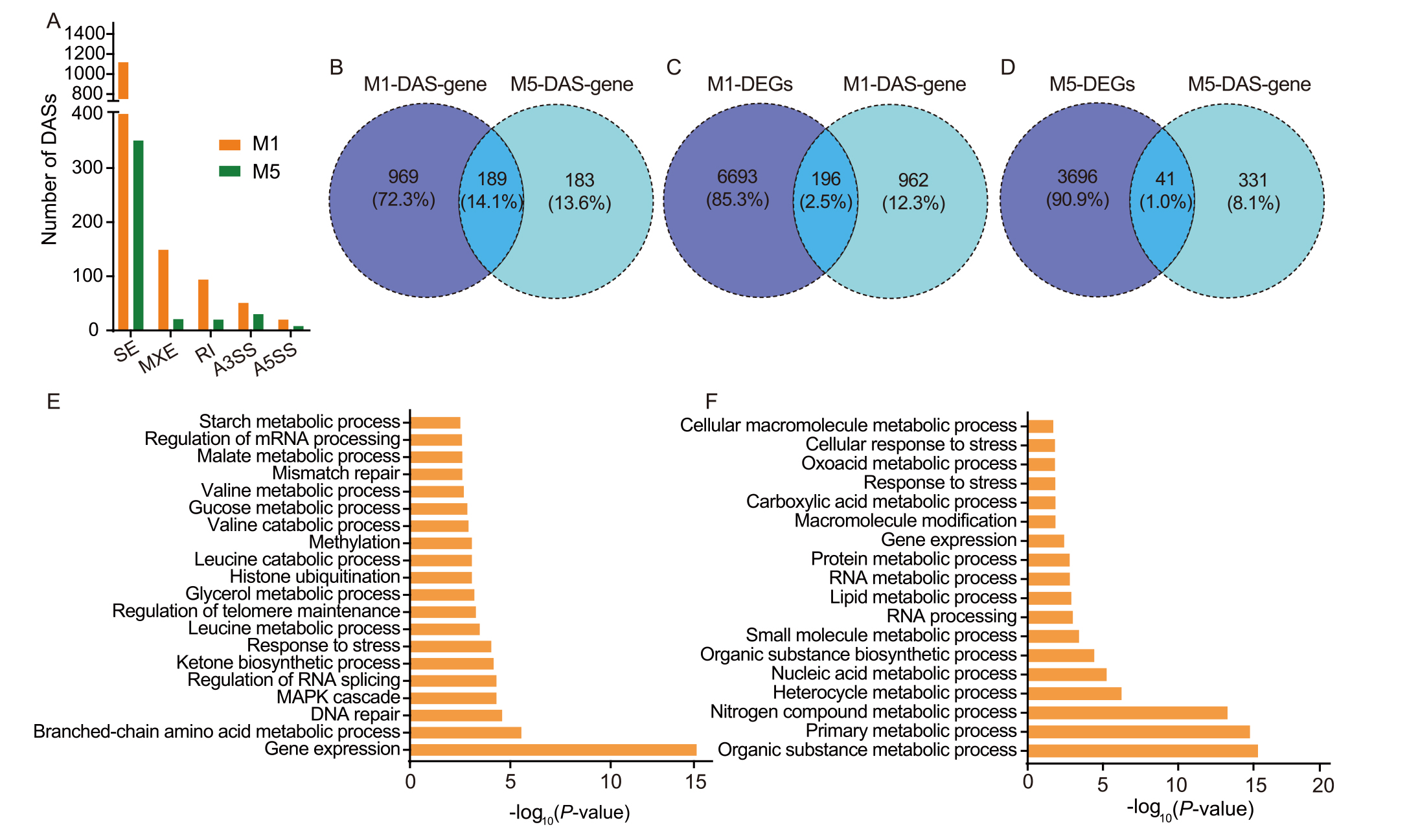
Fig. 10. Spaceflight induces differentially alternative splicing (DAS) in different generations of rice. A, Number of five different types of DAS events identified in M1 and M5 generations. SE, Skipped exon; MXE, Mutually exclusive exons; RI, Retained intron; A3SS, Alternative 3′ splice site; A5SS, Alternative 5′ splice site. B, Venn diagram of unique and shared DAS genes between M1 and M5 generations. C and D, Venn diagrams show the overlap of DAS genes and differentially expressed genes (DEGs) in M1 (C) and M5 (D) generations. E and F, Top 20 enriched Gene Ontology terms of DAS genes in M1 (E) and M5 (F) generations.
