
Rice Science ›› 2025, Vol. 32 ›› Issue (4): 525-536.DOI: 10.1016/j.rsci.2025.06.002
• Research Papers • Previous Articles Next Articles
Zhou Lin,#, Jiang Hong,#, Huang Long, Li Ziang, Yao Zhonghao, Li Linhan, Ji Kangwei, Li Yijie, Tang Haijuan, Cheng Jinping, Bao Yongmei, Huang Ji, Zhang Hongsheng( ), Chen Sunlu(
), Chen Sunlu( )
)
Received:2025-02-11
Accepted:2025-06-10
Online:2025-07-28
Published:2025-08-06
Contact:
Zhang Hongsheng, Chen Sunlu
About author:First author contact:#These authors contributed equally to this work
Zhou Lin, Jiang Hong, Huang Long, Li Ziang, Yao Zhonghao, Li Linhan, Ji Kangwei, Li Yijie, Tang Haijuan, Cheng Jinping, Bao Yongmei, Huang Ji, Zhang Hongsheng, Chen Sunlu. Genome-Wide Association Study of Brown Rice Weight Identifies an RNA-Binding Protein Antagonistically Regulating Grain Weight and Panicle Number[J]. Rice Science, 2025, 32(4): 525-536.
Add to citation manager EndNote|Ris|BibTeX
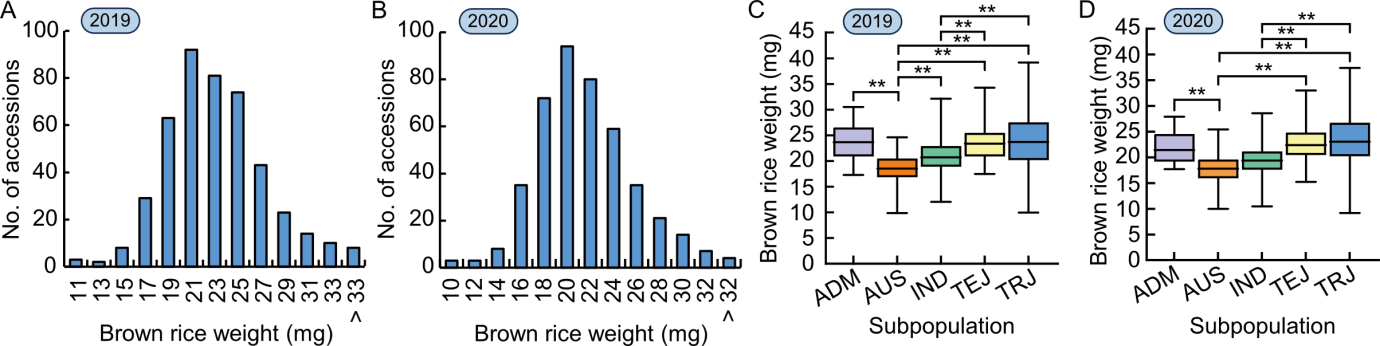
Fig. 1. Variation of brown rice weight (BRW) in the rice population. A and B, Frequency distributions of BRW of the rice population in 2019 (A) and 2020 (B). C and D, BRW comparisons among subpopulations in 2019 (C) and 2020 (D). ADM, Admixed; AUS, aus; IND, indica; TEJ, temperate japonica; TRJ, tropical japonica. **, P < 0.01 by the Student’s t-test.
| Year | Mean (mg) | SD (mg) | CV (%) | Maximum (mg) | Minimum (mg) | Ratio | Skewness | Kurtosis | h2 |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 22.29 | 4.15 | 18.62 | 39.18 | 9.87 | 3.97 | 0.53 | 0.94 | 0.93 |
| 2020 | 21.10 | 4.01 | 19.00 | 37.38 | 9.18 | 4.07 | 0.56 | 0.98 | 0.92 |
Table 1. Statistical analysis of brown rice weight.
