
Rice Science ›› 2025, Vol. 32 ›› Issue (2): 217-227.DOI: 10.1016/j.rsci.2024.12.012
• Research Papers • Previous Articles Next Articles
Wang Shuman1, Zhang Linqi1, Gao Ruiren1, Wei Guangbo1, Dong Weiguo1, Xu Jiming1, Wang Zhiye1,2( )
)
Received:2024-10-06
Accepted:2024-12-17
Online:2025-03-28
Published:2025-04-14
Contact:
Wang Zhiye (wangzhiye1@zju.edu.cn)
Wang Shuman, Zhang Linqi, Gao Ruiren, Wei Guangbo, Dong Weiguo, Xu Jiming, Wang Zhiye. Establishing Programmable CRISPR/Cas13b-Mediated Knockdown System in Rice[J]. Rice Science, 2025, 32(2): 217-227.
Add to citation manager EndNote|Ris|BibTeX
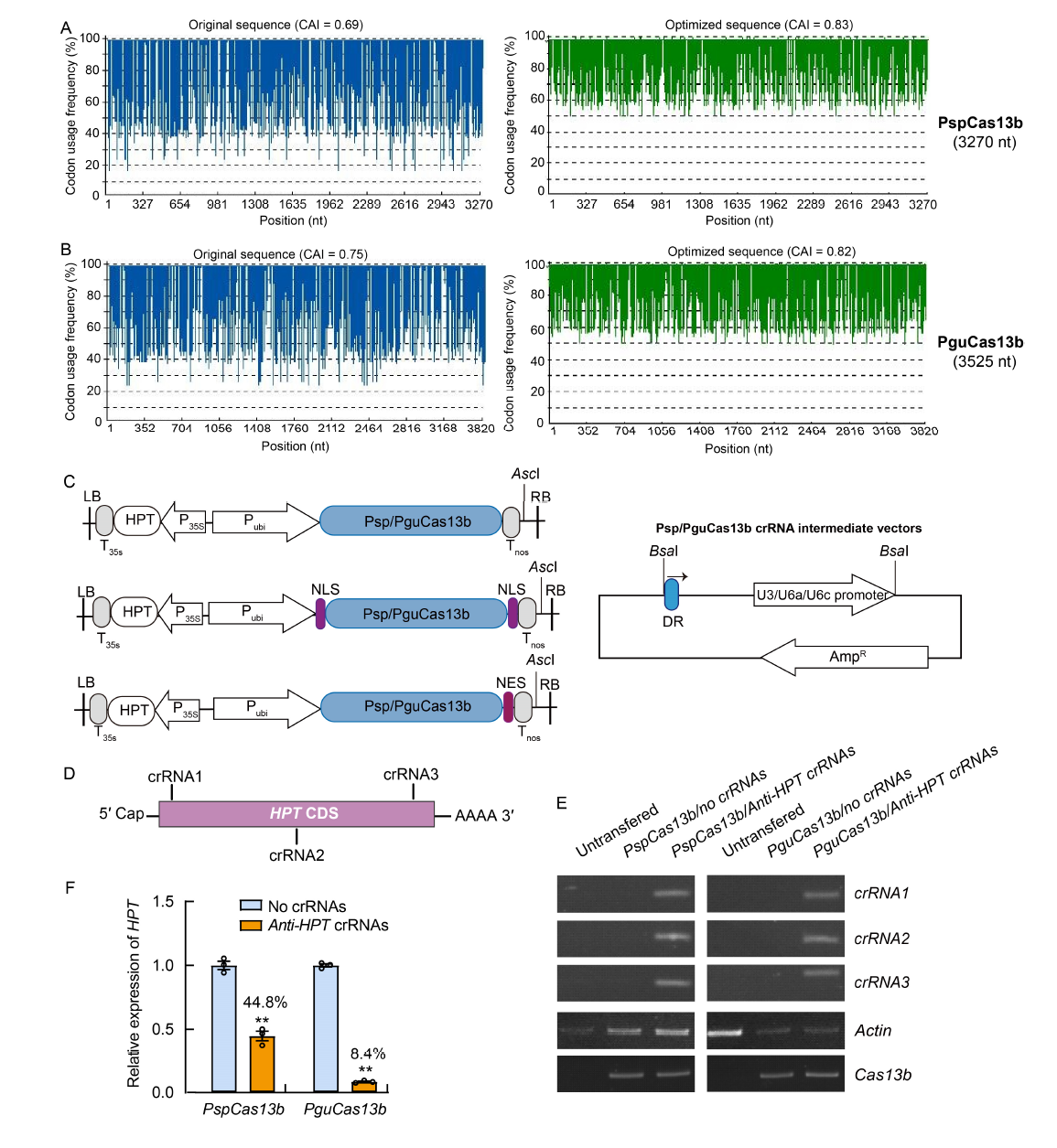
Fig. 1. CRISPR/Cas13b-mediated knockdown system in rice. A and B, Plant codon-optimized PspCas13b (A) and PguCas13b (B) have high codon adaptation indexes (CAI). CAI values of the original (blue) and optimized sequences (green) of PspCas13b and PguCas13b are presented. CAI values of the optimized sequences are in the range of 0.8-1.0, indicating ideal expression. C, Overall structure of Cas13b binary vectors and crRNA intermediate vectors. The arrow above the direct