
Rice Science ›› 2025, Vol. 32 ›› Issue (1): 118-130.DOI: 10.1016/j.rsci.2024.09.001
• Research Papers • Previous Articles
Jiang Nan1, Qiu Jiehua1, Tian Dagang2, Shi Huanbin1, Liu Zhiquan1, Wen Hui1, Xie Shuwei1, Chen Huizhe1, Wu Meng3, Kou Yanjun1( )
)
Received:2024-06-15
Accepted:2024-09-18
Online:2025-01-28
Published:2025-03-25
Contact:
Kou Yanjun
Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight[J]. Rice Science, 2025, 32(1): 118-130.
Add to citation manager EndNote|Ris|BibTeX
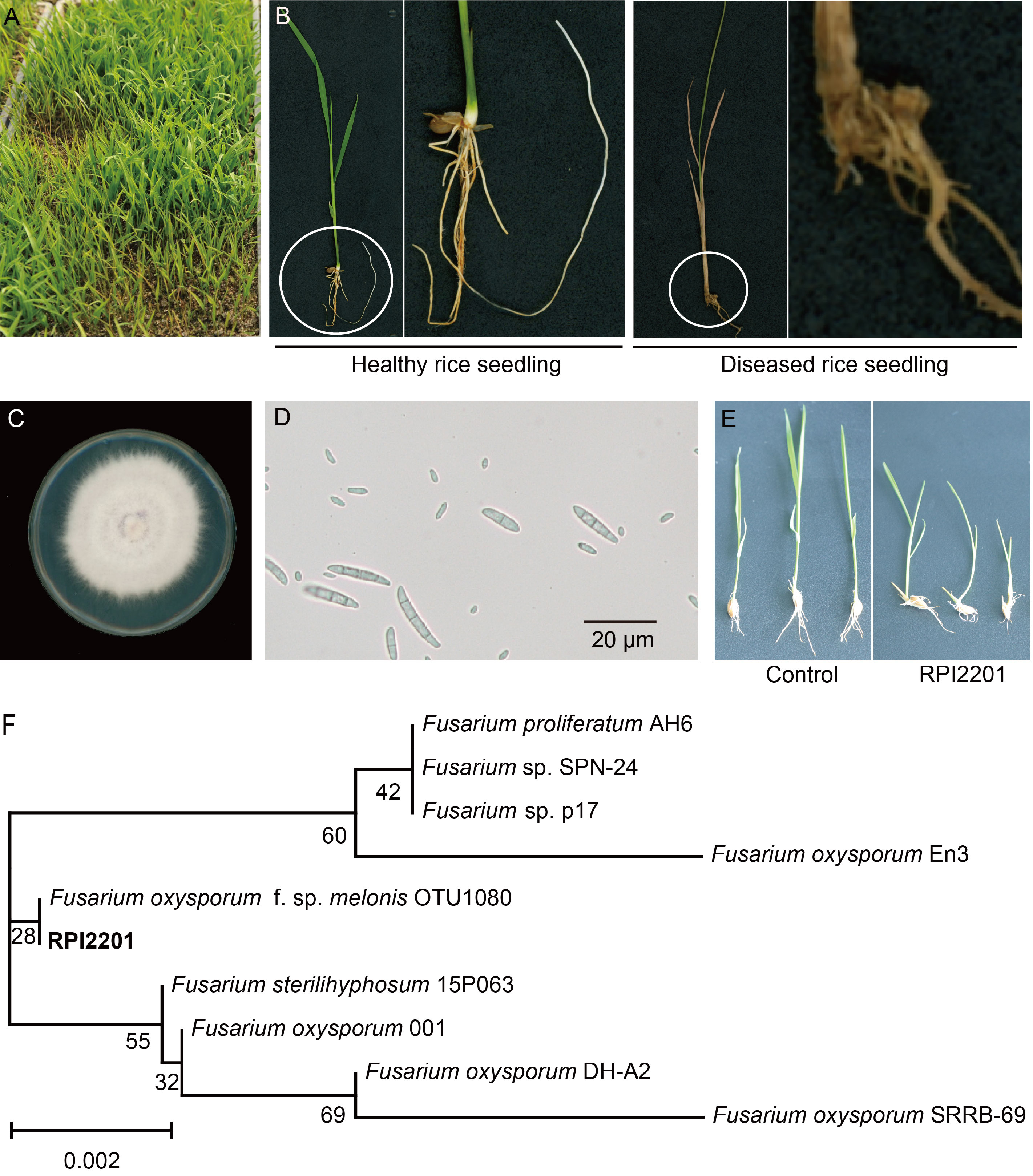
Fig. 1. Isolation and identification of pathogenic strain RPI 2201. A, Rice nursery tray with seedling blight. B, Rice plants with and without seedling blight. The position within the circle is the area that has been locally magnified. C, Colony morphology of RPI2201 strain. D, Photograph of RPI2201’s spores. E, Rice seedlings inoculated with RPI2201 strain. F, Phylogenetic analysis of RPI2201 strain based on the sequencing of PCR products (primers ITS1 and ITS2 are listed in Table S1).

Fig. 2. Combination of two different Bacillus strains better suppresses rice seedling blight than applying them individually. A, Phenotype of rice seedlings in the presence of Fusarium oxysporum with applying T40 and T208 strains individually or in combination. B‒E, Disease incidence of seedling blight (B), seedling emergence rate (C), plant height (D), and rice seedling biomass (E) under Bacillus strain treatments. Different lowercase letters above the bars indicate significant differences at the 0.05 probability level according to the Duncan’s test (n = 8).
