
Rice Science ›› 2024, Vol. 31 ›› Issue (5): 526-544.DOI: 10.1016/j.rsci.2024.04.008
• Review • Previous Articles Next Articles
Kunhikrishnan Hemalatha Dhanyalakshmi1, Reshma Mohan2, Sasmita Behera3, Uday Chand Jha4, Debashis Moharana3, Ahalya Behera3, Sini Thomas5, Preman Rejitha Soumya2, Rameswar Prasad Sah3( ), Radha Beena2(
), Radha Beena2( )
)
Received:2024-02-15
Accepted:2024-04-17
Online:2024-09-28
Published:2024-10-11
Contact:
Radha Beena (beena.r@kau.in);Kunhikrishnan Hemalatha Dhanyalakshmi, Reshma Mohan, Sasmita Behera, Uday Chand Jha, Debashis Moharana, Ahalya Behera, Sini Thomas, Preman Rejitha Soumya, Rameswar Prasad Sah, Radha Beena. Next Generation Nutrition: Genomic and Molecular Breeding Innovations for Iron and Zinc Biofortification in Rice[J]. Rice Science, 2024, 31(5): 526-544.
Add to citation manager EndNote|Ris|BibTeX
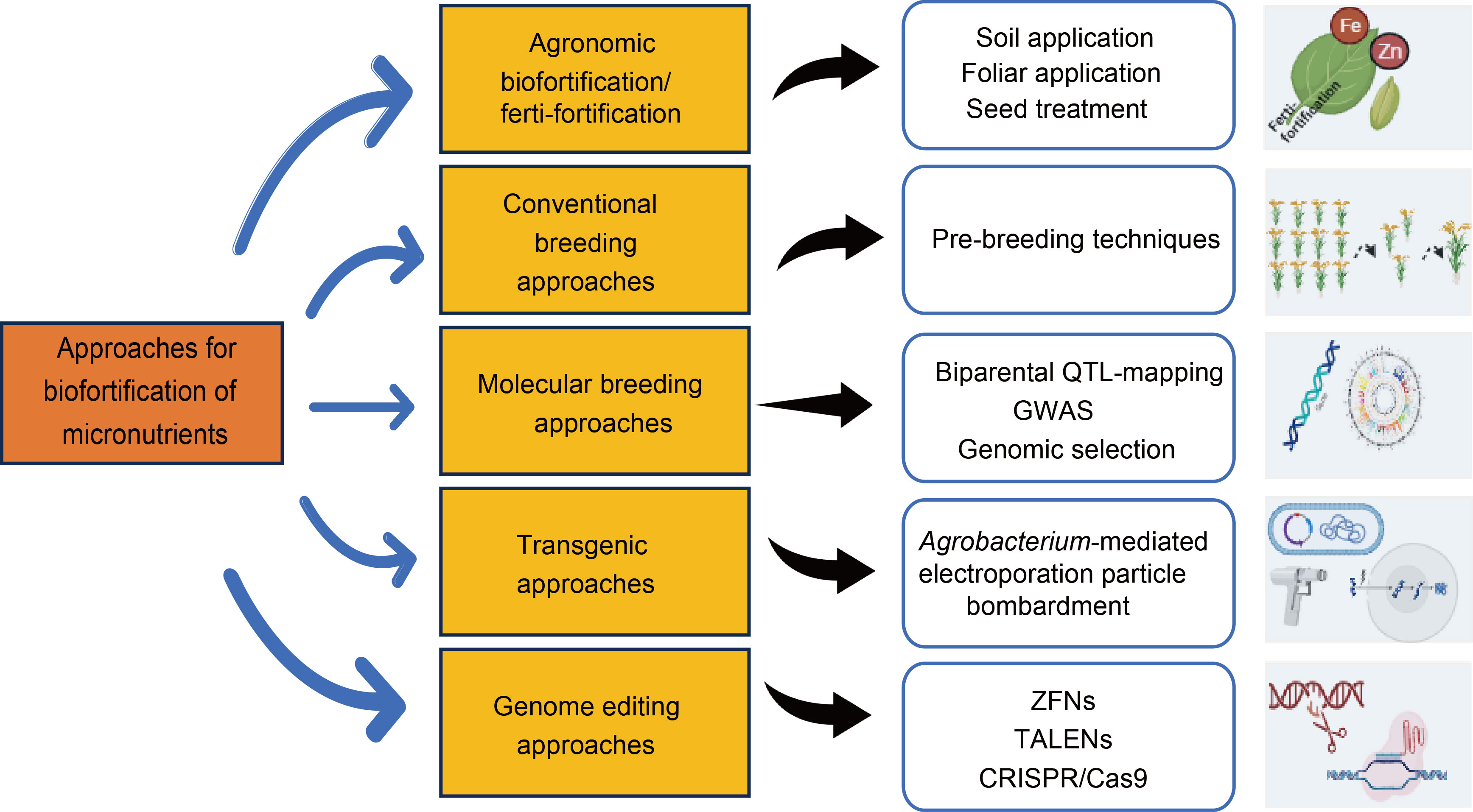
Fig. 1. Different approaches of biofortification in rice. GWAS, Genome-wide association study; ZFNs, Zinc finger nucleases; TALENs, Transcription activator-like effector nucleases.