| Year | Mean (mg) | SD (mg) | CV (%) | Maximum (mg) | Minimum (mg) | Ratio | Skewness | Kurtosis | h2 |
|---|---|---|---|---|---|---|---|---|---|
| 2019 | 22.29 | 4.15 | 18.62 | 39.18 | 9.87 | 3.97 | 0.53 | 0.94 | 0.93 |
| 2020 | 21.10 | 4.01 | 19.00 | 37.38 | 9.18 | 4.07 | 0.56 | 0.98 | 0.92 |
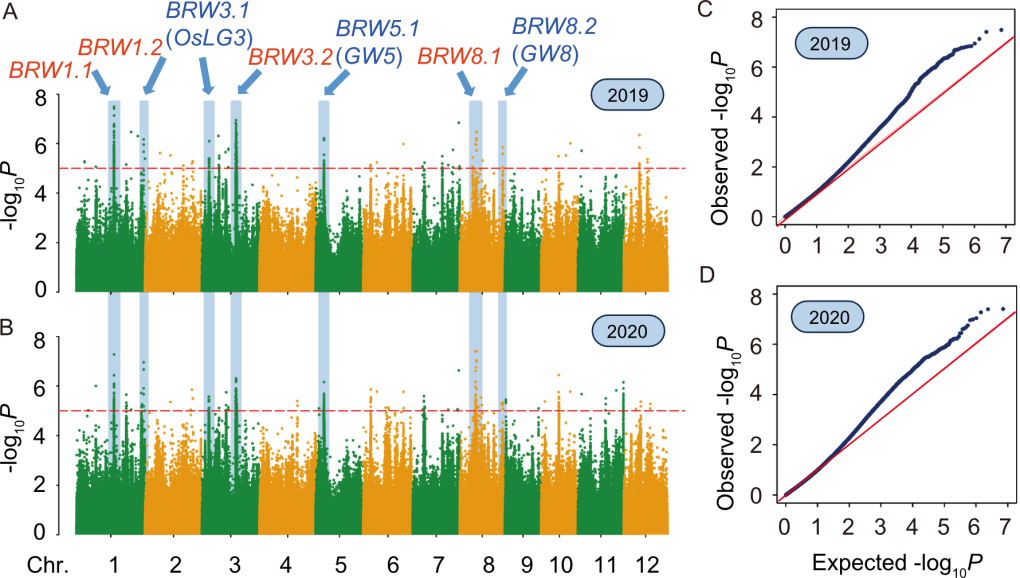
Fig. 2. Genome-wide association study (GWAS) of brown rice weight. A and B, Manhattan plots of GWAS results in 2019 (A) and 2020 (B). The x-axis shows the chromosome (Chr.) positions, and the y-axis indicates the -log10 (Transformed P-value) (-log10P). Red dashed lines denote the significance threshold. Blue-highlighted regions with arrows mark seven consistently detected loci across experimental replicates. Blue fonts denote the loci co-localized with known grain size- and grain weight-related genes, and red fonts indicate novel loci. C and D, Quantile-Quantile plots of GWAS results in 2019 (C) and 2020 (D).
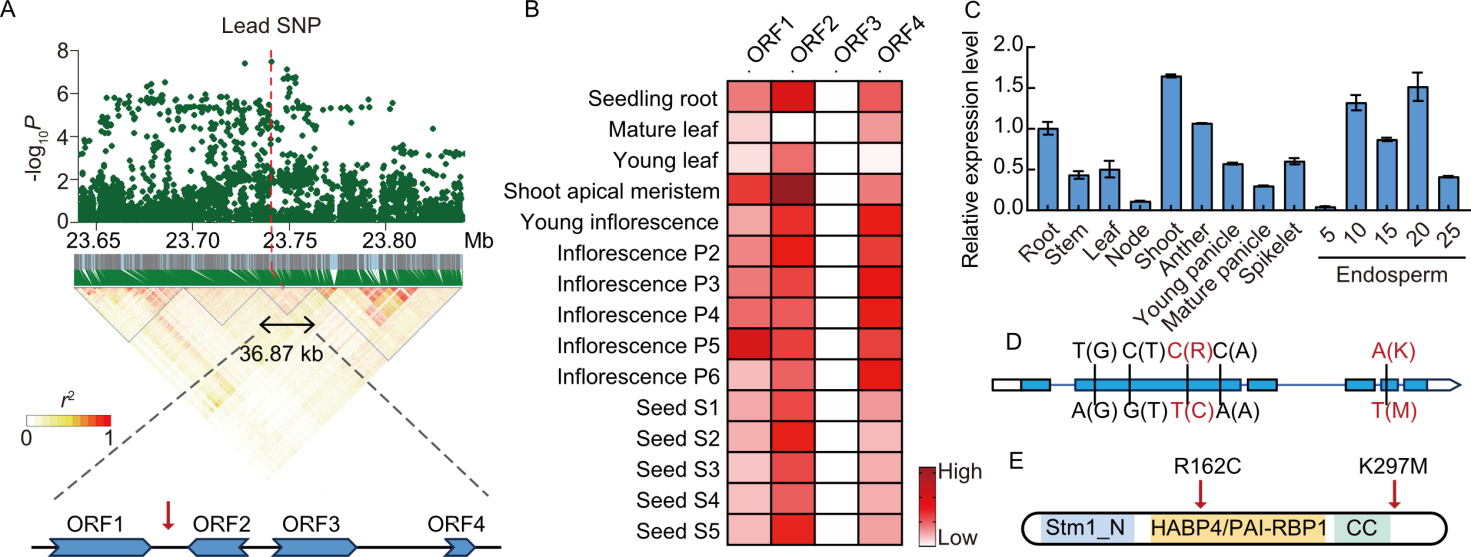
Fig. 3. Candidate gene analysis of BRW1.1. A, Linkage disequilibrium (LD) block analysis of BRW1.1. Manhattan plot of genome-wide associated study result for BRW1.1 is shown at the top, with grey and red dashed lines indicating the significance threshold and the lead single nucleotide polymorphism (SNP) position, respectively. y-axis indicates the -log10(Transformed P-value) (-log10P). LD heatmap of BRW1.1 for pairwise correlations (r2) between SNPs is displayed at the middle with barcodes denoting SNP positions. Four open reading frames (ORFs) in the LD block of the lead SNP are shown at the bottom. A red arrow points the lead SNP location at the genomic region of four ORFs. B, Expression heatmap of ORFs across tissues. Inflorescences P2-P6 represent