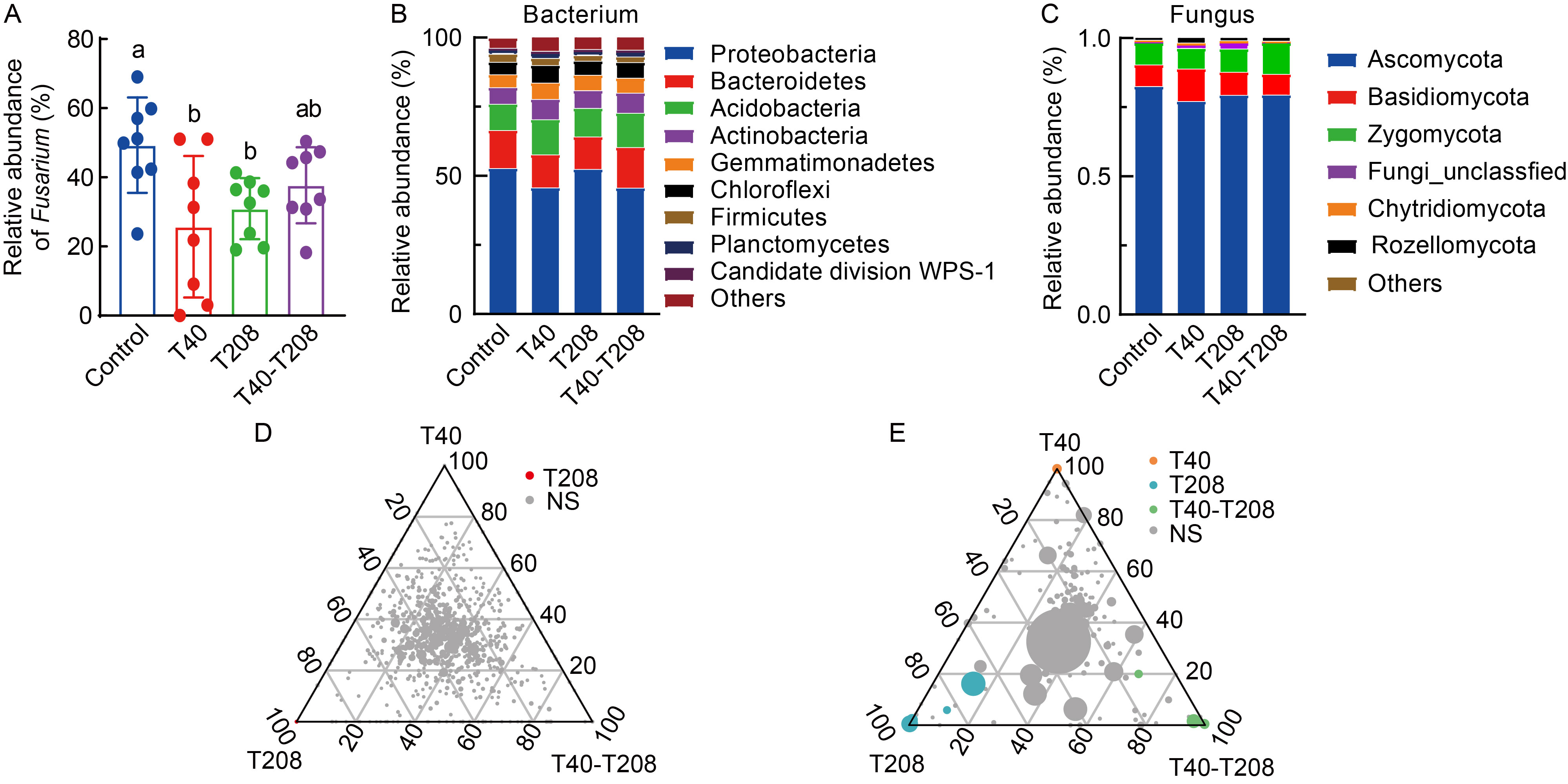
Fig. 3. Microbial community compositions in rhizosphere soil under different treatments. A, Relative abundance of Fusarium spp. treated with the T40 and T208 strains individually or in combination. Different lowercase letters above the bars indicate significant differences among the treatments according to the Duncan’s test (P < 0.05, n = 8). B and C, Stacked bar charts showing the relative abundance of various bacterial (B) and fungal (C) phyla communities. D and E, Ternary plots of bacterial and fungal amplicon sequence variants (ASVs). Each circle represents one ASV, and the size indicates its relative abundance. Circles marked NS indicate ASVs that did not significantly enrich in any of the three treatments.