| QTL/Locus | Mapping population | Marker | Chromosome | Nutritional component | Reference |
|---|---|---|---|---|---|
| QTLFe9 | Inbred | RM215 (SSR) | 9 | Fe | Islam et al, |
| QTLZn4 | RM551 (SSR) | 4 | Zn | ||
| qFe3.3 | Inbred | RM7 (SSR) | 3 | Fe | Pradhan et al, |
| qFe7.3 | RM1132 (SSR) | 7 | |||
| qZn2.2 | RM300 (SSR) | 2 | Zn | ||
| qZn8.3 | RM80 (SSR) | 8 | |||
| qZn12.3 | 12 | Zn | |||
| qFe1.1 | BIL | RM562‒RM11943 (SSR) | 1 | Fe | Dixit et al, |
| qFe1.2, qZn1.1 | RM294A‒RM12276 (SSR) | 1 | Fe and Zn | ||
| qFe6.1, qZn6.1 | RM8226‒RM400 (SSR) | 6 | Fe and Zn | ||
| qFe6.2, qZn6.2 | RM400‒RM162 (SSR) | 6 | Fe and Zn | ||
| qFe2 | F5 RIL | RM555 | 2 | Fe | Pippal et al, |
| qFe12 | F5 RIL | RM12 | 12 | Fe | |
| qFe2 | F6 RIL | RM263 | 2 | Fe | |
| qFe12 | F6 RIL | RM327 | 12 | Fe | |
| qZn2.1 | F5 RIL | RM1092 | 2 | Zn | |
| qZn2.2 | F5 RIL | RM406 | 2 | Zn | |
| qZn2.1 | F6 RIL | RM555 | 2 | Zn | |
| qZn2.2 | F6 RIL | RM263.2 | 2 | Zn | |
| qZn6 | F6 RIL | RM162 | 6 | Zn | |
| qZn10 | F6 RIL | RM474 | 10 | Zn | |
| qZn12.1 | F6 RIL | RM1080 | 12 | Zn | |
| qZn12.2 | F6 RIL | RM2734 | 12 | Zn | |
| qFe9.1 | DH | SNP-9809545-9819278 | 9 | Fe | Calayugan et al, |
| qFe12.1 | DH | SNP-12702072-12732307 | 12 | Fe | |
| qZn1.1 | DH | SNP-id1008679-439764 | 1 | Zn | |
| qZn5.1 | DH | SNP-4904312-4908650 | 5 | Zn | |
| qZn9.1 | DH | SNP-9809545-9819278 | 9 | Zn | |
| qZn12.1 | DH | SNP-c12p4887439-12172332 | 12 | Zn |
Table 1. QTLs identified for zinc (Zn) and iron (Fe) content in rice through biparental mapping and association mapping.
| QTL/Locus | Mapping population | Marker | Chromosome | Nutritional component | Reference |
|---|---|---|---|---|---|
| QTLFe9 | Inbred | RM215 (SSR) | 9 | Fe | Islam et al, |
| QTLZn4 | RM551 (SSR) | 4 | Zn | ||
| qFe3.3 | Inbred | RM7 (SSR) | 3 | Fe | Pradhan et al, |
| qFe7.3 | RM1132 (SSR) | 7 | |||
| qZn2.2 | RM300 (SSR) | 2 | Zn | ||
| qZn8.3 | RM80 (SSR) | 8 | |||
| qZn12.3 | 12 | Zn | |||