inflorescences of different lengths (P2, 3-6 cm; P3, 7-10 cm; P4, 11-15 cm; P5, 16-22 cm; P6, 23-30 cm). Seeds S1-S5 represent seeds at different days after pollination (DAP) (S1, 0-2 DAP; S2, 3-4 DAP; S3, 5-10 DAP; S4, 11-20 DAP; S5, 21-29 DAP). C, Expression profile of ORF2 examined by real-time qPCR assays. Actin was used as internal control. Endosperm 5, 10, 15, 20, and 25 repsent endosperms at 5, 10, 15, 20, and 25 DAP. D, SNPs in the ORF2 coding sequence. Missense and synonymous mutations are indicated in red and black, respectively. The corresponding amino acids are shown in parenthesis. E, Predicted protein domains of ORF2. Locations of missense mutations are marked by red arrows. CC, Coiled-coil.

Fig. 4. Brown rice phenotypes of BRW1.1 knockout mutants. A, Sequencing results of Zhonghua 11 (WT) and BRW1.1 knockout lines (brw1.1-1, brw1.1-2, and brw1.1-3). Genome editing targets are indicated by red dashed lines. Locations of premature termination codons are provided in parenthesis. PAM, Protospacer adjacent motif (AGG). B, Brown rice morphology of WT and BRW1.1 knockout lines. Brown rice length, width, and thickness are shown respectively. Scale bars, 1 cm. C, Measurements of brown rice weight, length, width, and thickness of WT and BRW1.1 knockout lines. Data are mean ± SE (n = 30). **, P < 0.01 by the Student’s t-test; ns, No significance.

Fig. 5. Grain phenotypes of BRW1.1 knockout mutants. A, Grain length, width, and thickness of Zhonghua 11 (WT) and BRW1.1 knockout lines (brw1.1-1, brw1.1-2, and brw1.1-3). Scale bars, 1 cm. B, Measurements of 1000-grain weight, and grain length, width, and thickness of BRW1.1 knockout lines. Data are mean ± SE (n = 30). *, P < 0.05; **, P < 0.01 by the Student’s t-test.