repeat (DR) indicates the direction of the DR sequence. RB, Right border; LB, Left border; HPT, Hygromycin B phosphotransferase; NLS, Nuclear localization signal; NES, Nuclear export signal. T, Terminator; P, Promoter; AmpR, Ampicillin resistance. D, Target sites of anti-HPT crRNAs in the coding sequence (CDS) region of HPT gene. E, Semi-RT-PCR results validate the expression of corresponding Cas13b and anti-HPT crRNAs in rice protoplast cells. Cas13b serves as loading control. F, qRT-PCR results showing that the CRISPR/Cas13b-knockdown system efficiently repressed exogenous HPT transcripts in rice protoplast cells. Protoplast cells without anti-HPT crRNAs expression (No crRNAs) is served as an internal control. The relative HPT signals were normalized to those of the no-crRNAs control, for which the ratio was arbitrarily set to 1. Data are Mean ± SE (n = 3). **, P < 0.01, determined using an unpaired two-tailed Student’s t-test.
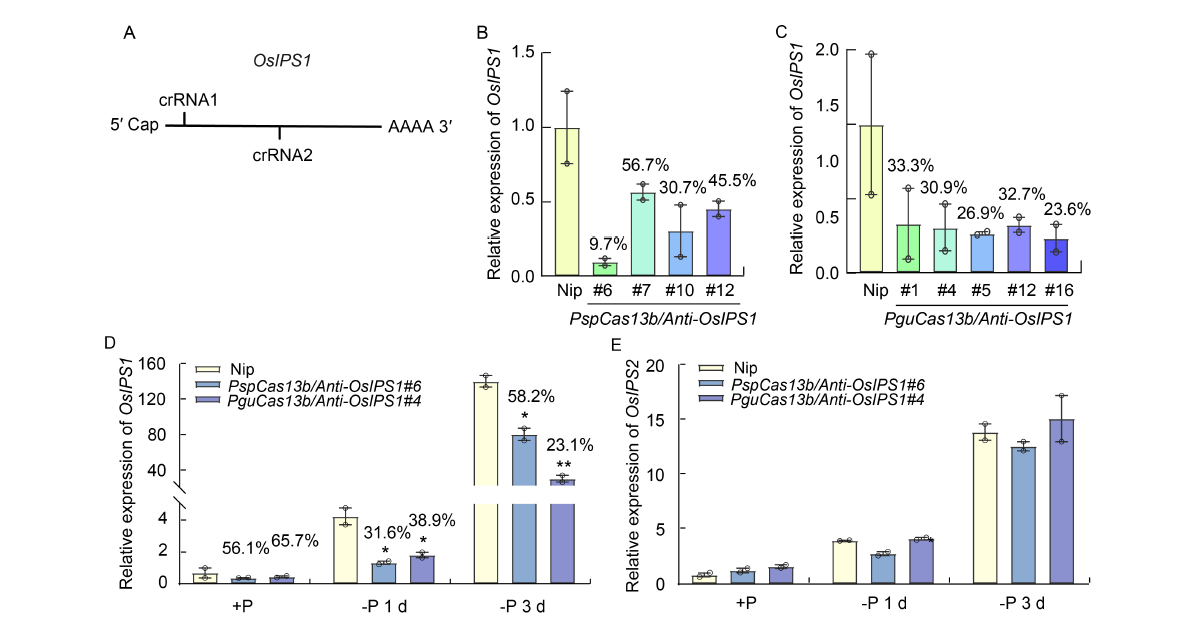