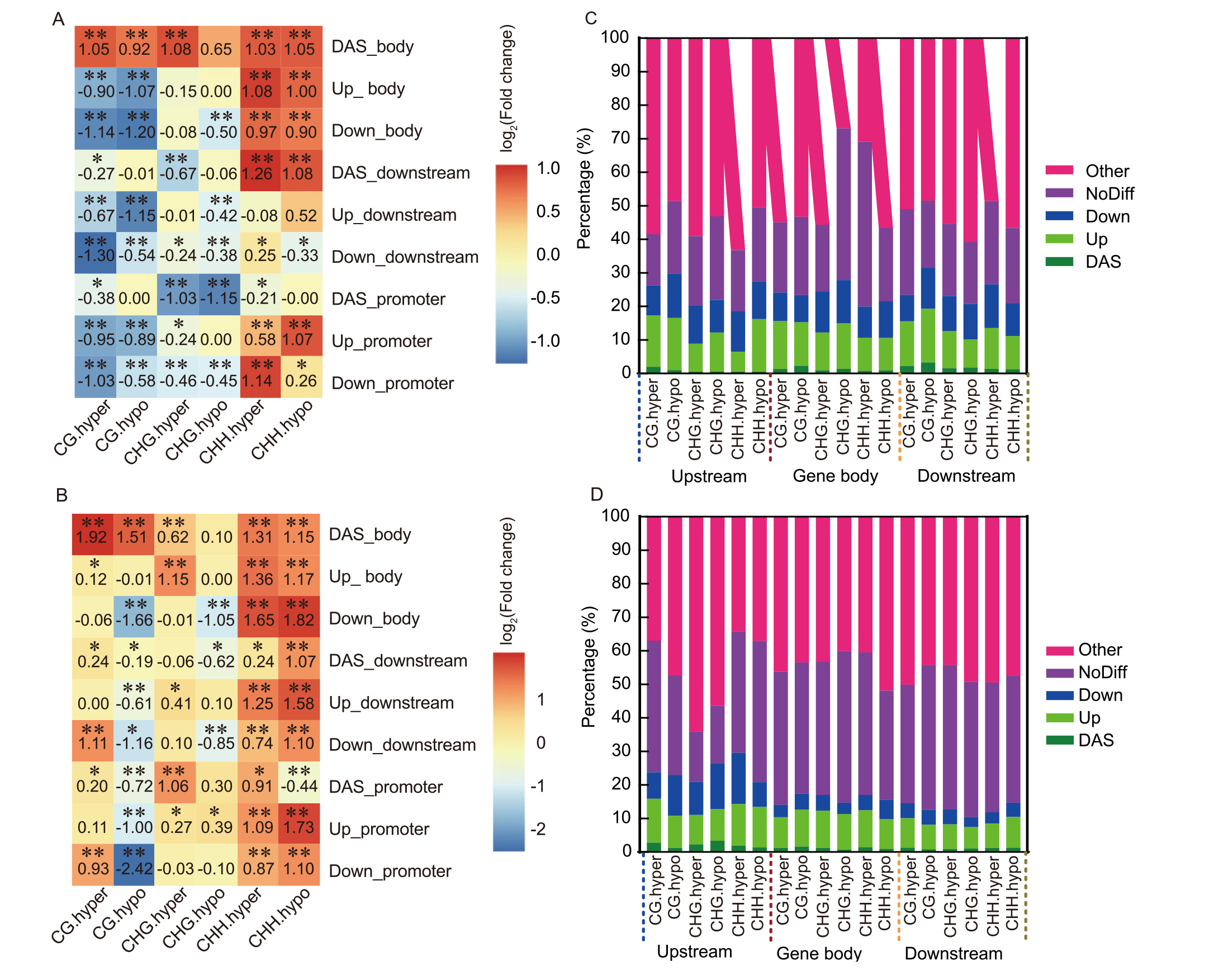
Fig. 11. Differentially methylated cytosines (DmCs) are biased among different types of genes. A and B, Comparison of DmC densities between up- or downregulated genes and differential alternative splicing (DAS) genes with randomly selected genes in upstream, body, or downstream regions in M1 (A) and M5 (B) generations. The value in the square is transformed from the P value estimated by Student’s t-test and adjusted by the false discovery rate (FDR) method. If the DmC densities of the differentially expresed gene or DAS gene are larger than those of the randomly selected genes, the value is set as log10(Padj) (minus value). If the densities are less than those of the randomly selected genes, the value is set as -log10(Padj) to distinguish this difference. C and D, Bar plots showing the ratios of different types of genes in the top 10% genes with abundant DmCs in gene body or in two flanking regions in M1 (C) and M5 (D) generations. Up, Upregulated genes; Down, Downregulated genes; NoDiff, Genes with no significant differential expression; Other, Other non-differential genes. Hyper, Hypermethylated density; hypo, Hypomethylated density.