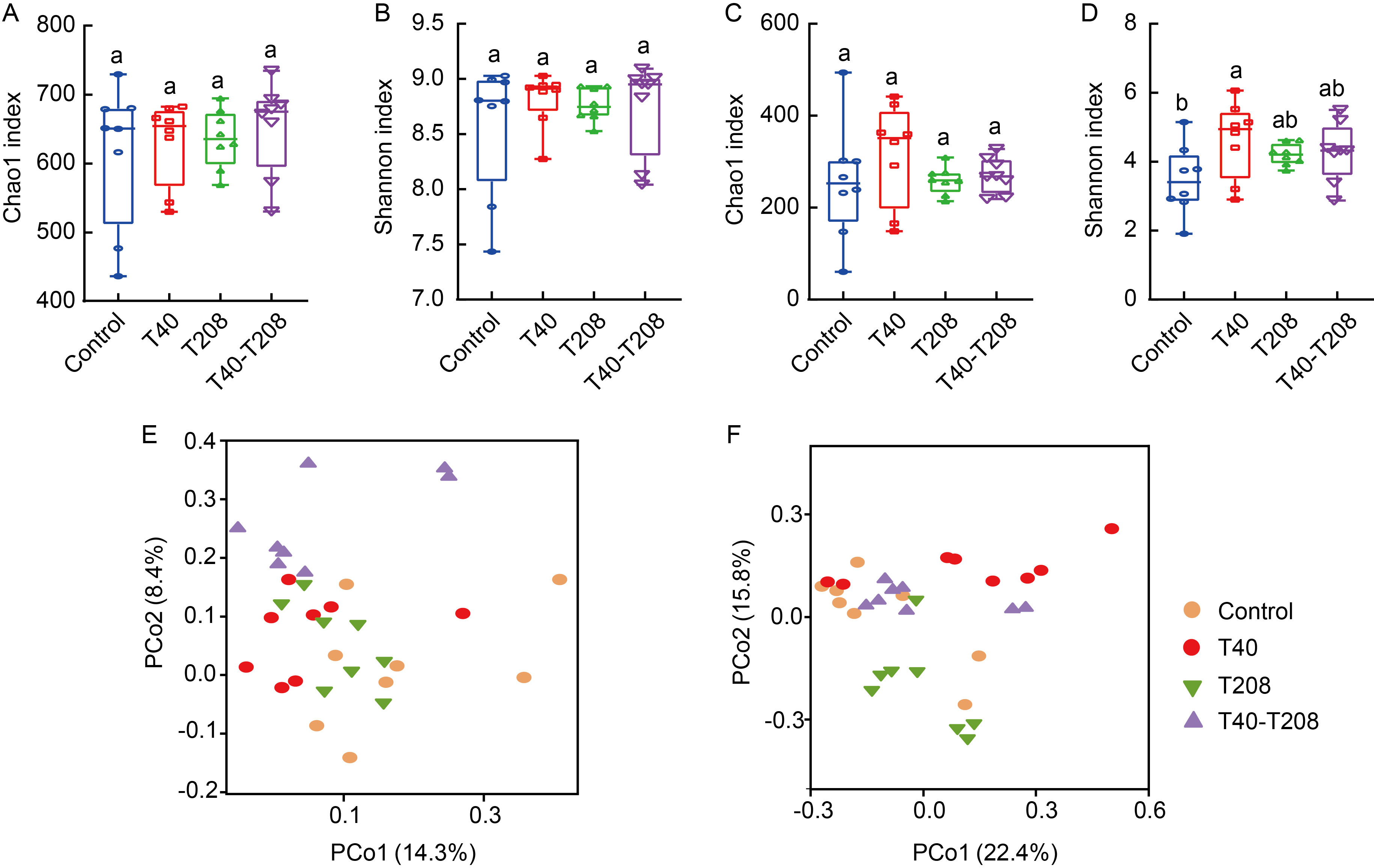
Fig. 4. Combination of T40 and T208 strains did not significantly change alpha diversities of microbial communities but significantly altered microbial community structures. A and B, Alpha-diversity of bacterial communities in rhizosphere soil of rice, expressed as richness (Chao1 index, A) and evenness (Shannon index, B). C and D, Alpha-diversity of fungal communities in rhizosphere soil of rice, expressed as richness (Chao1 index, C) and evenness (Shannon index, D). E and F, Beta-diversities of bacterial (E) and fungal (F) communities between different treatments. Different lowercase letters above the bars indicate significant differences among the treatments according to the Duncan’s test (P < 0.05, n = 8).
| Treatment comparison | P value | |||
|---|---|---|---|---|
| Bacterium | Fungus | |||
| Control vs T40 | 0.017 | < 0.01 | ||
| Control vs T208 | < 0.01 | < 0.01 | ||
| Control vs T40-T208 | < 0.01 | 0.012 | ||
| T40 vs T208 | 0.014 | < 0.01 | ||
| T40 vs T40-T208 | 0.013 | 0.033 | ||
| T208 vs T40-T208 | < 0.01 | 0.028 | ||
Table 1. Application of different Bacillus strains, either alone or in combination, significantly changed microbial community structures as determined by principal coordinates analysis based on Bray- Curtis dissimilarity.
| Treatment comparison | P value | |||
|---|---|---|---|---|
| Bacterium | Fungus | |||
| Control vs T40 | 0.017 | < 0.01 | ||
| Control vs T208 | < 0.01 | < 0.01 | ||
| Control vs T40-T208 | < 0.01 | 0.012 | ||
| T40 vs T208 | 0.014 | < 0.01 | ||
| T40 vs T40-T208 | 0.013 | 0.033 | ||
| T208 vs T40-T208 | < 0.01 | 0.028 | ||
| Treatment | Edge | Node | Degree | Diameter | Density | Modularity | Clustering coefficient | P | P/N |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 254 a | 364 b | 6.46 a | 19.4 b | 0.015 a | 0.65 c | 0.366 a | 1 204 a | 21.8 a |
| T40 | 1 372 a | 457 a | 5.76 a | 21.5 ab | 0.013 ab | 0.66 c | 0.314 b | 1 099 a | 4.5 b |
| T208 | 756 b | 446 a | 3.31 b | 23.1 a | 0.008 c | 0.89 a | 0.326 b | 474 b | 1.7 b |
| T40-T208 | 886 b | 422 a | 4.00 b | 20.1 ab | 0.009 bc | 0.82 b | 0.311 b | 687 b | 3.6 b |
Table 2. Combined use of different Bacillus strains significantly improved microbial network stability based on topological features of microbial subnetworks, including bacterial and fungal communities.