Fig. 2. CRISPR/Cas13b knockdown system efficiently represses endogenous long noncoding RNA (lncRNA). A, Target sites of anti-OsIPS1 crRNAs in OsIPS1. B and C, OsIPS1 was efficiently repressed in PspCas13b/Anti-OsIPS1 (B) and PguCas13b/Anti-OsIPS1 (C) transgenic plants compared with wild type Nipponbare (Nip) under phosphate deficient (-P) conditions for 7 d. D and E, RNA level of OsIPS1 (D), but not that its paralog OsIPS2 (E), was repressed in both PspCas13b/Anti-OsIPS1 and PguCas13b/Anti-OsIPS1 transgenic plants with varying levels of OsIPS1 abundance under normal (+P) and deficient (-P) phosphate conditions for 1 and 3 d. In B-E, the relative signals of detected transcripts were normalized to those of the Nip control, for which the ratio was arbitrarily set to 1. In D and E, * and **, P < 0.05 and P < 0.01, determined using an unpaired two-tailed Student’s t-test.
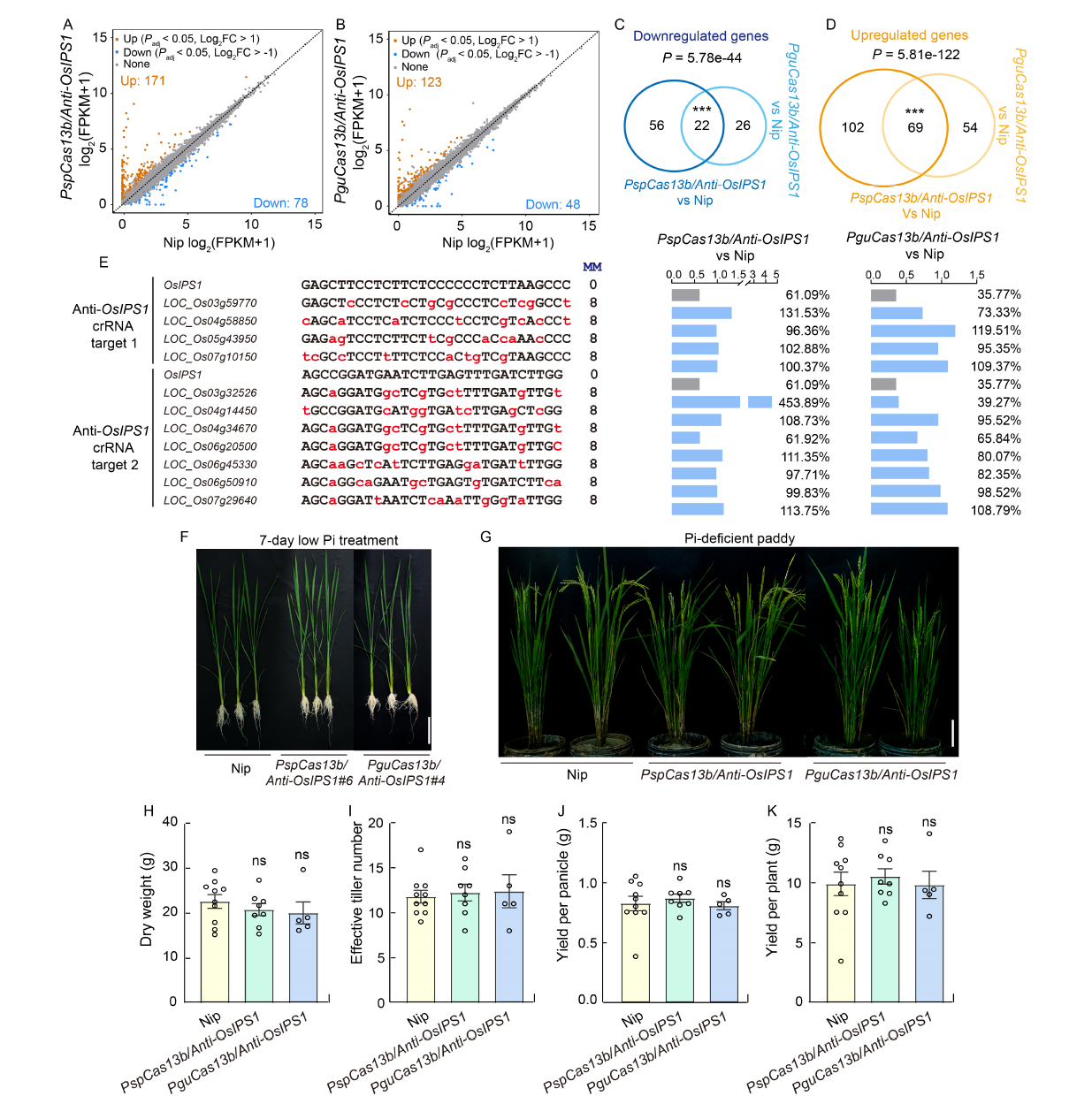
Fig. 3. CRISPR/Cas13b knockdown system has low collateral effects. A and B, Scatter plots of differential expression levels of genes between Nipponbare (Nip) and PspCas13b/Anti-OsIPS1#6 (A) or PguCas13b/Anti- OsIPS1#4 (B). Padj, Adjusted P-value; FC, Fold change; FPKM, Fragments per kilobase of transcript per million mapped