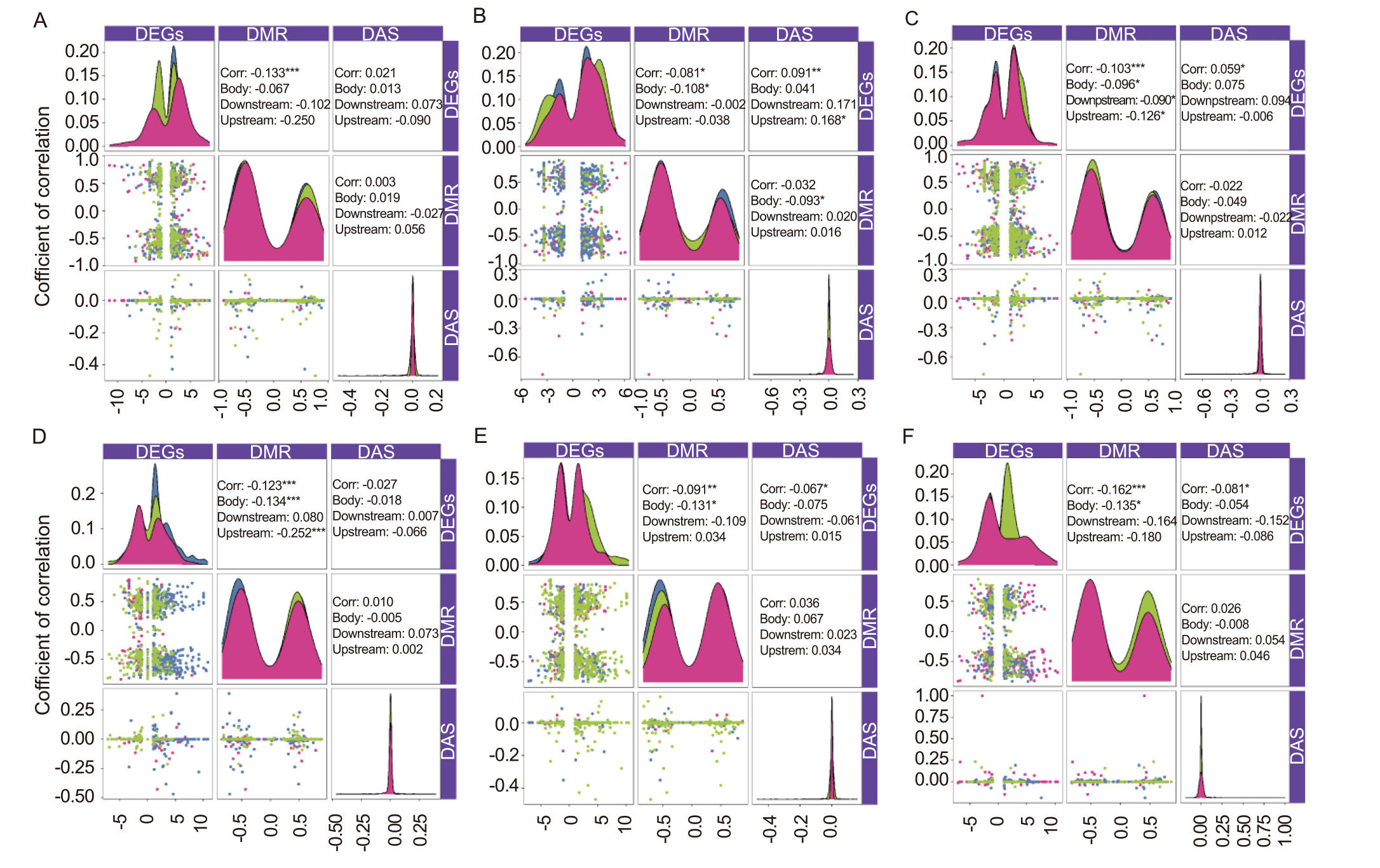
Fig. 12. Correlations among DMRs (differentially methylated regions), DEGs (differentially expressed genes), and DAS (differentially alternative splicing) in different methylation environments. A-C, Correlations among DMRs, DEGs, and DAS in different methylation environments in M1 generation (A, CpG; B, CHG; C, CHH). D-F, Correlations among DMRs, DEGs, and DAS in different methylation environments in M5 generation (D, CpG; E, CHG; F, CHH). Corr represents the coefficient of correlation obtained by Pearson’s. *, **, and *** represent significant differences at the 0.05, 0.01, and 0.001 levels, respectively.
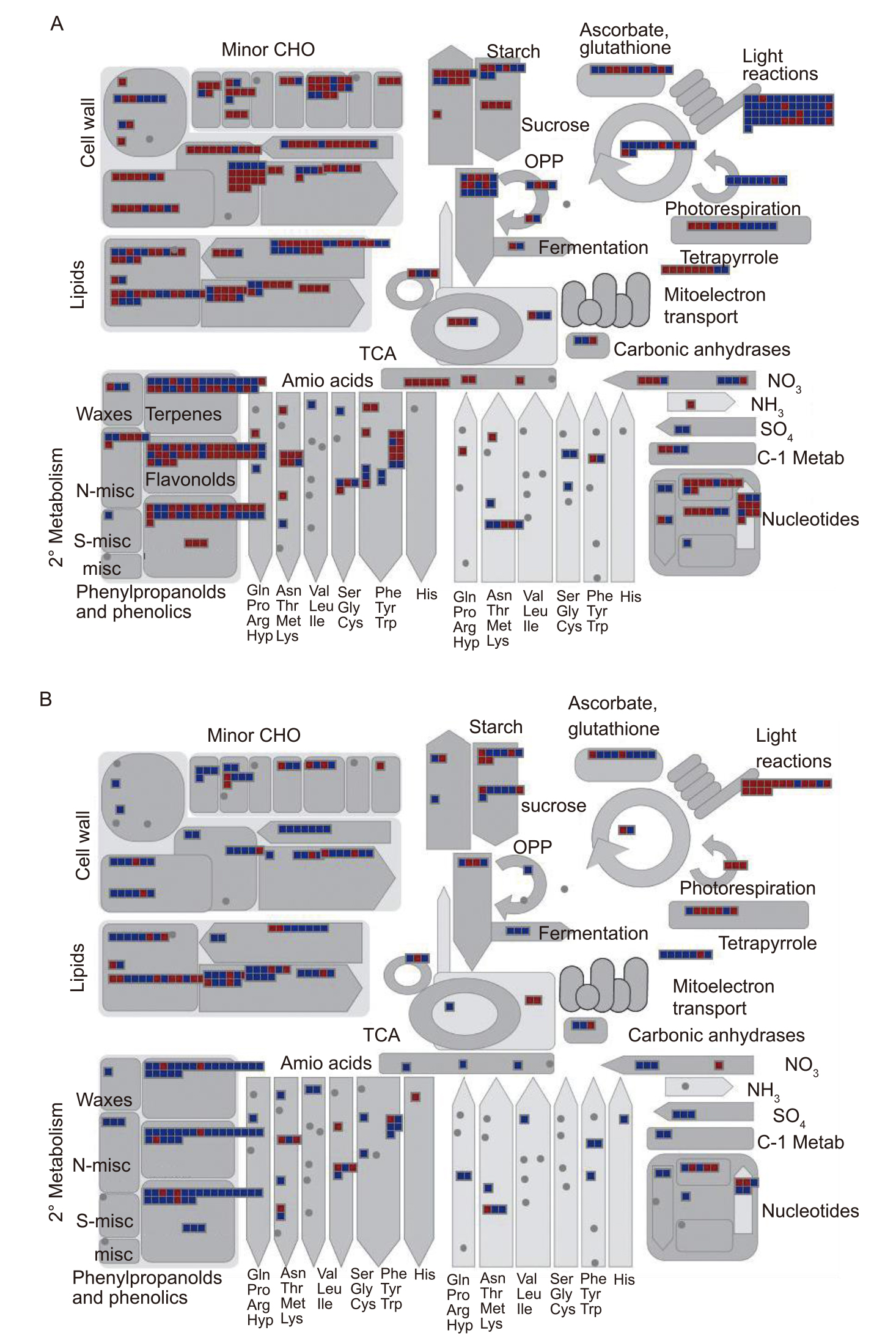
Fig. 13. Overview of metabolism involving differentially expressed genes (DEGs) in M1 (A) and M5 (B) generations. Squares represent DEGs annotated into specific metabolic pathways with red ones for upregulation and blue ones for downregulation. TCA, Tricarboxylic acid cycle; CHO, Carbohydrate; OPP, Oxidative pentose phosphate pathway; misc, Miscellaneous.
| [1] | Aboul-Maaty N A F, Oraby H A S. 2019. Extraction of high-quality genomic DNA from different plant orders applying a modified CTAB-based method. Bull Natl Res Cent, 43(1): 25. |
| [2] | Aebi H. 1984. Catalase in vitro. Methods Enzymol, 105: 121-126. |
| [3] | Bailey R W. 1958. The reaction of pentoses with anthrone. Biochem J, 68(4): 669-672. |
| [4] | Beisel N S, Noble J, Barbazuk W B, et al. 2019. Spaceflight-induced alternative splicing during seedling development in Arabidopsis thaliana NPJ Microgravity, 5: 9. |
| [5] | Belfield E J, Gan X C, Mithani A, et al. 2012. Genome-wide analysis of mutations in mutant lineages selected following fast-neutron irradiation mutagenesis of Arabidopsis thaliana. Genome Res, 22(7): 1306-1315. |
| [6] | Bewick A J, Ji L X, Niederhuth C E, et al. 2016. On the origin and evolutionary consequences of gene body DNA methylation. Proc Natl Acad Sci USA, 113(32): 9111-9116. |
| [7] | Bilichak A, Kovalchuk I. 2016. Transgenerational response to stress in plants and its application for breeding. J Exp Bot, 67(7): 2081-2092. |
| [8] | Bischof S. 2021. Which factors shape the rice DNA methylome? Plant Cell, 33(9): 2904-2905. |
| [9] | Boyko A, Kathiria P, Zemp F J, et al. 2007. Transgenerational changes in the genome stability and methylation in pathogen- infected plants: Virus-induced plant genome instability. Nucleic Acids Res, 35(5): 1714-1725. |
| [10] | Castillo F J, Penel C, Greppin H. 1984. Peroxidase release induced by ozone in Sedum album leaves: Involvement of Ca2+. Plant Physiol, 74(4): 846-851. |
| [11] | Chaouch S, Queval G, Noctor G. 2012. AtRbohF is a crucial modulator of defence-associated metabolism and a key actor in the interplay between intracellular oxidative stress and pathogenesis responses in Arabidopsis. Plant J, 69(4): 613-627. |
| [12] | Chinnusamy V, Zhu J K. 2009. Epigenetic regulation of stress responses in plants. Curr Opin Plant Biol, 12(2): 133-139. |
| [13] | Choi W G, Barker R J, Kim S H, et al. 2019. Variation in the transcriptome of different ecotypes of Arabidopsis thaliana reveals signatures of oxidative stress in plant responses to spaceflight. Am J Bot, 106(1): 123-136. |
| [14] | Cui J, Xia W Y, Wei S Y, et al. 2019. Photosynthetic performance of rice seedlings originated from seeds exposed to spaceflight conditions. Photochem Photobiol, 95(5): 1205-1212. |