| Treatment | Edge | Node | Degree | Diameter | Density | Modularity | Clustering coefficient | P | P/N |
|---|---|---|---|---|---|---|---|---|---|
| Control | 1 254 a | 364 b | 6.46 a | 19.4 b | 0.015 a | 0.65 c | 0.366 a | 1 204 a | 21.8 a |
| T40 | 1 372 a | 457 a | 5.76 a | 21.5 ab | 0.013 ab | 0.66 c | 0.314 b | 1 099 a | 4.5 b |
| T208 | 756 b | 446 a | 3.31 b | 23.1 a | 0.008 c | 0.89 a | 0.326 b | 474 b | 1.7 b |
| T40-T208 | 886 b | 422 a | 4.00 b | 20.1 ab | 0.009 bc | 0.82 b | 0.311 b | 687 b | 3.6 b |
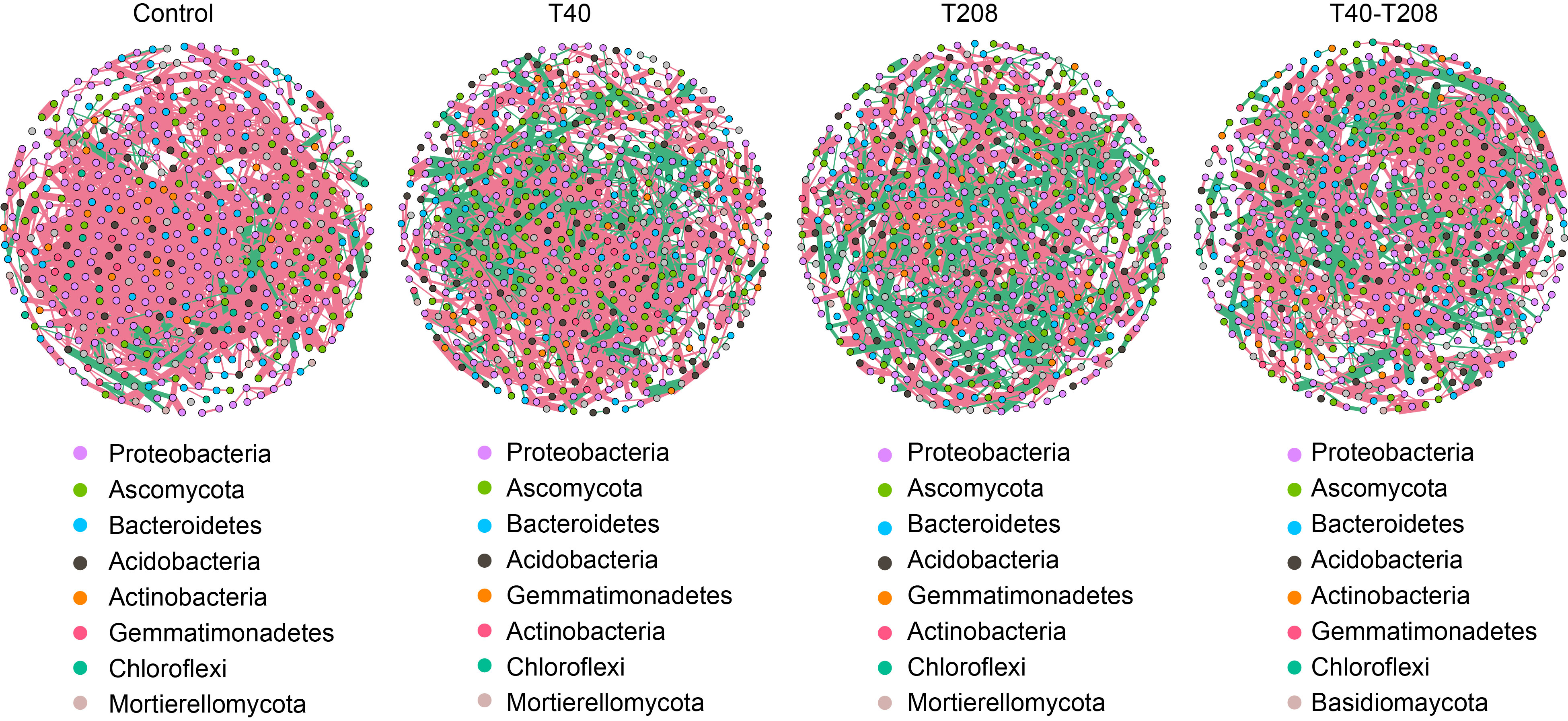
Fig. 5. Combining different Bacillus strains enhances microbial network stability. Co-occurrence networks of microbial communities, including both bacterial and fungal species in the samples. A connection represents a significant (P < 0.01) correlation between two amplicon sequence variants. The size of each node indicates the number of connections (i.e., degree), and the thickness of each connection (i.e., edge) between two nodes represents the value of the Spearman correlation coefficient. A blue edge indicates a positive correlation, while a red edge indicates a negative correlation.