| qFe1.1 | BIL | RM562‒RM11943 (SSR) | 1 | Fe | Dixit et al, |
| qFe1.2, qZn1.1 | RM294A‒RM12276 (SSR) | 1 | Fe and Zn | ||
| qFe6.1, qZn6.1 | RM8226‒RM400 (SSR) | 6 | Fe and Zn | ||
| qFe6.2, qZn6.2 | RM400‒RM162 (SSR) | 6 | Fe and Zn | ||
| qFe2 | F5 RIL | RM555 | 2 | Fe | Pippal et al, |
| qFe12 | F5 RIL | RM12 | 12 | Fe | |
| qFe2 | F6 RIL | RM263 | 2 | Fe | |
| qFe12 | F6 RIL | RM327 | 12 | Fe | |
| qZn2.1 | F5 RIL | RM1092 | 2 | Zn | |
| qZn2.2 | F5 RIL | RM406 | 2 | Zn | |
| qZn2.1 | F6 RIL | RM555 | 2 | Zn | |
| qZn2.2 | F6 RIL | RM263.2 | 2 | Zn | |
| qZn6 | F6 RIL | RM162 | 6 | Zn | |
| qZn10 | F6 RIL | RM474 | 10 | Zn | |
| qZn12.1 | F6 RIL | RM1080 | 12 | Zn | |
| qZn12.2 | F6 RIL | RM2734 | 12 | Zn | |
| qFe9.1 | DH | SNP-9809545-9819278 | 9 | Fe | Calayugan et al, |
| qFe12.1 | DH | SNP-12702072-12732307 | 12 | Fe | |
| qZn1.1 | DH | SNP-id1008679-439764 | 1 | Zn | |
| qZn5.1 | DH | SNP-4904312-4908650 | 5 | Zn | |
| qZn9.1 | DH | SNP-9809545-9819278 | 9 | Zn | |
| qZn12.1 | DH | SNP-c12p4887439-12172332 | 12 | Zn |
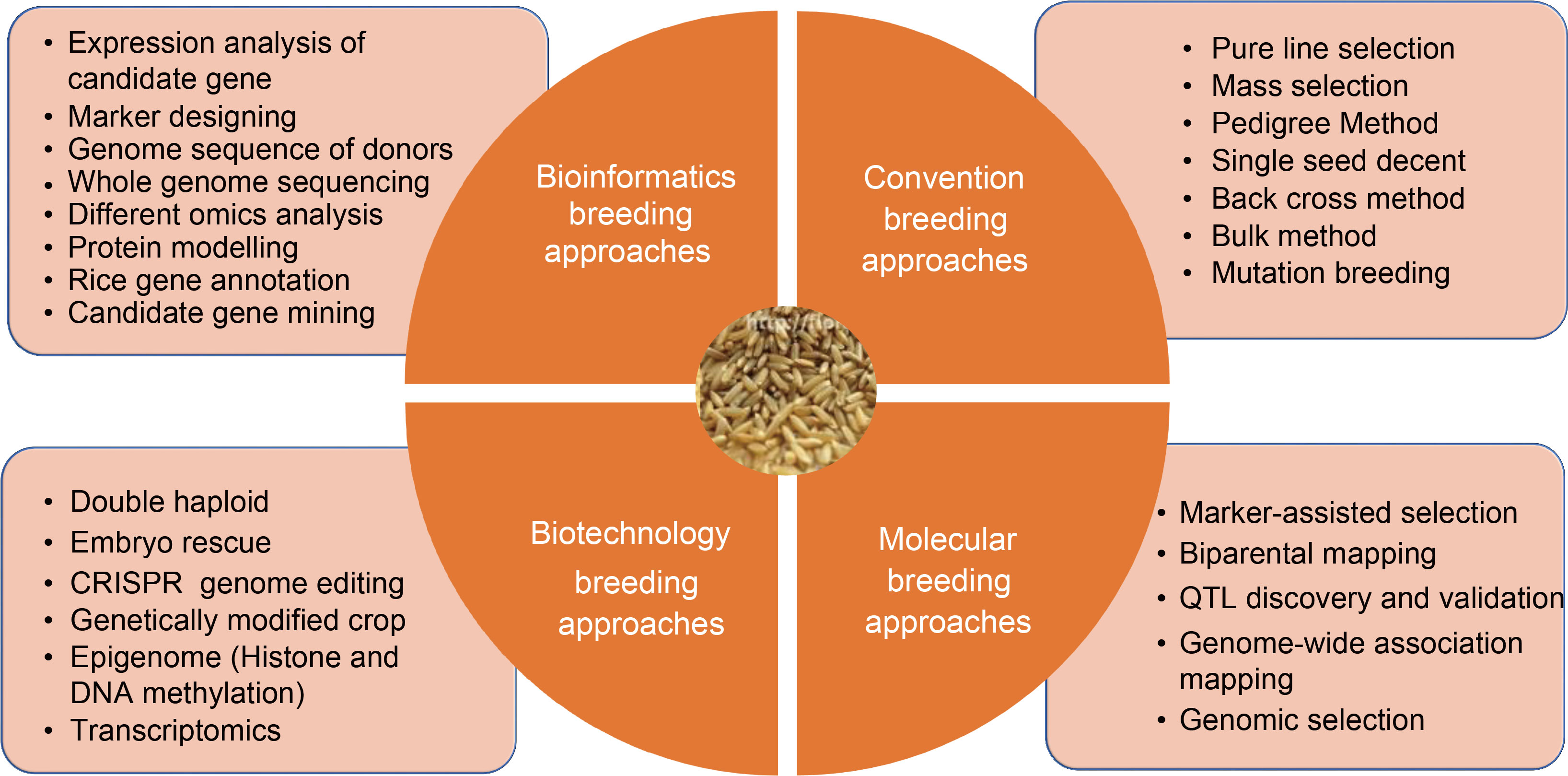
Fig. 2. Schematic representation of breeding for biofortified rice crop. Combinations of conventional and modern breeding approaches (molecular, transgenic, and bioinformatics) along with precision phenotyping methods, hasten the genetic gain for biofortified rice crop.
| Trait | Gene | Additional information | Reference |
|---|---|---|---|
| Fe | OsFER1, OsFER2 | Indica variety genome from genomic BLAST | Gross et al, |
| OsYSL1, OsMTP1 | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, | |
| Zn | OsARD2, OsIRT1, OsNAS1, OsNAS2 | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, |
| Fe and Zn | OsNAS gene family | Transformation of Nipponbare | Johnson et al, |
| OsNAS3, OsNRAMP1, heavy metal Fe transport, APRT | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, |
Table 2. List of candidate genes for enhancing zinc (Zn) and iron (Fe) content in rice grains.