| [15] | do Amaral M N, Auler P A, Rossatto T, et al. 2020. Long-term somatic memory of salinity unveiled from physiological, biochemical and epigenetic responses in two contrasting rice genotypes. Physiol Plant, 170(2): 248-268. |
| [16] | Dong C L, He F, Berkowitz O, et al. 2018. Alternative splicing plays a critical role in maintaining mineral nutrient homeostasis in rice (Oryza sativa). Plant Cell, 30(10): 2267-2285. |
| [17] | Erturk F A, Agar G, Arslan E, et al. 2014. Determination of genomic instability and DNA methylation effects of Cr on maize (Zea mays L.) using RAPD and CRED-RA analysis. Acta Physiol Plant, 36(6): 1529-1537. |
| [18] | Foreman J, Demidchik V, Bothwell J H F, et al. 2003. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature, 422: 442-446. |
| [19] | Ganie S A, McMulkin N, Devoto A. 2024. The role of priming and memory in rice environmental stress adaptation: Current knowledge and perspectives. Plant Cell Environ, 47(5): 1895-1915. |
| [20] | Gimeno-Valiente F, López-Rodas G, Castillo J, et al. 2022. Alternative splicing, epigenetic modifications and cancer: A dangerous triangle, or a hopeful one? Cancers, 14(3): 560. |
| [21] | Gómez X, Sanon S, Zambrano K, et al. 2021. Key points for the development of antioxidant cocktails to prevent cellular stress and damage caused by reactive oxygen species (ROS) during manned space missions. NPJ Microgravity, 7(1): 35. |
| [22] | Han J P, Köster P, Drerup M M, et al. 2019. Fine-tuning of RBOHF activity is achieved by differential phosphorylation and Ca2+ binding. New Phytol, 221(4): 1935-1949. |
| [23] | Heath R L, Packer L. 1968. Photoperoxidation in isolated chloroplasts: I: Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys, 125(1): 189-198. |
| [24] | Henry I M, Nagalakshmi U, Lieberman M C, et al. 2014. Efficient genome-wide detection and cataloging of EMS-induced mutations using exome capture and next-generation sequencing. Plant Cell, 26(4): 1382-1397. |
| [25] | Hu C H, Wei X Y, Yuan B, et al. 2018. Genome-wide identification and functional analysis of NADPH oxidase family genes in wheat during development and environmental stress responses. Front Plant Sci, 9: 906. |
| [26] | Hu L J, Li N, Xu C M, et al. 2014. Mutation of a major CG methylase in rice causes genome-wide hypomethylation, dysregulated genome expression, and seedling lethality. Proc Natl Acad Sci USA, 111(29): 10642-10647. |
| [27] | Huang H L, Ullah F, Zhou D X, et al. 2019. Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci, 10: 800. |
| [28] | Jabre I, Reddy A S N, Kalyna M, et al. 2019. Does co-transcriptional regulation of alternative splicing mediate plant stress responses? Nucleic Acids Res, 47(6): 2716-2726. |
| [29] | Jiang S Y, Ramachandran S. 2010. Assigning biological functions to rice genes by genome annotation, expression analysis and mutagenesis. Biotechnol Lett, 32(12): 1753-1763. |
| [30] | Kashkush K, Feldman M, Levy A A. 2003. Transcriptional activation of retrotransposons alters the expression of adjacent genes in wheat. Nat Genet, 33(1): 102-106. |
| [31] | Kim G J, Chandrasekaran K, Morgan W F. 2006. Mitochondrial dysfunction, persistently elevated levels of reactive oxygen species and radiation-induced genomic instability: A review. Mutagenesis, 21(6): 361-367. |
| [32] | Kim S, Kim T H. 2020. Alternative splicing for improving abiotic stress tolerance and agronomic traits in crop plants. J Plant Biol, 63(6): 409-420. |