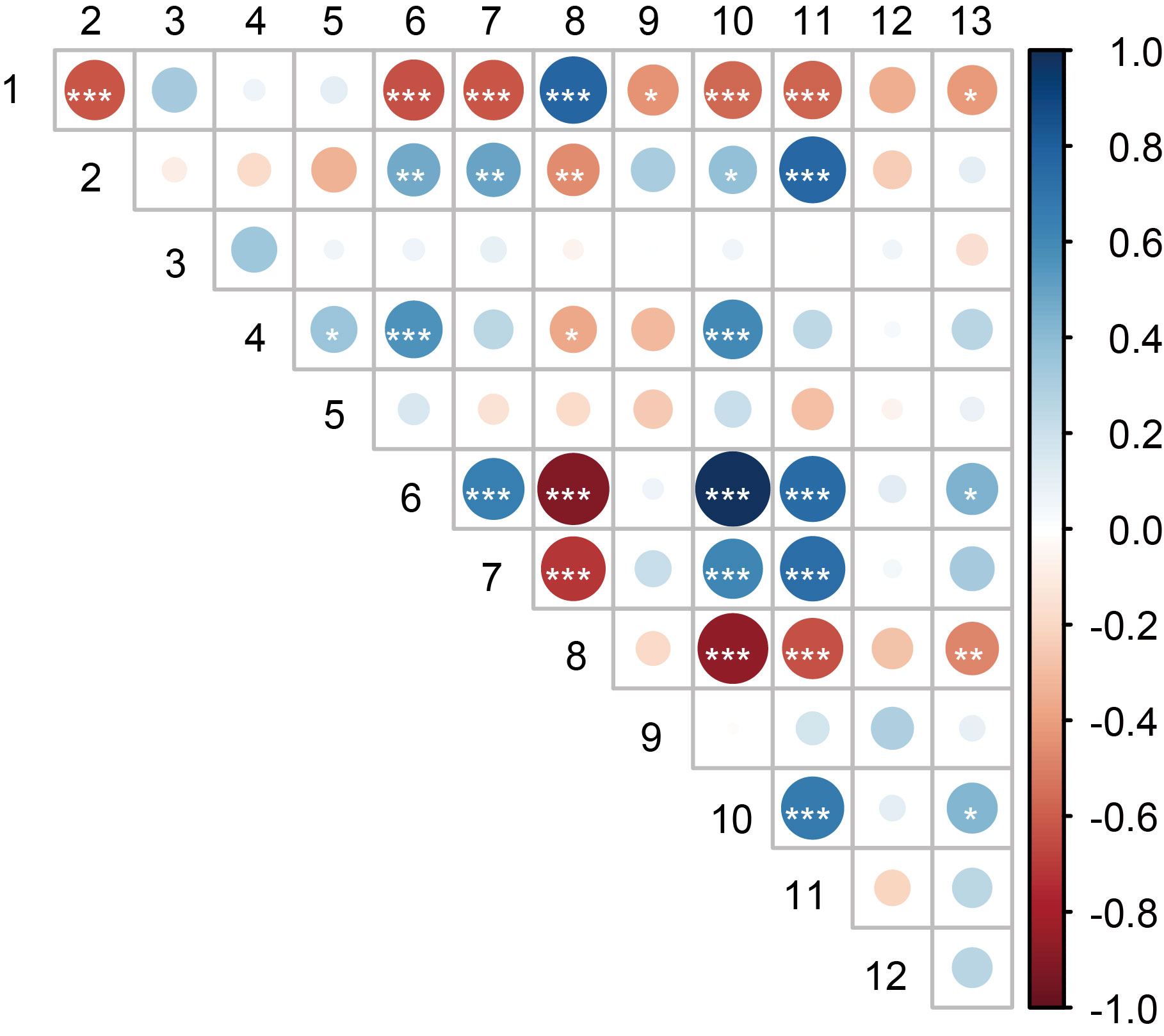
Fig. 6. Spearman’s correlation analysis between rice seedling biomass and major factors that explored by a random forest classification approach. 1, Rice seedling biomass; 2, Disease incidence; 3, β-nearest taxon index; 4, Fungal Chao1 index; 5, Fungal Shannon index; 6, Average degree; 7, Density; 8, Modularity; 9, Average clustering coefficient; 10, Positive correlation within the co-occurrence networks; 11, The ratio of positive to negative correlations in the co-occurrence networks; 12, ITS32; 13, ITS193. Asterisks (*) represent significant differences (Student’s t-test, *, P < 0.05; **, P < 0.01; ***, P < 0.001).