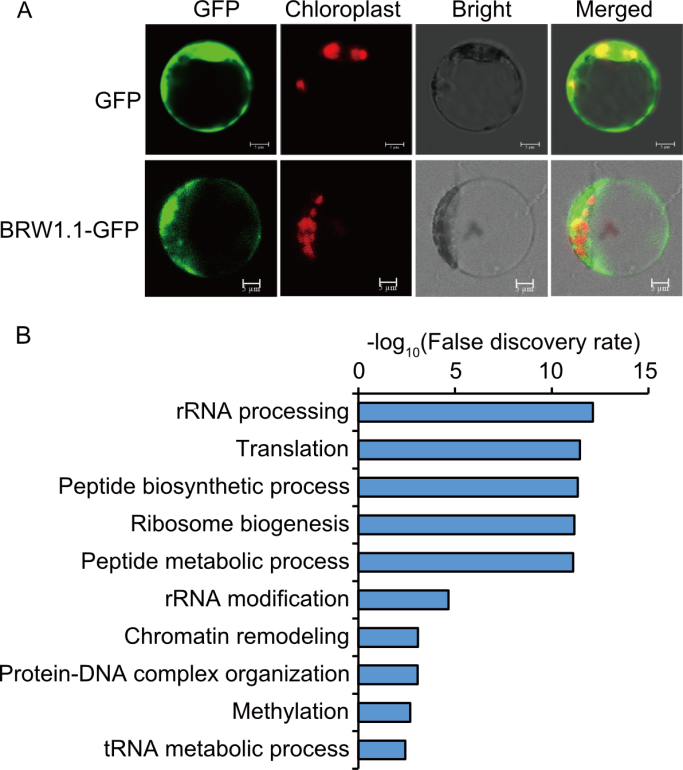
Fig. 6. Molecular function of BRW1.1. A, Subcellular localization of BRW1.1-GFP (green fluorescent protein) fusion protein in rice protoplasts. Chloroplasts autofluorescence indicates protoplast vigor. Scale bars, 5 μm. B, Gene Ontology enrichment of the top 100 co-expressed genes of BRW1.1.

Fig. 7. BRW1.1-mediated tradeoff between grain weight and panicle number. A, Plant architecture of BRW1.1 knockout lines (brw1.1-1, brw1.1-2, and brw1.1-3). Scale bars, 15 cm. B, Measurements of panicle number, grain number per panicle, and plant height of BRW1.1 knockout lines. Data are mean ± SE (n = 30). *, P < 0.05; **, P < 0.01 by the Student’s t-test; ns, No significance. C, Major BRW1.1 haplotypes (Hap. A-E) in the population of 3K Rice Genome Project. Single nucleotide polymorphisms within the coding sequence are displayed on the upper, and haplotype variation is shown at the lower. Accession numbers of different haplotypes are indicated in parenthesis. D, 1000-grain weight and panicle number of haplotypes. **, P < 0.01 by the Student’s t-test. E, Distribution of haplotypes in subpopulations. ADM, Admixed; AUS, aus; IND, indica; TEJ, temperate japonica; TRJ, tropical japonica.
| [1] | Alexander D H, Novembre J, Lange K. 2009. Fast model-based estimation of ancestry in unrelated individuals. Genome Res, 19(9): 1655-1664. |
| [2] | Bai X F, Huang Y, Hu Y, et al. 2017. Duplication of an upstream silencer of FZP increases grain yield in rice. Nat Plants, 3(11): 885-893. |
| [3] | Bianchini G, Sánchez-Baracaldo P. 2024. TreeViewer: Flexible, modular software to visualise and manipulate phylogenetic trees. Ecol Evol, 14(2): e10873. |
| [4] | Browning K S, Bailey-Serres J. 2015. Mechanism of cytoplasmic mRNA translation. Arabidopsis Book, 13: e0176. |
| [5] | Chang C C, Chow C C, Tellier L C, et al. 2015. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience, 4: 7. |
| [6] | Chen R J, Xiao N, Lu Y, et al. 2023. A de novo evolved gene contributes to rice grain shape difference between indica and japonica. Nat Commun, 14(1): 5906. |
| [7] | Chiou W Y, Kawamoto T, Himi E, et al. 2019. LARGE GRAIN encodes a putative RNA-binding protein that regulates spikelet hull length in rice. Plant Cell Physiol, 60(3): 503-515. |
| [8] | Cho H, Cho H S, Hwang I. 2019. Emerging roles of RNA-binding proteins in plant development. Curr Opin Plant Biol, 51: 51-57. |
| [9] | Cooper M, van Eeuwijk F A, Hammer G L, et al. 2009. Modeling QTL for complex traits: Detection and context for plant breeding. Curr Opin Plant Biol, 12(2): 231-240. |
| [10] | Corley M, Burns M C, Yeo G W. 2020. How RNA-binding proteins interact with RNA: Molecules and mechanisms. Mol Cell, 78(1): 9-29. |
| [11] | Davalos V, Blanco S, Esteller M. 2018. SnapShot: Messenger RNA modifications. Cell, 174(2): 498. |
| [12] | Dong S S, He W M, Ji J J, et al. 2021. LDBlockShow: A fast and convenient tool for visualizing linkage disequilibrium and haplotype blocks based on variant call format files. Brief Bioinform, 22(4): bbaa227. |
| [13] | Du Z X, Huang Z, Li J B, et al. 2021. qTGW12a, a naturally varying QTL, regulates grain weight in rice. Theor Appl Genet, 134(9): 2767-2776. |
| [14] | Duan P G, Xu J S, Zeng D L, et al. 2017. Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol Plant, 10(5): 685-694. |
| [15] | Gao C X. 2021. Genome engineering for crop improvement and future agriculture. Cell, 184(6): 1621-1635. |