| Trait | Gene | Additional information | Reference |
|---|---|---|---|
| Fe | OsFER1, OsFER2 | Indica variety genome from genomic BLAST | Gross et al, |
| OsYSL1, OsMTP1 | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, | |
| Zn | OsARD2, OsIRT1, OsNAS1, OsNAS2 | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, |
| Fe and Zn | OsNAS gene family | Transformation of Nipponbare | Johnson et al, |
| OsNAS3, OsNRAMP1, heavy metal Fe transport, APRT | F6 recombinant inbred lines derived from the cross of Madhukar and Swarna | Anuradha et al, |
| Gene | Localization/Expression pattern/Function | Reference |
|---|---|---|
| Genes associated with Strategy I | ||
| OsIRT1, OsIRT2 | Fe2+ transporters expressed in roots, leaves, and stems associated with Fe distribution and partitioning; Induced under low Fe conditions | Ishimaru et al, |
| OsFRO1, OsFRO2 | OsFRO1 is localized in the vacuolar membrane to reduce Fe3+ to Fe2+ and functions to maintain Fe homeostasis between vacuole and cytoplasm; OsFRO2 is a homolog of the Arabidopsis ferric chelate reductase (FRO) responsible for the reduction of Fe | Ishimaru et al, |
| OsPEZ1 | Localized in the stele of roots and associated with the transport of phenolics such as protocatechuic acid for the solubilization of apoplasmic Fe in the root xylem; Facilitate long-distance transport of Fe | Ishimaru et al, |
| OsPEZ2 | Expressed in the plasma membrane of root epidermis/exodermis; Solubilization of apoplasmic Fe; Secretion of protocatechuic acid and caffeic acid into the rhizosphere | Bashir et al, |
| OsFRDL1 | Plasma membrane-localized transporter for citrate; Expressed in most cells of enlarged vascular bundles, diffuse vascular bundles, and the interjacent parenchyma cell bridges of uppermost node I, as well as vascular tissues of the leaf blade, leaf sheath, peduncle, rachis, husk, and stamen; Essential for the distribution of Fe in the panicles through solubilizing Fe deposited in the apoplastic part of nodes in rice | Yokosho et al, |
| Genes associated with Strategy II | ||
| OsNAS1, OsNAS2, OsNAS3 | Biosynthesis of mugineic acid and long-distance transport of Fe; The expression pattern and tissue vary depending upon Fe sufficient and deficient conditions; The expression of OsNAS1 and OsNAS2 transcripts are markedly elevated in both roots and leaves in response to Fe deficiency, while the expression of OsNAS3 is induced in roots but suppressed in leaves | Inoue et al, |
| OsYSL2 | Transport of Fe2+-NA and Mn2+-NA; Predominantly expressed in phloem cells and associated with the phloem transport of Fe and Mn including its translocation to the grain | Koike et al, |
| OsNAAT1 | Mugineic acid biosynthesis in Fe-deficient roots; Expression induced in companion cells of Fe-sufficient shoots and Fe-deficient leaves indicates roles in Fe acquisition and long-distance transport via phloem | Inoue et al, |
| OsYSL18 | Fe3+-DMA transporter and is expressed in flowers and the phloem of lamina joints, indicating its role in the translocation of Fe in reproductive organs and phloem joints | Aoyama et al, |
| YSL15 | Fe3+-DMA transporter responsible for uptake of Fe from the rhizosphere and phloem transport; Expressed in root epidermis under Fe deficient condition, and in seeds | Inoue et al, Lee et al, |
| OsNAS3 | Biosynthesis of mugineic acid; 7-fold increase in bound Fe in seeds; Increased tolerance to Fe and Zn deficiencies and to excess metal (Zn, Cu, and Ni) toxicities; Expressed in roots, especially in vascular bundle, epidermis, exodermis, stem, and old leaf tissues under Fe excess condition; Mitigation of excess Fe | Lee et al, |
| OsTOM1 | DMA efflux transporter in roots; Higher expression in roots, leaf sheaths, and leaves under Fe deficient conditions | Nozoye et al, |
| OsYSL16 | Constitutively expressed in the plasma membranes and is highly expressed in the vascular tissues of the root, leaf, and spikelet, and in leaf mesophyll cells and associated with the Fe-homeostasis within plants | Lee et al, |
| OsTOM2 | Expressed in tissues involved in metal translocation; Strong expression in developing tissues during seed maturation and germination; Localized in the cell membrane; Transports DMA to cell exterior | Nozoye et al, |
| OsDMAS1 | Expression is restricted to cells participating in long distance transport and is highly up-regulated in the entire root under Fe-deficient conditions; In shoot tissue, its promoter drives expression in vascular bundles specifically under Fe-deficient conditions | Bashir et al, |
| OsYSL9 | Transport of Fe as both Fe2+-NA and Fe3+-DMA; Localized mainly in the plasma membrane; Induced in roots in response to Fe deficiency, and in the scutellum of the embryo and in endosperm cells surrounding the embryo during the grain filling stage | Senoura et al, |
| OsYSL13 | Highly expressed in leaves and leaf blades; Fe deficiency induces expression in both leaves and roots; Associated with Fe distribution within the plant and also to seeds | Zhang et al, |