| [1] | Alizadeh H, Behboudi K, Ahmadzadeh M, et al. 2013. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol Contr, 65(1): 14-23. |
| [2] | Backman P A, Wilson M, Murphy J F. 1997. Bacteria for biological control of plant diseases. In: Rechcigl J E. Environmentally Safe Approaches to Crop Disease Control. Boca Raton, USA: CRC Press: 95-109. |
| [3] | Berendsen R L, Pieterse C M J, Bakker P A H M. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci, 17(8): 478-486. |
| [4] | Bolyen E, Rideout J R, Dillon M R, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol, 37(8): 852-857. |
| [5] | Bubici G, Kaushal M, Prigigallo M I, et al. 2019. Biological control agents against Fusarium wilt of banana. Front Microbiol, 10: 616. |
| [6] | Callahan B J, McMurdie P J, Rosen M J, et al. 2016. DADA2: High-resolution sample inference from Illumina amplicon data. Nat Methods, 13(7): 581-583. |
| [7] | Cao Y, Ding W, Liu C. 2021. Unraveling the metabolite signature of endophytic Bacillus velezensis strain showing defense response towards Fusarium oxysporum. Agronomy, 11(4): 683. |
| [8] | Cheng T, Yao X Z, Wu C Y, et al. 2020. Endophytic Bacillus megaterium triggers salicylic acid-dependent resistance and improves the rhizosphere bacterial community to mitigate rice spikelet rot disease. Appl Soil Ecol, 156: 103710. |
| [9] | Daraz U, Li Y, Sun Q, et al. 2021. Inoculation of Bacillus spp. modulate the soil bacterial communities and available nutrients in the rhizosphere of vetiver plant irrigated with acid mine drainage. Chemosphere, 263: 128345. |
| [10] | Delgado-Baquerizo M, Maestre F T, Reich P B, et al. 2016. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat Commun, 7: 10541. |
| [11] | Devi A, Mohan S, Rajalakshmi J. 2014. Growth promotion activity and biological control for the management of leaf blight incited by Alternaria helianthi. Arch Phytopathol, 47(18): 2280-2287. |
| [12] | Dobrzyński J, Jakubowska Z, Kulkova I, et al. 2023. Biocontrol of fungal phytopathogens by Bacillus pumilus. Front Microbiol, 14: 1194606. |
| [13] | Elmqvist T, Folke C, Nyström M, et al. 2003. Response diversity, ecosystem change, and resilience. Front Ecol Environ, 1(9): 488-494. |
| [14] | Elnahal A S M, El-Saadony M T, Saad A M, et al. 2022. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur J Plant Pathol, 162(4): 759-792. |
| [15] | Etesami H, Jeong B R, Glick B R. 2023. Biocontrol of plant diseases by Bacillus spp. Physiol Mol Plant Pathol, 126: 102048. |
| [16] | Fan J B, Bai P F, Ning Y S, et al. 2018. The monocot-specific receptor-like kinase SDS2 controls cell death and immunity in rice. Cell Host Microbe, 23(4): 498-510.e5. |
| [17] | Faust K, Raes J. 2016. CoNet app: Inference of biological association networks using Cytoscape. F1000Research, 5: 1519. |
| [18] | Gadhave K R, Devlin P F, Ebertz A, et al. 2018. Soil inoculation with Bacillus spp. modifies root endophytic bacterial diversity, evenness, and community composition in a context-specific manner. Microb Ecol, 76(3): 741-750. |
| [19] | Gao G F, Peng D, Zhang Y H, et al. 2021. Dramatic change of bacterial assembly process and co-occurrence pattern in Spartina alterniflora salt marsh along an inundation frequency gradient. Sci Total Environ, 755(Pt 1): 142546. |
| [20] | Gurav R, Tang J C, Jadhav J. 2017. Novel chitinase producer Bacillus pumilus RST25 isolated from the shellfish processing industry revealed antifungal potential against phyto-pathogens. Int Biodeterior Biodegrad, 125: 228-234. |
| [21] | International Rice Genome Sequencing Project. 2005. The map- based sequence of the rice genome. Nature, 436: 793-800. |
| [22] | Jetiyanon K, Kloepper J W. 2002. Mixtures of plant growth- promoting rhizobacteria for induction of systemic resistance against multiple plant diseases. Biol Contr, 24(3): 285-291. |
| [23] | Jiang W Y, Liu J X, He Y, et al. 2024. Biological control ability and antifungal activities of Bacillus velezensis Bv S3 against Fusarium oxysporum that causes rice seedling blight. Agronomy, 14(1): 167. |
| [24] | Karjalainen R, Jensen D F. 2006. Special issue: Biological control of plant pathogens. BioControl, 51: 279-400. |
| [25] | Katan J. 2015. Soil solarization: The idea, the research and its development. Phytoparasitica, 43(1): 1-4. |
| [26] | Li Z F, Bai X L, Jiao S, et al. 2021. A simplified synthetic community rescues Astragalus mongholicus from root rot disease by activating plant-induced systemic resistance. Microbiome, 9(1): 217. |
| [27] | Liaw A, Wiener M. 2002. Classification and regression by randomForest. R News, 2(3): 18-22. |
| [28] | Liu J X, Cai Y N, Jiang W Y, et al. 2020. Population structure and genetic diversity of fungi causing rice seedling blight in Northeast China based on microsatellite markers. Plant Dis, 104(3): 868-874. |