| [33] | Kruse C P S, Meyers A D, Basu P, et al. 2020. Spaceflight induces novel regulatory responses in Arabidopsis seedling as revealed by combined proteomic and transcriptomic analyses. BMC Plant Biol, 20(1): 237. |
| [34] | Kue Foka I C, Ketehouli T, Zhou Y, et al. 2020. The emerging roles of diacylglycerol kinase (DGK) in plant stress tolerance, growth, and development. Agronomy, 10: 1375. |
| [35] | Lepage É, Zampini É, Brisson N. 2013. Plastid genome instability leads to reactive oxygen species production and plastid-to-nucleus retrograde signaling in Arabidopsis. Plant Physiol, 163(2): 867-881. |
| [36] | Lev Maor G, Yearim A, Ast G. 2015. The alternative role of DNA methylation in splicing regulation. Trends Genet, 31(5): 274-280. |
| [37] | Li H, Handsaker B, Wysoker A, et al. 2009. The sequence alignment/map format and SAMtools. Bioinformatics, 25(16): 2078-2079. |
| [38] | Li S, Zheng Y C, Cui H R, et al. 2016. Frequency and type of inheritable mutations induced by γ rays in rice as revealed by whole genome sequencing. J Zhejiang Univ Sci B, 17(12): 905-915. |
| [39] | Lisch D. 2013. How important are transposons for plant evolution? Nat Rev Genet, 14(1): 49-61. |
| [40] | Lutts S. 1996. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann Bot, 78(3): 389-398. |
| [41] | Manzano A, Carnero-Diaz E, Herranz R, et al. 2022. Recent transcriptomic studies to elucidate the plant adaptive response to spaceflight and to simulated space environments. iScience, 25(8): 104687. |
| [42] | Mao X Z, Cai T, Olyarchuk J G, et al. 2005. Automated genome annotation and pathway identification using the KEGG Orthology (KO) as a controlled vocabulary. Bioinformatics, 21(19): 3787-3793. |
| [43] | Matsukura S, Mizoi J, Yoshida T, et al. 2010. Comprehensive analysis of rice DREB2-type genes that encode transcription factors involved in the expression of abiotic stress-responsive genes. Mol Genet Genomics, 283(2): 185-196. |
| [44] | Migicovsky Z, Kovalchuk I. 2014. Transgenerational changes in plant physiology and in transposon expression in response to UV-C stress in Arabidopsis thaliana. Plant Signal Behav, 9(11): e976490. |
| [45] | Mittler R. 2017. ROS are good. Trends Plant Sci, 22(1): 11-19. |
| [46] | Molinier J, Ries G, Zipfel C, et al. 2006. Transgeneration memory of stress in plants. Nature, 442: 1046-1049. |
| [47] | Moreno-Villanueva M, Wong M, Lu T, et al. 2017. Interplay of space radiation and microgravity in DNA damage and DNA damage response. NPJ Microgravity, 3: 14. |
| [48] | Müller-Xing R, Xing Q, Goodrich J. 2014. Footprints of the Sun: Memory of UV and light stress in plants. Front Plant Sci, 5: 474. |
| [49] | Nestler J, Liu S Z, Wen T J, et al. 2014. Roothairless5, which functions in maize (Zea mays L.) root hair initiation and elongation encodes a monocot-specific NADPH oxidase. Plant J, 79(5): 729-740. |
| [50] | Pariset E, Bertucci A, Petay M, et al. 2020. DNA damage baseline predicts resilience to space radiation and radiotherapy. Cell Rep, 33(10): 108434. |
| [51] | Pastor V, Luna E, Mauch-Mani B, et al. 2013. Primed plants do not forget. Environ Exp Bot, 94: 46-56. |
| [52] | Peng Y, Zhang Y Y, Gui Y J, et al. 2019. Elimination of a retrotransposon for quenching genome instability in modern rice. Mol Plant, 12(10): 1395-1407. |
| [53] | Pogribny I, Raiche J, Slovack M, et al. 2004. Dose-dependence, sex- and tissue-specificity, and persistence of radiation-induced genomic DNA methylation changes. Biochem Biophys Res Commun, 320(4): 1253-1261. |