reads.C and D, Venn diagrams showing significant overlaps in downregulated genes (C) and upregulated genes (D) of PspCas13b/Anti-OsIPS1 and PguCas13b/Anti-OsIPS1. ***, P < 0.001 using a hypergeometric test. E, Sites and relative expression levels of crRNA-dependent off-target transcripts from Anti-OsIPS1 crRNA target 1 and Anti-OsIPS1 crRNA target 2 identified in PspCas13b/Anti-OsIPS1 and PguCas13b/Anti-OsIPS1 transgenic plants. MM, Mismatch number of off-target sites. The value of bars represents the mean of the biological replicates. F and G, Representative photographs of Nip, PspCas13b/Anti-OsIPS1#6, and PguCas13b/Anti-OsIPS1#4 transgenic plants following a 7-day low inorganic phosphate (Pi) treatment at the seedling stage (F) and under Pi-deficient conditions in a paddy field at the flowering stage (G). Scale bars, 10 cm. H-K, There were no differences in dry weight (H), effective tiller number (I), yield per panicle (J), and yield per plant (K) between Nip (n = 10), PspCas13b/Anti-OsIPS1#6 (n = 8) or PguCas13b/Anti-OsIPS1#4 (n = 5) transgenic plants. Data are Mean ± SE; ns, Not significant.