| OsENA1 | ENA is primarily expressed in root epidermis and cortex, while in shoots, the expression was induced near the root-to-shoot junction and absent in leaves; Localized in the plasma membrane and vesicular structures; Cellular trafficking between plasma membrane and intracellular compartments, and to cell exterior; Probable role in Fe homeostasis | Nozoye et al, |
| Transcriptional regulators | ||
| IDEF1 | Strongly expressed in pollen, ovary, aleurone layer, and embryo; Positively regulates Fe utilization- related genes under Fe deficiency; IDEF1 dependent induction of late embryogenesis abundant proteins like Osem, indicates the function of seed maturation-related genes in Fe-deficient vegetative organs; Binds to Fe and other divalent cations to sense the Fe status | Kobayashi et al, |
| OsIRO2 | Localized in the cytoplasm; Nuclear accumulation under Fe limited conditions; Positively regulates the expression of genes associated with the Strategy II Fe uptake; Positively regulated by IDEF1; Regulation of DMA uptake and translocation of Fe to grain during seed maturation | Ogo et al, |
| IDEF2 | NAC like transcription factor; Constitutively present in roots and leaves; Expressed in pollen, ovary, and the dorsal vascular region of the endosperm; Associated with Fe homeostasis | Ogo et al, |
| OsIRO3 | Negative regulator of Fe uptake and translocation; Directly represses the expression of OsIRO2 by interacting with OsPRI1 and OsPRI2; Repressor function due to the presence of an EAR motif | Zheng et al, Li et al, |
| OsbHLH33 | Negative regulator of Fe uptake and translocation | Wang et al, |
| OsPRI1/OsbHLH060 | Binds to the promoters of OsIRO2 and OsIRO3 and positively regulate their expression; Ubiquitously expressed in roots and shoots; Required for Fe translocation from roots to shoots; A target of OsHRZ1, degraded by 26S proteosome pathway | Zhang et al, |
| OsbHLH156 | Regulator of Strategy II; Expressed in the roots, and induced by Fe deficiency. Required for the nuclear localisation of IRO2 | Wang et al, |
| OsPRI2/OsbHLH058, OsPRI3/OsbHLH059 | Paralogs of OsPRI1; Bind to the promoters of OsIRO2 and OsIRO3; Induce the expression of OsYSL2 by associating with its promoter; Directly regulated by OsHRZ1 via 26S proteasomal degradation | Zhang et al, |
| Subcellular sequestration | ||
| OsVIT1, OsVIT2 | OsVIT1 expressed in flag leaf blade and sheath while OsVIT2 expressed in sheath; Fe translocation between source and sink organs. OsVIT2 is involved in the distribution of Fe to the grains through sequestering Fe into vacuoles in mestome sheaths, nodes, and aleurone layers | Zhang et al, Che et al, |
| Other genes | ||
| OsNAS1, OsNAS2, OsNAAT1, OsDMAS1 | Increased expression in both roots and shoots by Fe deficiency | Inoue et al, |
| OsZIP1, OsZIP3, | Induced by Zn deficiency; Expressed in the vascular bundles in shoots, vascular bundles, and epidermal cells in roots | Ramesh et al, |
| OsZIP4 | Identified in rice plants, induced by Zn deficiency, expressed in the meristem of Zn-deficient roots and shoots, and also in vascular bundles of the roots and shoots | Ramesh et al, |
| RT1, OsIRT2 | Involved in Zn uptake and transport | Ishimaru et al, |
| MIR | Induced in roots and shoots in response to Fe-deficiency; Localized in mitochondria; Associated with Fe homeostasis | Ishimaru et al, |
| OsZIP5 | Involved in Zn uptake and transport | Yang et al, |
| TOM1 | Involved in DMA secretion, expressed in all root cells | Nozoye et al, |
| HORZ1 | Protein with hemerythrin domain; Antagonistic to HRZ functions | Kobayashi et al, |
| OsHRZ1 | Fe binding sensor; Negative regulation of Fe acquisition under Fe-sufficient conditions; 26S proteasome mediated degradation of OsPRI1, OsPRI2, and OsPRI3 as potential Fe sensors, playing a negative role in Fe homeostasis | Kobayashi et al, |
| OsHRZ2 | Fe binding sensor; Negative regulation of Fe acquisition under Fe-sufficient conditions | Kobayashi et al, |
| OsHMA2 | Delivery of Zn to growing tissues; Localized to the pericycle of the roots and at the phloem of enlarged and diffuse vascular bundles in the nodes | Yamaji et al, |
| OsRMC | Receptor like protein; Involved in the regulation of Fe acquisition | Yang et al, |
| OsNRAMP1 | Associated with Fe uptake in ferrous form | Ogo et al, |
| OsIBP1.1, IBP1.2 | Bowman-Birk trypsin inhibitors; Interact with IDEF1 and prevent its degradation mediated by 26S proteasome-mediated pathway | Zhang et al, Li Q et al, |
| OsZIP7 | Involved in Zn transport to growing tissues; Expressed in parenchyma cells of vascular bundles in roots and nodes | Tan et al, |
| OsZIP9 | Uptake of Zn into roots; Expressed in the epidermal and exodermal cells of lateral roots; Localized to the plasma membrane | Yang et al, |
| OsZIP11 | Involved in Fe accumulation; Knocking out OsZIP11 by CRISPR/Cas9 approach lowered Fe accumulation in brown rice, besides reducing plant height and biomass, and causing chlorosis and over-accumulation of malondialdehyde in rice plantlets | Zhao et al, |
| OsbZIP48 | Involved in the regulation of the expression of Zn transporters OsZIP4 and OsZIP8 | Hu et al, |
Table 3. Genes associated with zinc (Zn) and iron (Fe) uptake and translocation in rice.