| [54] | Ramakrishnan M, Satish L, Sharma A, et al. 2022. Transposable elements in plants: Recent advancements, tools and prospects. Plant Mol Biol Report, 40(4): 628-645. |
| [55] | Reddy A S N, Marquez Y, Kalyna M, et al. 2013. Complexity of the alternative splicing landscape in plants. Plant Cell, 25(10): 3657-3683. |
| [56] | Regulski M, Lu Z Y, Kendall J, et al. 2013. The maize methylome influences mRNA splice sites and reveals widespread paramutation- like switches guided by small RNA. Genome Res, 23(10): 1651-1662. |
| [57] | Richards E J. 1997. DNA methylation and plant development. Trends Genet, 13(8): 319-323. |
| [58] | Sagi M, Fluhr R. 2006. Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol, 141(2): 336-340. |
| [59] | Saini S, Kaur N, Pati P K. 2018. Reactive oxygen species dynamics in roots of salt sensitive and salt tolerant cultivars of rice. Anal Biochem, 550: 99-108. |
| [60] | Sanchez D H, Paszkowski J. 2014. Heat-induced release of epigenetic silencing reveals the concealed role of an imprinted plant gene. PLoS Genet, 10(11): e1004806. |
| [61] | Schieber M, Chandel N S. 2014. ROS function in redox signaling and oxidative stress. Curr Biol, 24(10): R453-R462. |
| [62] | Shi Y, Chang Y L, Wu H T, et al. 2020. OsRbohB-mediated ROS production plays a crucial role in drought stress tolerance of rice. Plant Cell Rep, 39(12): 1767-1784. |
| [63] | Sokolova D O, Halych T V, Zhuk V V, et al. 2022. Association of the stimulation of plant antioxidant protection with traits of genome instability. Cytol Genet, 56(5): 431-440. |
| [64] | Sridharan D M, Asaithamby A, Bailey S M, et al. 2015. Understanding cancer development processes after HZE-particle exposure: Roles of ROS, DNA damage repair and inflammation. Radiat Res, 183(1): 1-26. |
| [65] | Sugimoto M, Oono Y, Gusev O, et al. 2014. Genome-wide expression analysis of reactive oxygen species gene network in Mizuna plants grown in long-term spaceflight. BMC Plant Biol, 14: 4. |
| [66] | Sun X H, Tian Y P, Wang J M, et al. 2020. Genome-wide analysis reveals the association between alternative splicing and DNA methylation across human solid tumors. BMC Med Genomics, 13(1): 4. |
| [67] | Suzuki N, Miller G, Morales J, et al. 2011. Respiratory burst oxidases: The engines of ROS signaling. Curr Opin Plant Biol, 14(6): 691-699. |
| [68] | Thiebaut F, Hemerly A S, Ferreira P C G. 2019. A role for epigenetic regulation in the adaptation and stress responses of non-model plants. Front Plant Sci, 10: 246. |
| [69] | Tian P, Lin Z T, Lin D B, et al. 2021. The pattern of DNA methylation alteration, and its association with the changes of gene expression and alternative splicing during phosphate starvation in tomato. Plant J, 108(3): 841-858. |
| [70] | Velikova M, Bankova V, Marcucci M C, et al. 2000. Chemical composition and biological activity of propolis from Brazilian Meliponinae. Z Naturforsch, 55: 785-789. |
| [71] | Wang G F, Li W Q, Li W Y, et al. 2013. Characterization of rice NADPH oxidase genes and their expression under various environmental conditions. Int J Mol Sci, 14(5): 9440-9458. |
| [72] | Wang X T, Hu L J, Wang X F, et al. 2016. DNA methylation affects gene alternative splicing in plants: An example from rice. Mol Plant, 9(2): 305-307. |
| [73] | Wibowo A, Becker C, Marconi G, et al. 2016. Hyperosmotic stress memory in Arabidopsis is mediated by distinct epigenetically labile sites in the genome and is restricted in the male germline by DNA glycosylase activity. elife, 5: e13546. |