| [29] | Liu J X, Zhang R S, Xu C Z, et al. 2022. Characterisation of Pythium aristosporum oomycete: A novel pathogen causing rice seedling blight in China. J Fungi, 8(9): 890. |
| [30] | Liu K, Newman M, McInroy J A, et al. 2017. Selection and assessment of plant growth-promoting rhizobacteria for biological control of multiple plant diseases. Phytopathology, 107(8): 928-936. |
| [31] | Liu K, McInroy J A, Hu C H, et al. 2018. Mixtures of plant- growth-promoting rhizobacteria enhance biological control of multiple plant diseases and plant-growth promotion in the presence of pathogens. Plant Dis, 102(1): 67-72. |
| [32] | Lu R K. 2000. Analytical Method for Soil and Agricultural Chemistry. Beijing: China Agricultural Science and Technology Press. (in Chinese) |
| [33] | Ma B, Wang H Z, Dsouza M, et al. 2016. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J, 10(8): 1891-1901. |
| [34] | Ma B, Wang J H, Liu C Z, et al. 2019. Preventive effects of fluoro-substituted benzothiadiazole derivatives and chitosan oligosaccharide against the rice seedling blight induced by Fusarium oxysporum. Plants, 8(12): 538. |
| [35] | Ma L J, Geiser D M, Proctor R H, et al. 2013. Fusarium pathogenomics. Annu Rev Microbiol, 67: 399-416. |
| [36] | Miljaković D, Marinković J, Balešević-Tubić S. 2020. The significance of Bacillus spp. in disease suppression and growth promotion of field and vegetable crops. Microorganisms, 8(7): 1037. |
| [37] | Muñoz-Leoz B, Garbisu C, Charcosset J Y, et al. 2013. Non-target effects of three formulated pesticides on microbially-mediated processes in a clay-loam soil. Sci Total Environ, 449: 345-354. |
| [38] | Park C H, Chen S B, Shirsekar G, et al. 2012. The Magnaporthe oryzae effector AvrPiz-t targets the RING E3 ubiquitin ligase APIP6 to suppress pathogen-associated molecular pattern-triggered immunity in rice. Plant Cell, 24(11): 4748-4762. |
| [39] | Pinkerton J N, Ivors K L, Reeser P W, et al. 2002. The use of soil solarization for the management of soilborne plant pathogens in strawberry and red raspberry production. Plant Dis, 86(6): 645-651. |
| [40] | Prismantoro D, Akbari S I, Permadi N, et al. 2024. The multifaceted roles of Trichoderma in managing rice diseases for enhanced productivity and sustainability. J Agric Food Res, 18: 101324. |
| [41] | Raupach G S, Kloepper J W. 1998. Mixtures of plant growth- promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology, 88(11): 1158-1164. |
| [42] | Seenivasan N, David P M M, Vivekanandan P, et al. 2012. Biological control of rice root-knot nematode, Meloidogyne graminicola through mixture of Pseudomonas fluorescens strains. Biocontrol Sci Technol, 22(6): 611-632. |
| [43] | Shahzad R, Khan A L, Bilal S, et al. 2017. Plant growth-promoting endophytic bacteria versus pathogenic infections: An example of Bacillus amyloliquefaciens RWL-1 and Fusarium oxysporum f.sp. lycopersici in tomato. PeerJ, 5: e3107. |
| [44] | Sharma N, Reinke R, Sacks E J. 2021. Comparison of methods to evaluate rice (Oryza sativa) germplasm for tolerance to low temperature at the seedling stage. Agronomy, 11(2): 385. |
| [45] | Smith L J, Smith M K, Tree D, et al. 2008. Development of a small-plant bioassay to assess banana grown from tissue culture for consistent infection by Fusarium oxysporum f. sp. cubense. Austral Plant Pathol, 37(2): 171-179. |
| [46] | Stegen J C, Lin X J, Konopka A E, et al. 2012. Stochastic and deterministic assembly processes in subsurface microbial communities. ISME J, 6(9): 1653-1664. |
| [47] | Sun H W, Xiang X, Li Q, et al. 2021. Comparative genome analysis of Bacillus thuringiensis strain HD521 and HS18-1. Sci Rep, 11(1): 16590. |
| [48] | Sun X L, Xu Z H, Xie J Y, et al. 2022. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J, 16(3): 774-787. |
| [49] | Sun Z B, Yu S F, Hu Y F, Wen Y C. 2022. Biological control of the cucumber downy mildew pathogen Pseudoperonospora cubensis. Horticulturae, 8(5): 410. |
| [50] | Veneklaas E J, Stevens J, Cawthray G R, et al. 2003. Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil, 248: 187-197. |
| [51] | Wang C N, Wu R L, Li Y Y, et al. 2020. Effects of pesticide residues on bacterial community diversity and structure in typical greenhouse soils with increasing cultivation years in Northern China. Sci Total Environ, 710: 136321. |
| [52] | Wang H T, Wu C F, Zhang H Q, Xiao M L, Ge T D, Zhou Z C, Liu Y J, Peng S G, Peng P Q, Chen J P. 2022. Characterization of the belowground microbial community and co-occurrence networks of tobacco plants infected with bacterial wilt disease. World J Microbiol Biotechnol, 38(9): 155. |
| [53] | Wang J W, He Y H, Li T, et al. 2022. Complex biochemical synergistic interactions between two rhizobacteria grown in consortium, Bacillus subtilis SL-44 and Enterobacter hormaechei Wu-15. Rhizosphere, 24: 100587. |