| Gene | Localization/Expression pattern/Function | Reference |
|---|---|---|
| Genes associated with Strategy I | ||
| OsIRT1, OsIRT2 | Fe2+ transporters expressed in roots, leaves, and stems associated with Fe distribution and partitioning; Induced under low Fe conditions | Ishimaru et al, |
| OsFRO1, OsFRO2 | OsFRO1 is localized in the vacuolar membrane to reduce Fe3+ to Fe2+ and functions to maintain Fe homeostasis between vacuole and cytoplasm; OsFRO2 is a homolog of the Arabidopsis ferric chelate reductase (FRO) responsible for the reduction of Fe | Ishimaru et al, |
| OsPEZ1 | Localized in the stele of roots and associated with the transport of phenolics such as protocatechuic acid for the solubilization of apoplasmic Fe in the root xylem; Facilitate long-distance transport of Fe | Ishimaru et al, |
| OsPEZ2 | Expressed in the plasma membrane of root epidermis/exodermis; Solubilization of apoplasmic Fe; Secretion of protocatechuic acid and caffeic acid into the rhizosphere | Bashir et al, |
| OsFRDL1 | Plasma membrane-localized transporter for citrate; Expressed in most cells of enlarged vascular bundles, diffuse vascular bundles, and the interjacent parenchyma cell bridges of uppermost node I, as well as vascular tissues of the leaf blade, leaf sheath, peduncle, rachis, husk, and stamen; Essential for the distribution of Fe in the panicles through solubilizing Fe deposited in the apoplastic part of nodes in rice | Yokosho et al, |
| Genes associated with Strategy II | ||
| OsNAS1, OsNAS2, OsNAS3 | Biosynthesis of mugineic acid and long-distance transport of Fe; The expression pattern and tissue vary depending upon Fe sufficient and deficient conditions; The expression of OsNAS1 and OsNAS2 transcripts are markedly elevated in both roots and leaves in response to Fe deficiency, while the expression of OsNAS3 is induced in roots but suppressed in leaves | Inoue et al, |
| OsYSL2 | Transport of Fe2+-NA and Mn2+-NA; Predominantly expressed in phloem cells and associated with the phloem transport of Fe and Mn including its translocation to the grain | Koike et al, |
| OsNAAT1 | Mugineic acid biosynthesis in Fe-deficient roots; Expression induced in companion cells of Fe-sufficient shoots and Fe-deficient leaves indicates roles in Fe acquisition and long-distance transport via phloem | Inoue et al, |
| OsYSL18 | Fe3+-DMA transporter and is expressed in flowers and the phloem of lamina joints, indicating its role in the translocation of Fe in reproductive organs and phloem joints | Aoyama et al, |
| YSL15 | Fe3+-DMA transporter responsible for uptake of Fe from the rhizosphere and phloem transport; Expressed in root epidermis under Fe deficient condition, and in seeds | Inoue et al, Lee et al, |
| OsNAS3 | Biosynthesis of mugineic acid; 7-fold increase in bound Fe in seeds; Increased tolerance to Fe and Zn deficiencies and to excess metal (Zn, Cu, and Ni) toxicities; Expressed in roots, especially in vascular bundle, epidermis, exodermis, stem, and old leaf tissues under Fe excess condition; Mitigation of excess Fe | Lee et al, |
| OsTOM1 | DMA efflux transporter in roots; Higher expression in roots, leaf sheaths, and leaves under Fe deficient conditions | Nozoye et al, |
| OsYSL16 | Constitutively expressed in the plasma membranes and is highly expressed in the vascular tissues of the root, leaf, and spikelet, and in leaf mesophyll cells and associated with the Fe-homeostasis within plants | Lee et al, |
| OsTOM2 | Expressed in tissues involved in metal translocation; Strong expression in developing tissues during seed maturation and germination; Localized in the cell membrane; Transports DMA to cell exterior | Nozoye et al, |
| OsDMAS1 | Expression is restricted to cells participating in long distance transport and is highly up-regulated in the entire root under Fe-deficient conditions; In shoot tissue, its promoter drives expression in vascular bundles specifically under Fe-deficient conditions | Bashir et al, |
| OsYSL9 | Transport of Fe as both Fe2+-NA and Fe3+-DMA; Localized mainly in the plasma membrane; Induced in roots in response to Fe deficiency, and in the scutellum of the embryo and in endosperm cells surrounding the embryo during the grain filling stage | Senoura et al, |
| OsYSL13 | Highly expressed in leaves and leaf blades; Fe deficiency induces expression in both leaves and roots; Associated with Fe distribution within the plant and also to seeds | Zhang et al, |
| OsENA1 | ENA is primarily expressed in root epidermis and cortex, while in shoots, the expression was induced near the root-to-shoot junction and absent in leaves; Localized in the plasma membrane and vesicular structures; Cellular trafficking between plasma membrane and intracellular compartments, and to cell exterior; Probable role in Fe homeostasis | Nozoye et al, |
| Transcriptional regulators | ||
| IDEF1 | Strongly expressed in pollen, ovary, aleurone layer, and embryo; Positively regulates Fe utilization- related genes under Fe deficiency; IDEF1 dependent induction of late embryogenesis abundant proteins like Osem, indicates the function of seed maturation-related genes in Fe-deficient vegetative organs; Binds to Fe and other divalent cations to sense the Fe status | Kobayashi et al, |
| OsIRO2 | Localized in the cytoplasm; Nuclear accumulation under Fe limited conditions; Positively regulates the expression of genes associated with the Strategy II Fe uptake; Positively regulated by IDEF1; Regulation of DMA uptake and translocation of Fe to grain during seed maturation | Ogo et al, |