| [74] | Wong H L, Pinontoan R, Hayashi K, et al. 2007. Regulation of rice NADPH oxidase by binding of Rac GTPase to its N-terminal extension. Plant Cell, 19(12): 4022-4034. |
| [75] | Xu P P, Chen H Y, Jin J, et al. 2018. Single-base resolution methylome analysis shows epigenetic changes in Arabidopsis seedlings exposed to microgravity spaceflight conditions on board the SJ-10 recoverable satellite. NPJ Microgravity, 4: 12. |
| [76] | Xu P P, Chen H Y, Hu J B, et al. 2021. Potential evidence for transgenerational epigenetic memory in Arabidopsis thaliana following spaceflight. Commun Biol, 4(1): 835. |
| [77] | Yamauchi T, Johzuka-Hisatomi Y, Terada R, et al. 2014. The MET1b gene encoding a maintenance DNA methyltransferase is indispensable for normal development in rice. Plant Mol Biol, 85(3): 219-232. |
| [78] | Yatagai F, Honma M, Dohmae N, et al. 2019. Biological effects of space environmental factors: A possible interaction between space radiation and microgravity. Life Sci Space Res, 20: 113-123. |
| [79] | Zeng D Y, Cui J, Yin Y S, et al. 2020a. Proteomic analysis in different development stages on SP0 generation of rice seeds after space flight. Life Sci Space Res, 26: 34-45. |
| [80] | Zeng D Y, Cui J, Yin Y S, et al. 2020b. Effects of space flight on expression of key proteins in rice leaves. Rice Sci, 27(5): 423-433. |
| [81] | Zeng D Y, Cui J, Yin Y S, et al. 2021. Metabolomics analysis in different development stages on SP0 generation of rice seeds after spaceflight. Front Plant Sci, 12: 700267. |
| [82] | Zeng D Y, Cui J, Yin Y S, et al. 2022a. Combining proteomics and metabolomics to analyze the effects of spaceflight on rice progeny. Front Plant Sci, 13: 900143. |
| [83] | Zeng D Y, Cui J, Yin Y S, et al. 2022b. The memory of rice response to spaceflight stress: From the perspective of metabolomics and proteomics. Int J Mol Sci, 23(6): 3390. |
| [84] | Zhang J, Zhang Y Z, Jiang J, et al. 2020. The crosstalk between epigenetic mechanisms and alternative RNA processing regulation. Front Genet, 11: 998. |
| [85] | Zhang P, Deng H, Xiao F M, et al. 2013. Alterations of alternative splicing patterns of Ser/Arg-rich (SR) genes in response to hormones and stresses treatments in different ecotypes of rice (Oryza sativa). J Integr Agric, 12(5): 737-748. |
| [86] | Zhang Z F, Xiao B Z. 2018. Comparative alternative splicing analysis of two contrasting rice cultivars under drought stress and association of differential splicing genes with drought response QTLs. Euphytica, 214(4): 73. |
| [87] | Zheng K Z, Wang L L, Zeng L J, et al. 2021. The effect of RNA polymerase V on 24-nt siRNA accumulation depends on DNA methylation contexts and histone modifications in rice. Proc Natl Acad Sci USA, 118(30): e2100709118. |
| [88] | Zheng X G, Chen L, Xia H, et al. 2017. Transgenerational epimutations induced by multi-generation drought imposition mediate rice plant’s adaptation to drought condition. Sci Rep, 7: 39843. |
| [1] | Chen Su, Ma Feilong, Chen Jiaoyang, Qi Man, Wei Qianshu, Tao Zhihuan, Sun Bo. Function of R2R3-Type Myeloblastosis Transcription Factors in Plants [J]. Rice Science, 2025, 32(3): 307-321. |
| [2] | Yang Yajun, Lu Yanhui, Tian Junce, Zheng Xusong, Guo Jiawen, Liu Xiaowei, Lü Zhongxian, Xu Hongxing. Sustainable Management Strategies for Rice Leaffolder, Cnaphalocrocis medinalis (Guenée): Progress and Prospects [J]. Rice Science, 2025, 32(3): 322-338. |