| [1] | Abudayyeh O O, Gootenberg J S, Konermann S, et al. 2016. C2c2 is a single-component programmable RNA-guided RNA-targeting CRISPR effector. Science, 353: aaf5573. |
| [2] | Abudayyeh O O, Gootenberg J S, Essletzbichler P, et al. 2017. RNA targeting with CRISPR-Cas13. Nature, 550: 280-284. |
| [3] | Ai Y X, Liang D M, Wilusz J E. 2022. CRISPR/Cas13 effectors have differing extents of off-target effects that limit their utility in eukaryotic cells. Nucleic Acids Res, 50(11): e65. |
| [4] | Aman R, Ali Z, Butt H, et al. 2018a. RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol, 19: 1. |
| [5] | Aman R, Mahas A, Butt H, et al. 2018b. Engineering RNA virus interference via the CRISPR/Cas 13 machinery in Arabidopsis. Viruses, 10(12): 732. |
| [6] | Barrangou R, Fremaux C, Deveau H, et al. 2007. CRISPR provides acquired resistance against viruses in prokaryotes. Science, 315: 1709-1712. |
| [7] | Cox D B T, Gootenberg J S, Abudayyeh O O, et al. 2017. RNA editing with CRISPR-Cas13. Science, 358: 1019-1027. |
| [8] | East-Seletsky A, O’Connell M R, Knight S C, et al. 2016. Two distinct RNase activities of CRISPR-C2c2 enable guide-RNA processing and RNA detection. Nature, 538: 270-273. |
| [9] | Franco-Zorrilla J M, Valli A, Todesco M, et al. 2007. Target mimicry provides a new mechanism for regulation of microRNA activity. Nat Genet, 39: 1033-1037. |
| [10] | Freije C A, Myhrvold C, Boehm C K, et al. 2019. Programmable inhibition and detection of RNA viruses using Cas13. Mol Cell, 76(5): 826-837.e11. |
| [11] | Gao C X. 2021. Genome engineering for crop improvement and future agriculture. Cell, 184(6): 1621-1635. |
| [12] | Guo M N, Ruan W Y, Li C Y, et al. 2015. Integrative comparison of the role of the PHOSPHATE RESPONSE1 subfamily in phosphate signaling and homeostasis in rice. Plant Physiol, 168(4): 1762-1776. |
| [13] | Hou X L, Wu P, Jiao F C, et al. 2005. Regulation of the expression of OsIPS1 and OsIPS2 in rice via systemic and local Pi signalling and hormones. Plant Cell Environ, 28(3): 353-364. |
| [14] | Hu Y P, Chen Y C, Xu J, et al. 2022. Metagenomic discovery of novel CRISPR-Cas13 systems. Cell Discov, 8(1): 107. |
| [15] | Kavuri N R, Ramasamy M, Qi Y P, et al. 2022. Applications of CRISPR/Cas13-based RNA editing in plants. Cells, 11(17): 2665. |
| [16] | Kelley C P, Haerle M C, Wang E T. 2022. Negative autoregulation mitigates collateral RNase activity of repeat-targeting CRISPR- Cas13d in mammalian cells. Cell Rep, 40(7): 111226. |
| [17] | Konermann S, Lotfy P, Brideau N J, et al. 2018. Transcriptome engineering with RNA-targeting type VI-D CRISPR effectors. Cell, 173(3): 665-676.e14. |
| [18] | Li B S, Sun C, Li J Y, et al. 2024. Targeted genome-modification tools and their advanced applications in crop breeding. Nat Rev Genet, 25(9): 603-622. |
| [19] | Ma X L, Zhang Q Y, Zhu Q L, et al. 2015. A robust CRISPR/Cas9 system for convenient, high-efficiency multiplex genome editing in monocot and dicot plants. Mol Plant, 8(8): 1274-1284. |
| [20] | Mahas A, Aman R, Mahfouz M. 2019. CRISPR-Cas13d mediates robust RNA virus interference in plants. Genome Biol, 20(1): 263. |
| [21] | Miao Y S, Jiang L W. 2007. Transient expression of fluorescent fusion proteins in protoplasts of suspension cultured cells. Nat Protoc, 2: 2348-2353. |
| [22] | Pickar-Oliver A, Gersbach C A. 2019. The next generation of CRISPR-Cas technologies and applications. Nat Rev Mol Cell Biol, 20(8): 490-507. |