| [16] | Gilbert C, Svejstrup J Q. 2006. RNA immunoprecipitation for determining RNA-protein associations in vivo. Curr Protoc Mol Biol, 75(1): 27.4.1-27.4.11. |
| [17] | Guo H M, Cui Y C, Huang L J, et al. 2022. The RNA binding protein OsLa influences grain and anther development in rice. Plant J, 110(5): 1397-1414. |
| [18] | Guo T, Chen K, Dong N Q, et al. 2018. GRAIN SIZE AND NUMBER1 negatively regulates the OsMKKK10-OsMKK4-OsMPK6 cascade to coordinate the trade-off between grain number per panicle and grain size in rice. Plant Cell, 30(4): 871-888. |
| [19] | Hu Z J, Lu S J, Wang M J, et al. 2018. A novel QTL qTGW3 encodes the GSK3/SHAGGY-like kinase OsGSK5/OsSK41 that interacts with OsARF4 to negatively regulate grain size and weight in rice. Mol Plant, 11(5): 736-749. |
| [20] | Huang X H, Han B. 2014. Natural variations and genome-wide association studies in crop plants. Annu Rev Plant Biol, 65: 531-551. |
| [21] | Ishimaru K, Hirotsu N, Madoka Y, et al. 2013. Loss of function of the IAA-glucose hydrolase gene TGW6 enhances rice grain weight and increases yield. Nat Genet, 45(6): 707-711. |
| [22] | Jiao Y Q, Wang Y H, Xue D W, et al. 2010. Regulation of OsSPL14 by OsmiR156 defines ideal plant architecture in rice. Nat Genet, 42(6): 541-544. |
| [23] | Khan N, Zhang Y F, Wang J Y, et al. 2022. TaGSNE, a WRKY transcription factor, overcomes the trade-off between grain size and grain number in common wheat and is associated with root development. J Exp Bot, 73(19): 6678-6696. |
| [24] | Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol, 33(7): 1870-1874. |
| [25] | Lee K, Kang H. 2016. Emerging roles of RNA-binding proteins in plant growth, development, and stress responses. Mol Cells, 39(3): 179-185. |
| [26] | Li J Y, Li H Y, Chen J L, et al. 2020. Toward precision genome editing in crop plants. Mol Plant, 13(6): 811-813. |
| [27] | Li L F, Li J J, Liu K K, et al. 2024. DGW1, encoding an hnRNP-like RNA binding protein, positively regulates grain size and weight by interacting with GW6 mRNA. Plant Biotechnol J, 22(2): 512-526. |
| [28] | Li W J, Yan J J, Zhang Y, et al. 2023. Serine protease NAL1 exerts pleiotropic functions through degradation of TOPLESS-related corepressor in rice. Nat Plants, 9(7): 1130-1142. |
| [29] | Liu H J, Yan J B. 2019. Crop genome-wide association study: A harvest of biological relevance. Plant J, 97(1): 8-18. |
| [30] | Liu J F, Chen J, Zheng X M, et al. 2017. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat Plants, 3: 17043. |
| [31] | Liu J X, Wu M W, Liu C M. 2022. Cereal endosperms: Development and storage product accumulation. Annu Rev Plant Biol, 73: 255-291. |
| [32] | Lorković Z J. 2009. Role of plant RNA-binding proteins in development, stress response and genome organization. Trends Plant Sci, 14(4): 229-236. |
| [33] | Lyu J, Wang D K, Duan P G, et al. 2020. Control of grain size and weight by the GSK2-LARGE1/OML4 pathway in rice. Plant Cell, 32(6): 1905-1918. |
| [34] | Mackay T F C, Stone E A, Ayroles J F. 2009. The genetics of quantitative traits: Challenges and prospects. Nat Rev Genet, 10(8): 565-577. |
| [35] | Mason J M, Arndt K M. 2004. Coiled coil domains: Stability, specificity, and biological implications. ChemBioChem, 5(2): 170-176. |
| [36] | Minkenberg B, Zhang J W, Xie K B, et al. 2019. CRISPR-PLANT v2: An online resource for highly specific guide RNA spacers based on improved off-target analysis. Plant Biotechnol J, 17(1): 5-8. |