| [3] | Xie Yuhao, Xie Wenya, Zhao Jianhua, Xue Xiang, Cao Wenlei, Shi Xiaopin, Wang Zhou, Wang Yiwen, Wang Guangda, Feng Zhiming, Hu Keming, Chen Xijun, Chen Zongxiang, Zuo Shimin. OsERF7 Negatively Regulates Resistance to Sheath Blight Disease by Inhibiting Phytoalexin Biosynthesis [J]. Rice Science, 2025, 32(3): 367-379. |
| [4] | Chaemyeong Lim, Sae Hyun Lee, Haeun Lee, So-Yon Park, Kiyoon Kang, Hyeryung Yoon, Tae-Jin Yang, Gary Stacey, Nam-Chon Paek, Sung-Hwan Cho. Global Transcriptome Analysis of Rice Seedlings in Response to Extracellular ATP [J]. Rice Science, 2025, 32(3): 380-399. |
| [5] | Sanchika Snehi, Ravi Kiran Kt, Sanket Rathi, Sameer Upadhyay, Suneetha Kota, Satish Kumar Sanwal, Lokeshkumar Bm, Arun Balasubramaniam, Nitish Ranjan Prakash, Pawan Kumar Singh. Discerning Genes to Deliver Varieties: Enhancing Vegetative- and Reproductive-Stage Flooding Tolerance in Rice [J]. Rice Science, 2025, 32(2): 160-176. |
| [6] | Nie Lixiao, Guo Xiayu, Wang Weiqin, Qi Yucheng, Ai Zhiyong, He Aibin. Regulation of Regeneration Rate to Enhance Ratoon Rice Production [J]. Rice Science, 2025, 32(2): 177-192. |
| [7] | Wang Shuman, Zhang Linqi, Gao Ruiren, Wei Guangbo, Dong Weiguo, Xu Jiming, Wang Zhiye. Establishing Programmable CRISPR/Cas13b-Mediated Knockdown System in Rice [J]. Rice Science, 2025, 32(2): 217-227. |
| [8] | Uthpal Krishna Roy, Babita Pal, Soumen Bhattacharjee. A Novel Approach for Screening Salinity-Tolerant Rice Germplasm by Exploring Redox-Regulated Cytological Fingerprint [J]. Rice Science, 2025, 32(2): 228-242. |
| [9] | He Zhenrui, Zhao Wenhua, Cheng Baoping, Yang Mei, Yang Yingqing, Zhu Yiming, Zhou Erxun. Molecular and Biological Characterization of Novel Mitovirus Infecting Phytopathogenic Fungus Ustilaginoidea virens [J]. Rice Science, 2025, 32(2): 243-258. |
| [10] | He Chen, Ruan Yunze, Jia Zhongjun. A Meta-Analysis of 30 Years in China and Micro-District Experiments Shows Organic Fertilizer Quantification Combined with Chemical Fertilizer Reduction Enhances Rice Yield on Saline-Alkali Land [J]. Rice Science, 2025, 32(2): 259-272. |
| [11] | Wang Mingyue, Zhao Weibo, Feng Xiaoya, Chen Yi, Li Junhao, Fu Jinmei, Yan Yingchun, Chu Zhaohui, Huang Wenchao. Disruption of Energy Metabolism and Reactive Oxygen Species Homeostasis in Honglian Type-Cytoplasmic Male Sterility (HL-CMS) Rice Pollen [J]. Rice Science, 2025, 32(1): 81-93. |
| [12] | Intan Farahanah, Shariza Sahudin, Hannis Fadzillah Mohsin, Siti Alwani Ariffin, Liyana Dhamirah Aminuddin. Understanding Investigational Perspective of Antioxidant and Antibacterial Properties of Rice [J]. Rice Science, 2025, 32(1): 15-31. |
| [13] | Jeberlin Prabina Bright, Hemant S. Maheshwari, Sugitha Thangappan, Kahkashan Perveen, Najat A. Bukhari, Debasis Mitra, Riyaz Sayyed, Andrea Mastinu. Biofilmed-PGPR: Next-Generation Bioinoculant for Plant Growth Promotion in Rice under Changing Climate [J]. Rice Science, 2025, 32(1): 94-106. |
| [14] | Wang Haoran, Chen Guoqing, Feng Guozhong. Expanding Viral Diversity in Rice Fields by Next-Generation Sequencing [J]. Rice Science, 2025, 32(1): 44-51. |
| [15] | Surangkana Chimthai, Sulaiman Cheabu, Wanchana Aesomnuk, Siriphat Ruengphayak, Siwaret Arikit, Apichart Vanavichit, Chanate Malumpong. Breeding for Heat Tolerant Aromatic Rice Varieties and Identification of Novel QTL Regions Associated with Heat Tolerance During Reproductive Phase by QTL-Seq [J]. Rice Science, 2025, 32(1): 67-80. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||