| [54] | Wang J W, Deng Z H, Gao X Z, et al. 2024. Combined control of plant diseases by Bacillus subtilis SL44 and Enterobacter hormaechei Wu15. Sci Total Environ, 934: 173297. |
| [55] | Wang X B, Liang G B. 2014. Control efficacy of an endophytic Bacillus amyloliquefaciens strain BZ6-1 against peanut bacterial Wilt, Ralstonia solanacearum. Biomed Res Int, 2014: 465435. |
| [56] | Wei Z, Gu Y A, Friman V P, et al. 2019. Initial soil microbiome composition and functioning predetermine future plant health. Sci Adv, 5(9): eaaw0759. |
| [57] | Wen T, Xie P H, Penton C R, et al. 2022. Specific metabolites drive the deterministic assembly of diseased rhizosphere microbiome through weakening microbial degradation of autotoxin. Microbiome, 10(1): 177. |
| [58] | Xu X M, Wang Y Q, Lei T, et al. 2022. Synergistic effects of Bacillus amyloliquefaciens SDTB009 and difenoconazole on Fusarium wilt of tomato. Plant Dis, 106(8): 2165-2171. |
| [59] | Xun W B, Li W, Xiong W, et al. 2019. Diversity-triggered deterministic bacterial assembly constrains community functions. Nat Commun, 10(1): 3833. |
| [60] | Xun W B, Liu Y P, Li W, et al. 2021. Specialized metabolic functions of keystone taxa sustain soil microbiome stability. Microbiome, 9(1): 35. |
| [61] | Yuan S F, Wang L L, Wu K, Shi J X, Wang M S, Yang X M, Shen Q R, Shen B. 2014. Evaluation of Bacillus-fortified organic fertilizer for controlling tobacco bacterial wilt in greenhouse and field experiments. Appl Soil Ecol, 75: 86-94. |
| [62] | Zhao Y B, Guan D W, Liu X, et al. 2022. Profound change in soil microbial assembly process and co-occurrence pattern in co-inoculation of Bradyrhizobium japonicum 5038 and Bacillus aryabhattai MB35-5 on soybean. Front Microbiol, 13: 846359. |
| [1] | Yu Shicong, Luo Ruxian, Zheng Shuqin, Ning Jing, Shi Yuanzhu, Guo Daiming, Jia Liangmeng, Wang Sen, Xiao Guizong, Guo Pengwang, Li Yang, Ma Xiaoding. CHOLINE TRANSPORTER-RELATED 4 (CTR4) Is Involved in Drought and Saline Tolerance in Rice [J]. Rice Science, 2025, 32(1): 52-66. |
| [2] | Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance [J]. Rice Science, 2024, 31(6): 688-699. |
| [3] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [4] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [5] | Xia Xiaodong, Zhang Xiaobo, Wang Zhonghao, Cheng Benyi, Sun Huifeng, Xu Xia, Gong Junyi, Yang Shihua, Wu Jianli, Shi Yongfeng, Xu Rugen. Mapping and Functional Analysis of LE Gene in a Lethal Etiolated Rice Mutant at Seedling Stage [J]. Rice Science, 2023, 30(6): 567-576. |
| [6] | Md. Dhin Islam, Adam H. Price, Paul D. Hallett. Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth [J]. Rice Science, 2023, 30(5): 459-472. |
| [7] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [8] | Yousef Alhaj Hamoud, Hiba Shaghaleh, Wang Ruke, Willy Franz Gouertoumbo, Amar Ali Adam hamad, Mohamed Salah Sheteiwy, Wang Zhenchang, Guo Xiangping. Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation [J]. Rice Science, 2022, 29(5): 473-488. |
| [9] | Chen Wei, Cai Yicong, Shakeel Ahmad, Wang Yakun, An Ruihu, Tang Shengjia, Guo Naihui, Wei Xiangjin, Tang Shaoqing, Shao Gaoneng, Jiao Guiai, Xie Lihong, Hu Shikai, Sheng Zhonghua, Hu Peisong. NRL3 Interacts with OsK4 to Regulate Heading Date in Rice [J]. Rice Science, 2022, 29(3): 237-246. |
| [10] | Yong Yang, Qiujun Lin, Xinyu Chen, Weifang Liang, Yuwen Fu, Zhengjin Xu, Yuanhua Wu, Xuming Wang, Jie Zhou, Chulang Yu, Chengqi Yan, Qiong Mei, Jianping Chen. Characterization and Proteomic Analysis of Novel Rice Lesion Mimic Mutant with Enhanced Disease Resistance [J]. Rice Science, 2021, 28(5): 466-478. |
| [11] | Mishra Rukmini, Zheng Wei, Kumar Joshi Raj, Kaijun Zhao. Genome Editing Strategies Towards Enhancement of Rice Disease Resistance [J]. Rice Science, 2021, 28(2): 133-145. |
| [12] | Yanchang Luo, Tingchen Ma, Teo Joanne, Zhixiang Luo, Zefu Li, Jianbo Yang, Zhongchao Yin. Marker-Assisted Breeding of Thermo-Sensitive Genic Male Sterile Line 1892S for Disease Resistance and Submergence Tolerance [J]. Rice Science, 2021, 28(1): 89-98. |
| [13] | Shuting Yuan, Chunjue Xu, Wei Yan, Zhenyi Chang, Xingwang Deng, Zhufeng Chen, Jianxin Wu, Xiaoyan Tang. Alternative Splicing of OsRAD1 Defines C-Terminal Domain Essential for Protein Function in Meiosis [J]. Rice Science, 2020, 27(4): 289-301. |
| [14] | Vijayaraghavareddy Preethi, Xinyou Yin, C. Struik Paul, Makarla Udayakumar, Sreeman Sheshshayee. Responses of Lowland, Upland and Aerobic Rice Genotypes to Water Limitation During Different Phases [J]. Rice Science, 2020, 27(4): 345-354. |
| [15] | Hussain Kashif, Yingxing Zhang, Anley Workie, Riaz Aamir, Abbas Adil, Hasanuzzaman Rani Md., Hong Wang, Xihong Shen, Liyong Cao, Shihua Cheng. Association Mapping of Quantitative Trait Loci for Grain Size in Introgression Line Derived from Oryza rufipogon [J]. Rice Science, 2020, 27(3): 246-254. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||