| [37] | Miura K, Ikeda M, Matsubara A, et al. 2010. OsSPL14 promotes panicle branching and higher grain productivity in rice. Nat Genet, 42(6): 545-549. |
| [38] | Ouyang X, Zhong X Y, Chang S Q, et al. 2022. Partially functional NARROW LEAF1 balances leaf photosynthesis and plant architecture for greater rice yield. Plant Physiol, 189(2): 772-789. |
| [39] | Price M N, Dehal P S, Arkin A P. 2009. FastTree: Computing large minimum evolution trees with profiles instead of a distance matrix. Mol Biol Evol, 26(7): 1641-1650. |
| [40] | Raudvere U, Kolberg L, Kuzmin I, et al. 2019. g: Profiler: A web server for functional enrichment analysis and conversions of gene lists (2019 update). Nucleic Acids Res, 47: W191-W198. |
| [41] | Ren D, Liu H, Sun X J, et al. 2024. Post-transcriptional regulation of grain weight and shape by the RBP-A-J-K complex in rice. J Integr Plant Biol, 66(1): 66-85. |
| [42] | Ren D Y, Rao Y C, Huang L C, et al. 2016. Fine mapping identifies a new QTL for brown rice rate in rice (Oryza sativa L.). Rice, 9(1): 4. |
| [43] | Robinson M R, Wray N R, Visscher P M. 2014. Explaining additional genetic variation in complex traits. Trends Genet, 30(4): 124-132. |
| [44] | Ruan B P, Shang L G, Zhang B, et al. 2020. Natural variation in the promoter of TGW2 determines grain width and weight in rice. New Phytol, 227(2): 629-640. |
| [45] | Salvi S, Tuberosa R. 2015. The crop QTLome comes of age. Curr Opin Biotechnol, 32: 179-185. |
| [46] | Sato Y, Namiki N, Takehisa H, et al. 2013a. RiceFREND: A platform for retrieving coexpressed gene networks in rice. Nucleic Acids Res, 41: D1214-D1221. |
| [47] | Sato Y, Takehisa H, Kamatsuki K, et al. 2013b. RiceXPro Version 3.0: Expanding the informatics resource for rice transcriptome. Nucleic Acids Res, 41: D1206-D1213. |
| [48] | Shi Y G. 2017. Mechanistic insights into precursor messenger RNA splicing by the spliceosome. Nat Rev Mol Cell Biol, 18(11): 655-670. |
| [49] | Si L Z, Chen J Y, Huang X H, et al. 2016. OsSPL13 controls grain size in cultivated rice. Nat Genet, 48(4): 447-456. |
| [50] | Song X G, Meng X B, Guo H Y, et al. 2022. Targeting a gene regulatory element enhances rice grain yield by decoupling panicle number and size. Nat Biotechnol, 40(9): 1403-1411. |
| [51] | Song X J, Kuroha T, Ayano M, et al. 2015. Rare allele of a previously unidentified histone H4 acetyltransferase enhances grain weight, yield, and plant biomass in rice. Proc Natl Acad Sci USA, 112(1): 76-81. |
| [52] | Tilman D, Balzer C, Hill J, et al. 2011. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA, 108: 20260-20264. |
| [53] | Ule J, Jensen K B, Ruggiu M, et al. 2003. CLIP identifies Nova-regulated RNA networks in the brain. Science, 302: 1212-1215. |
| [54] | Wang C C, Yu H, Huang J, et al. 2020. Towards a deeper haplotype mining of complex traits in rice with RFGB v2.0. Plant Biotechnol J, 18(1): 14-16. |
| [55] | Wang J W, Qi M F, Liu J, et al. 2015. CARMO: A comprehensive annotation platform for functional exploration of rice multi-omics data. Plant J, 83(2): 359-374. |
| [56] | Wang J Y, Chitsaz F, Derbyshire M K, et al. 2023. The conserved domain database in 2023. Nucleic Acids Res, 51: D384-D388. |
| [57] | Wang S K, Wu K, Yuan Q B, et al. 2012. Control of grain size, shape and quality by OsSPL16 in rice. Nat Genet, 44(8): 950-954. |
| [58] | Wang W S, Mauleon R, Hu Z Q, et al. 2018. Genomic variation in 3, 010 diverse accessions of Asian cultivated rice. Nature, 557: 43-49. |