| [23] | Sharma V K, Marla S, Zheng W G, et al. 2022. CRISPR guides induce gene silencing in plants in the absence of Cas. Genome Biol, 23: 6. |
| [24] | Sharp P M, Li W H. 1987. The codon adaptation index: A measure of directional synonymous Codon usage bias, and its potential applications. Nucleic Acids Res, 15(3): 1281-1295. |
| [25] | Shi P G, Murphy M R, Aparicio A O, et al. 2023. Collateral activity of the CRISPR/RfxCas13d system in human cells. Commun Biol, 6: 334. |
| [26] | Shmakov S, Abudayyeh O O, Makarova K S, et al. 2015. Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell, 60(3): 385-397. |
| [27] | Smargon A A, Shi Y J, Yeo G W. 2020. RNA-targeting CRISPR systems from metagenomic discovery to transcriptomic engineering. Nat Cell Biol, 22(2): 143-150. |
| [28] | Tong H W, Huang J, Xiao Q Q, et al. 2023. High-fidelity Cas13 variants for targeted RNA degradation with minimal collateral effects. Nat Biotechnol, 41: 108-119. |
| [29] | Wang Q X, Liu X, Zhou J H, et al. 2019. The CRISPR-Cas13a gene-editing system induces collateral cleavage of RNA in glioma cells. Adv Sci, 6(20): 1901299. |
| [30] | Wen W, Meinkotht J L, Tsien R Y, et al. 1995. Identification of a signal for rapid export of proteins from the nucleus. Cell, 82(3): 463-473. |
| [31] | Wiedenheft B, Sternberg S H, Doudna J A. 2012. RNA-guided genetic silencing systems in bacteria and archaea. Nature, 482: 331-338. |
| [32] | Wierzbicki A T, Blevins T, Swiezewski S. 2021. Long noncoding RNAs in plants. Annu Rev Plant Biol, 72: 245-271. |
| [33] | Wilson C, Chen P J, Miao Z, et al. 2020. Programmable m6A modification of cellular RNAs with a Cas13-directed methyltransferase. Nat Biotechnol, 38(12): 1431-1440. |
| [34] | Xu B B, Zhu Y D, Cao C C, et al. 2022. Recent advances in RNA structurome. Sci China Life Sci, 65(7): 1285-1324. |
| [35] | Xu C L, Zhou Y S, Xiao Q Q, et al. 2021. Programmable RNA editing with compact CRISPR-Cas13 systems from uncultivated microbes. Nat Methods, 18(5): 499-506. |
| [36] | Yang H, Patel D J. 2024. Structures, mechanisms and applications of RNA-centric CRISPR-Cas13. Nat Chem Biol, 20: 673-688. |
| [37] | Yang L Z, Wang Y, Li S Q, et al. 2019. Dynamic imaging of RNA in living cells by CRISPR-Cas13 systems. Mol Cell, 76(6): 981-997.e7. |
| [38] | Yu Y C, Pan Z Y, Wang X, et al. 2022. Targeting of SPCSV- RNase3 via CRISPR-Cas13 confers resistance against sweet potato virus disease. Mol Plant Pathol, 23(1): 104-117. |
| [39] | Zhan X H, Zhang F J, Zhong Z Y, et al. 2019. Generation of virus-resistant potato plants by RNA genome targeting. Plant Biotechnol J, 17(9): 1814-1822. |
| [40] | Zhang T, Zhao Y L, Ye J J, et al. 2019. Establishing CRISPR/ Cas13a immune system conferring RNA virus resistance in both dicot and monocot plants. Plant Biotechnol J, 17(7): 1185-1187. |
| [41] | Zhou J, Jiao F C, Wu Z C, et al. 2008. OsPHR2 is involved in phosphate- starvation signaling and excessive phosphate accumulation in shoots of plants. Plant Physiol, 146(4): 1673-1686. |
| [1] | Sanchika Snehi, Ravi Kiran Kt, Sanket Rathi, Sameer Upadhyay, Suneetha Kota, Satish Kumar Sanwal, Lokeshkumar Bm, Arun Balasubramaniam, Nitish Ranjan Prakash, Pawan Kumar Singh. Discerning Genes to Deliver Varieties: Enhancing Vegetative- and Reproductive-Stage Flooding Tolerance in Rice [J]. Rice Science, 2025, 32(2): 160-176. |
| [2] | Nie Lixiao, Guo Xiayu, Wang Weiqin, Qi Yucheng, Ai Zhiyong, He Aibin. Regulation of Regeneration Rate to Enhance Ratoon Rice Production [J]. Rice Science, 2025, 32(2): 177-192. |