| IDEF2 | NAC like transcription factor; Constitutively present in roots and leaves; Expressed in pollen, ovary, and the dorsal vascular region of the endosperm; Associated with Fe homeostasis | Ogo et al, |
| OsIRO3 | Negative regulator of Fe uptake and translocation; Directly represses the expression of OsIRO2 by interacting with OsPRI1 and OsPRI2; Repressor function due to the presence of an EAR motif | Zheng et al, Li et al, |
| OsbHLH33 | Negative regulator of Fe uptake and translocation | Wang et al, |
| OsPRI1/OsbHLH060 | Binds to the promoters of OsIRO2 and OsIRO3 and positively regulate their expression; Ubiquitously expressed in roots and shoots; Required for Fe translocation from roots to shoots; A target of OsHRZ1, degraded by 26S proteosome pathway | Zhang et al, |
| OsbHLH156 | Regulator of Strategy II; Expressed in the roots, and induced by Fe deficiency. Required for the nuclear localisation of IRO2 | Wang et al, |
| OsPRI2/OsbHLH058, OsPRI3/OsbHLH059 | Paralogs of OsPRI1; Bind to the promoters of OsIRO2 and OsIRO3; Induce the expression of OsYSL2 by associating with its promoter; Directly regulated by OsHRZ1 via 26S proteasomal degradation | Zhang et al, |
| Subcellular sequestration | ||
| OsVIT1, OsVIT2 | OsVIT1 expressed in flag leaf blade and sheath while OsVIT2 expressed in sheath; Fe translocation between source and sink organs. OsVIT2 is involved in the distribution of Fe to the grains through sequestering Fe into vacuoles in mestome sheaths, nodes, and aleurone layers | Zhang et al, Che et al, |
| Other genes | ||
| OsNAS1, OsNAS2, OsNAAT1, OsDMAS1 | Increased expression in both roots and shoots by Fe deficiency | Inoue et al, |
| OsZIP1, OsZIP3, | Induced by Zn deficiency; Expressed in the vascular bundles in shoots, vascular bundles, and epidermal cells in roots | Ramesh et al, |
| OsZIP4 | Identified in rice plants, induced by Zn deficiency, expressed in the meristem of Zn-deficient roots and shoots, and also in vascular bundles of the roots and shoots | Ramesh et al, |
| RT1, OsIRT2 | Involved in Zn uptake and transport | Ishimaru et al, |
| MIR | Induced in roots and shoots in response to Fe-deficiency; Localized in mitochondria; Associated with Fe homeostasis | Ishimaru et al, |
| OsZIP5 | Involved in Zn uptake and transport | Yang et al, |
| TOM1 | Involved in DMA secretion, expressed in all root cells | Nozoye et al, |
| HORZ1 | Protein with hemerythrin domain; Antagonistic to HRZ functions | Kobayashi et al, |
| OsHRZ1 | Fe binding sensor; Negative regulation of Fe acquisition under Fe-sufficient conditions; 26S proteasome mediated degradation of OsPRI1, OsPRI2, and OsPRI3 as potential Fe sensors, playing a negative role in Fe homeostasis | Kobayashi et al, |
| OsHRZ2 | Fe binding sensor; Negative regulation of Fe acquisition under Fe-sufficient conditions | Kobayashi et al, |
| OsHMA2 | Delivery of Zn to growing tissues; Localized to the pericycle of the roots and at the phloem of enlarged and diffuse vascular bundles in the nodes | Yamaji et al, |
| OsRMC | Receptor like protein; Involved in the regulation of Fe acquisition | Yang et al, |
| OsNRAMP1 | Associated with Fe uptake in ferrous form | Ogo et al, |
| OsIBP1.1, IBP1.2 | Bowman-Birk trypsin inhibitors; Interact with IDEF1 and prevent its degradation mediated by 26S proteasome-mediated pathway | Zhang et al, Li Q et al, |
| OsZIP7 | Involved in Zn transport to growing tissues; Expressed in parenchyma cells of vascular bundles in roots and nodes | Tan et al, |
| OsZIP9 | Uptake of Zn into roots; Expressed in the epidermal and exodermal cells of lateral roots; Localized to the plasma membrane | Yang et al, |
| OsZIP11 | Involved in Fe accumulation; Knocking out OsZIP11 by CRISPR/Cas9 approach lowered Fe accumulation in brown rice, besides reducing plant height and biomass, and causing chlorosis and over-accumulation of malondialdehyde in rice plantlets | Zhao et al, |
| OsbZIP48 | Involved in the regulation of the expression of Zn transporters OsZIP4 and OsZIP8 | Hu et al, |
| Strategy | Trait improved | Reference |
|---|---|---|
| Over-expression of soyabean ferritin gene SoyferH1 through Agrobacterium-mediated transformation | 2-fold increase in Fe content in brown rice compared with type plants | Goto et al, |
| Improvement of Iron Absorption and Transport (IDS3) | 1.3- and 1.4-fold of Fe increase in brown rice and polished rice, respectively | Suzuki et al, Masuda et al, |
| Over-expression of HvNAS1 in rice | 2.3- and 1.5-fold increases in Fe and Zn concentrations in polished rice grains | Masuda et al, |
| Constitutive expression of AtNAS1 with endosperm-specific expression of genes encoding ferritin and phytase | 6.3-fold increase in Fe concentration in rice endosperm | Wirth et al, |
| Over-expression of OsYSL2 gene under the sucrose transporter (OsSUT1) promoter in rice | 4.0-fold increase in Fe concentration in polished rice | Ishimaru et al, |
| Over-expression of OsNAS1, OsNAS2, and OsNAS3 genes | 2.3- to 4.0-fold increase in Fe concentration in polished rice | Johnson et al, |
| Over-expression of OsIRO2 | 2.0-fold increase of Fe concentration in brown rice | Ogo et al, |
| RNAi-mediated silencing of OsHRZ2 in rice | 3.8- and 2.9-fold more Fe levels in brown rice and polished rice, respectively | Ogo et al, Kobayashi et al, |