| [59] | Wei S B, Li X, Lu Z F, et al. 2022. A transcriptional regulator that boosts grain yields and shortens the growth duration of rice. Science, 377: eabi8455. |
| [60] | Wei X, Qiu J, Yong K C, et al. 2021. A quantitative genomics map of rice provides genetic insights and guides breeding. Nat Genet, 53(2): 243-253. |
| [61] | Wei X, Chen M J, Zhang Q, et al. 2024. Genomic investigation of 18, 421 lines reveals the genetic architecture of rice. Science, 385: eadm8762. |
| [62] | Wu Y, Wang Y, Mi X F, et al. 2016. The QTL GNP1 encodes GA20ox1, which increases grain number and yield by increasing cytokinin activity in rice panicle meristems. PLoS Genet, 12(10): e1006386. |
| [63] | Würschum T. 2012. Mapping QTL for agronomic traits in breeding populations. Theor Appl Genet, 125(2): 201-210. |
| [64] | Xie Q, Sparkes D L. 2021. Dissecting the trade-off of grain number and size in wheat. Planta, 254(1): 3. |
| [65] | Xing H L, Dong L, Wang Z P, et al. 2014. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol, 14: 327. |
| [66] | Yan L, Jiao B Y, Duan P G, et al. 2024. Control of grain size and weight by the RNA-binding protein EOG1 in rice and wheat. Cell Rep, 43(11): 114856. |
| [67] | Yano K, Morinaka Y, Wang F M, et al. 2019. GWAS with principal component analysis identifies a gene comprehensively controlling rice architecture. Proc Natl Acad Sci USA, 116: 21262-21267. |
| [68] | Ying J Z, Ma M, Bai C, et al. 2018. TGW3, a major QTL that negatively modulates grain length and weight in rice. Mol Plant, 11(5): 750-753. |
| [69] | Yu J P, Xiong H Y, Zhu X Y, et al. 2017. OsLG3 contributing to rice grain length and yield was mined by Ho-LAMap. BMC Biol, 15(1): 28. |
| [70] | Yue Z C, Wang Z P, Yao Y L, et al. 2024. Variation in WIDTH OF LEAF AND GRAIN contributes to grain and leaf size by controlling LARGE2 stability in rice. Plant Cell, 36(9): 3201-3218. |
| [71] | Zargar S M, Raatz B, Sonah H, et al. 2015. Recent advances in molecular marker techniques: Insight into QTL mapping, GWAS and genomic selection in plants. J Crop Sci Biotechnol, 18(5): 293-308. |
| [72] | Zhai L Y, Wang F, Yan A, et al. 2020. Pleiotropic effect of GNP1 underlying grain number per panicle on sink, source and flow in rice. Front Plant Sci, 11: 933. |
| [73] | Zhang L, Yu H, Ma B, et al. 2017. A natural tandem array alleviates epigenetic repression of IPA1 and leads to superior yielding rice. Nat Commun, 8: 14789. |
| [74] | Zhao H, Li J C, Yang L, et al. 2021. An inferred functional impact map of genetic variants in rice. Mol Plant, 14(9): 1584-1599. |
| [75] | Zhou X, Stephens M. 2014. Efficient multivariate linear mixed model algorithms for genome-wide association studies. Nat Methods, 11(4): 407-409. |
| [76] | Zhou X Y, Huang X H. 2019. Genome-wide association studies in rice: How to solve the low power problems? Mol Plant, 12(1): 10-12. |
| [1] | Ratan Kumar Ganapati, Chen Kai, Zhao Xiuqin, Zheng Tianqing, Zhang Fan, Zhai Laiyuan, Xu Jianlong. Genome-Wide Association Study and Haplotype Analysis Jointly Identify New Candidate Genes for Alkaline Tolerance at Seedling Stage in Rice [J]. Rice Science, 2025, 32(4): 537-548. |
| [2] | Fu Yiwei, Wu Jiayelu, Wu Mingming, Ye Shenghai, Zhai Rongrong, Ye Jing, Zhu Guofu, Yu Faming, Lu Yanting, Zhang Xiaoming. Progress on Molecular Mechanism of Heat Tolerance in Rice [J]. Rice Science, 2024, 31(6): 673-687. |
| [3] | Ren Jian, Hu Kelin, Feng Puyu, William D. Batchelor, Liu Haitao, Lü Shihua. Simulating Responses of Rice Yield and Nitrogen Fates to Ground Cover Rice Production System under Different Types of Precipitation Years [J]. Rice Science, 2024, 31(6): 725-739. |