| [3] | Uthpal Krishna Roy, Babita Pal, Soumen Bhattacharjee. A Novel Approach for Screening Salinity-Tolerant Rice Germplasm by Exploring Redox-Regulated Cytological Fingerprint [J]. Rice Science, 2025, 32(2): 228-242. |
| [4] | He Zhenrui, Zhao Wenhua, Cheng Baoping, Yang Mei, Yang Yingqing, Zhu Yiming, Zhou Erxun. Molecular and Biological Characterization of Novel Mitovirus Infecting Phytopathogenic Fungus Ustilaginoidea virens [J]. Rice Science, 2025, 32(2): 243-258. |
| [5] | He Chen, Ruan Yunze, Jia Zhongjun. A Meta-Analysis of 30 Years in China and Micro-District Experiments Shows Organic Fertilizer Quantification Combined with Chemical Fertilizer Reduction Enhances Rice Yield on Saline-Alkali Land [J]. Rice Science, 2025, 32(2): 259-272. |
| [6] | Wang Mingyue, Zhao Weibo, Feng Xiaoya, Chen Yi, Li Junhao, Fu Jinmei, Yan Yingchun, Chu Zhaohui, Huang Wenchao. Disruption of Energy Metabolism and Reactive Oxygen Species Homeostasis in Honglian Type-Cytoplasmic Male Sterility (HL-CMS) Rice Pollen [J]. Rice Science, 2025, 32(1): 81-93. |
| [7] | Intan Farahanah, Shariza Sahudin, Hannis Fadzillah Mohsin, Siti Alwani Ariffin, Liyana Dhamirah Aminuddin. Understanding Investigational Perspective of Antioxidant and Antibacterial Properties of Rice [J]. Rice Science, 2025, 32(1): 15-31. |
| [8] | Jeberlin Prabina Bright, Hemant S. Maheshwari, Sugitha Thangappan, Kahkashan Perveen, Najat A. Bukhari, Debasis Mitra, Riyaz Sayyed, Andrea Mastinu. Biofilmed-PGPR: Next-Generation Bioinoculant for Plant Growth Promotion in Rice under Changing Climate [J]. Rice Science, 2025, 32(1): 94-106. |
| [9] | Wang Haoran, Chen Guoqing, Feng Guozhong. Expanding Viral Diversity in Rice Fields by Next-Generation Sequencing [J]. Rice Science, 2025, 32(1): 44-51. |
| [10] | Surangkana Chimthai, Sulaiman Cheabu, Wanchana Aesomnuk, Siriphat Ruengphayak, Siwaret Arikit, Apichart Vanavichit, Chanate Malumpong. Breeding for Heat Tolerant Aromatic Rice Varieties and Identification of Novel QTL Regions Associated with Heat Tolerance During Reproductive Phase by QTL-Seq [J]. Rice Science, 2025, 32(1): 67-80. |
| [11] | Durga Prasad Mullangie, Kalaimagal Thiyagarajan, Manonmani Swaminathan, Jagadeesan Ramalingam, Sritharan Natarajan, Senthilkumar Govindan. Breeding Resilience: Exploring Lodging Resistance Mechanisms in Rice [J]. Rice Science, 2024, 31(6): 659-672. |
| [12] | Fu Yiwei, Wu Jiayelu, Wu Mingming, Ye Shenghai, Zhai Rongrong, Ye Jing, Zhu Guofu, Yu Faming, Lu Yanting, Zhang Xiaoming. Progress on Molecular Mechanism of Heat Tolerance in Rice [J]. Rice Science, 2024, 31(6): 673-687. |
| [13] | Yang Yigang, Xu Ya’nan, Bai Yeran, Zhang Yuanpei, Han Wei, Makoto Saito, Lü Guohua, Song Jiqing, Bai Wenbo. Mixed-Oligosaccharides Promote Seedling Growth of Direct-Seeded Rice under Salt and Alkaline Stress [J]. Rice Science, 2024, 31(6): 712-724. |
| [14] | Ren Jian, Hu Kelin, Feng Puyu, William D. Batchelor, Liu Haitao, Lü Shihua. Simulating Responses of Rice Yield and Nitrogen Fates to Ground Cover Rice Production System under Different Types of Precipitation Years [J]. Rice Science, 2024, 31(6): 725-739. |
| [15] | Tao Yi, Xiao Deshun, Ye Chang, Liu Kancheng, Tang Xinxin, Ma Hengyu, Chu Guang, Yu Kai, Xu Chunmei, Wang Danying. Compound Microbial Agent Improves Soil Redox Status to Reduce Methane Emissions from Paddy Fields [J]. Rice Science, 2024, 31(6): 740-750. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||