| [4] | Deng Bowen, Zhang Yanni, Zhang Fan, Wang Wensheng, Xu Jianlong, Zhang Yu, Bao Jinsong. Genome-Wide Association Study of Cooked Rice Textural Attributes and Starch Physicochemical Properties in indica Rice [J]. Rice Science, 2024, 31(3): 300-316. |
| [5] | Amrit Kumar Nayak, Anilkumar C, Sasmita Behera, Rameswar Prasad Sah, Gera Roopa Lavanya, Awadhesh Kumar, Lambodar Behera, Muhammed Azharudheen Tp. Genetic Dissection of Grain Size Traits Through Genome-Wide Association Study Based on Genic Markers in Rice [J]. Rice Science, 2022, 29(5): 462-472. |
| [6] | Liu Kai, Li Minjuan, Zhang Bin, Yin Xuming, Xia Xinjie, Wang Manling, Cui Yanchun. Poaceae Orthologs of Rice OsSGL, DUF1645 Domain-Containing Genes, Positively Regulate Drought Tolerance, Grain Length and Weight in Rice [J]. Rice Science, 2022, 29(3): 257-267. |
| [7] | Rakotoson Tatiana, Dusserre Julie, Letourmy Philippe, Frouin Julien, Ramonta Ratsimiala Isabelle, Victorine Rakotoarisoa Noronirina, Cao Tuong-Vi, Vom Brocke Kirsten, Ramanantsoanirina Alain, Ahmadi Nourollah, Raboin Louis-Marie. Genome-Wide Association Study of Nitrogen Use Efficiency and Agronomic Traits in Upland Rice [J]. Rice Science, 2021, 28(4): 379-390. |
| [8] | Matsue Yuji, Takasaki Katsuya, Abe Jun. Water Management for Improvement of Rice Yield, Appearance Quality and Palatability with High Temperature During Ripening Period [J]. Rice Science, 2021, 28(4): 409-416. |
| [9] | Xiaoqin Zeng, Hui Zhuang, Qinglan Cheng, Jun Tang, Fayu Yang, Mingjiang Huang, Ziyi Wang, Zhongcheng Li, Honghui Zhu, Rui Chen, Guanghua He, Yunfeng Li. SB1 Encoding RING-Like Zinc-Finger Protein Regulates Branch Development as a Transcription Repressor [J]. Rice Science, 2021, 28(3): 243-256. |
| [10] | Yang Lv, Yueying Wang, Jahan Noushin, Haitao Hu, Ping Chen, Lianguang Shang, Haiyan Lin, Guojun Dong, Jiang Hu, Zhenyu Gao, Qian Qian, Yu Zhang, Longbiao Guo. Genome-Wide Association Analysis and Allelic Mining of Grain Shape-Related Traits in Rice [J]. Rice Science, 2019, 26(6): 384-392. |
| [11] | Wenhui Wang, Linlin Wang, Yujun Zhu, Yeyang Fan, Jieyun Zhuang. Fine-Mapping of qTGW1.2a, a Quantitative Trait Locus for 1000-Grain Weight in Rice [J]. Rice Science, 2019, 26(4): 220-228. |
| [12] | Matiar Rahaman Muhammad, Joseph Stout Michael. Comparative Efficacies of Next-Generation Insecticides Against Yellow Stem Borer and Their Effects on Natural Enemies in Rice Ecosystem [J]. Rice Science, 2019, 26(3): 157-166. |
| [13] | Zongxiang Chen, Zhiming Feng, Houxiang Kang, Jianhua Zhao, Tianxiao Chen, Qianqian Li, Hongbing Gong, Yafang Zhang, Xijun Chen, Xuebiao Pan, Wende Liu, Guoliang Wang, Shimin Zuo. Identification of New Resistance Loci Against Sheath Blight Disease in Rice Through Genome-Wide Association Study [J]. Rice Science, 2019, 26(1): 21-31. |
| [14] | Lee Jae-Sung, Wissuwa Matthias, B. Zamora Oscar, M. Ismail Abdelbagi. Novel Sources of aus Rice for Zinc Deficiency Tolerance Identified Through Association Analysis Using High-Density SNP Array [J]. Rice Science, 2018, 25(5): 293-296. |
| [15] | Yaobin Qin, Peng Cheng, Yichen Cheng, Yue Feng, Derun Huang, Tingxu Huang, Xianjun Song, Jiezheng Ying. QTL-Seq Identified a Major QTL for Grain Length and Weight in Rice Using Near Isogenic F2 Population [J]. Rice Science, 2018, 25(3): 121-131. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||