| Transformation of rice nicotianamine synthase gene OsNAS3-D1 by Agrobacterium-mediated co-cultivation | Fe, Zn, and Cu content increased by 2.9-, 2.2-, and 1.7-fold compared with the controls, respectively | Lee et al, |
| Over-expression of rice ferritin gene Osfer2 through biolistic method | 2.09- and 1.37-fold increases in Fe and Zn content in rice | Paul et al, |
| Silencing OsVITs genes for increasing Fe translocation | 1.4- and 1.8-fold increase in Fe content in brown rice and polished rice, respectively | Zhang et al, Bashir et al, |
| Silencing IPK1 gene by RNAi technology | Accumulated 1.8-fold more Fe in the endosperm | Ali et al, |
| RNAi-mediated silencing of MIPS gene of the phytic acid metabolism pathway | Increased the content of Zn, Fe, Ca, and Mg in milled rice grains | Ali et al, |
| Transgene for a high-iron trait, soyfer1 gene, into a high- yielding indica rice cultivar, Swarna | 2.54-fold increase in Fe content and 1.54-fold increase in Zn content | Paul et al, |
| Combined expression of AtIRT1 with Pvferritin, AtIRT21, and AtNAS1 genes | Fe content of 10.46 mg/g dry weight in polished rice | Boonyaves et al, |
| Agrobacterium-mediated transformation of two genes (OsNAS2 and SferH1) into rice produced the most promising rice variety NASFer-274 | NASFer-274 polished rice contains 15 μg/g of Fe and 45.7 μg/g of Zn | Trijatmiko et al, |
| Transgenic plants developed by cloning of Fe homeostasis genes OsNAS1, OsNAS2, OsFer, OsVIT1, OsVIT2, OsZIP, OsIRO2, and OsIRT1 | 6-fold and 4-fold increase in Fe and Zn content in rice grains | Kawakami and Bhullar, |
| Knock out of OsITPK1‒6 genes through CRISPR/Cas9 | Low phytic acid accumulation in rice grain and a consequent increase in Fe, Zn, and other micronutrients | Jiang et al, |
| Knocked down of OsVIT2 gene | Increased Fe availability | Ludwig and Slamet-Loedin, |
| Incorporation of AtNRAMP3, AtNAS1, and PvFER genes in rice | 12.67 μg/g Fe and 45.60 μg/g Zn content in polished rice grains | Wu et al, |
Table 4. Zinc (Zn) and iron (Fe) fortification of rice achieved by different transgenic approaches.
| Strategy | Trait improved | Reference |
|---|---|---|
| Over-expression of soyabean ferritin gene SoyferH1 through Agrobacterium-mediated transformation | 2-fold increase in Fe content in brown rice compared with type plants | Goto et al, |
| Improvement of Iron Absorption and Transport (IDS3) | 1.3- and 1.4-fold of Fe increase in brown rice and polished rice, respectively | Suzuki et al, Masuda et al, |
| Over-expression of HvNAS1 in rice | 2.3- and 1.5-fold increases in Fe and Zn concentrations in polished rice grains | Masuda et al, |
| Constitutive expression of AtNAS1 with endosperm-specific expression of genes encoding ferritin and phytase | 6.3-fold increase in Fe concentration in rice endosperm | Wirth et al, |
| Over-expression of OsYSL2 gene under the sucrose transporter (OsSUT1) promoter in rice | 4.0-fold increase in Fe concentration in polished rice | Ishimaru et al, |
| Over-expression of OsNAS1, OsNAS2, and OsNAS3 genes | 2.3- to 4.0-fold increase in Fe concentration in polished rice | Johnson et al, |
| Over-expression of OsIRO2 | 2.0-fold increase of Fe concentration in brown rice | Ogo et al, |
| RNAi-mediated silencing of OsHRZ2 in rice | 3.8- and 2.9-fold more Fe levels in brown rice and polished rice, respectively | Ogo et al, Kobayashi et al, |
| Transformation of rice nicotianamine synthase gene OsNAS3-D1 by Agrobacterium-mediated co-cultivation | Fe, Zn, and Cu content increased by 2.9-, 2.2-, and 1.7-fold compared with the controls, respectively | Lee et al, |
| Over-expression of rice ferritin gene Osfer2 through biolistic method | 2.09- and 1.37-fold increases in Fe and Zn content in rice | Paul et al, |
| Silencing OsVITs genes for increasing Fe translocation | 1.4- and 1.8-fold increase in Fe content in brown rice and polished rice, respectively | Zhang et al, Bashir et al, |
| Silencing IPK1 gene by RNAi technology | Accumulated 1.8-fold more Fe in the endosperm | Ali et al, |
| RNAi-mediated silencing of MIPS gene of the phytic acid metabolism pathway | Increased the content of Zn, Fe, Ca, and Mg in milled rice grains | Ali et al, |
| Transgene for a high-iron trait, soyfer1 gene, into a high- yielding indica rice cultivar, Swarna | 2.54-fold increase in Fe content and 1.54-fold increase in Zn content | Paul et al, |
| Combined expression of AtIRT1 with Pvferritin, AtIRT21, and AtNAS1 genes | Fe content of 10.46 mg/g dry weight in polished rice | Boonyaves et al, |
| Agrobacterium-mediated transformation of two genes (OsNAS2 and SferH1) into rice produced the most promising rice variety NASFer-274 | NASFer-274 polished rice contains 15 μg/g of Fe and 45.7 μg/g of Zn | Trijatmiko et al, |
| Transgenic plants developed by cloning of Fe homeostasis genes OsNAS1, OsNAS2, OsFer, OsVIT1, OsVIT2, OsZIP, OsIRO2, and OsIRT1 | 6-fold and 4-fold increase in Fe and Zn content in rice grains | Kawakami and Bhullar, |
| Knock out of OsITPK1‒6 genes through CRISPR/Cas9 | Low phytic acid accumulation in rice grain and a consequent increase in Fe, Zn, and other micronutrients | Jiang et al, |
| Knocked down of OsVIT2 gene | Increased Fe availability | Ludwig and Slamet-Loedin, |
| Incorporation of AtNRAMP3, AtNAS1, and PvFER genes in rice | 12.67 μg/g Fe and 45.60 μg/g Zn content in polished rice grains | Wu et al, |
| Mutant material | Mutant type/Gene | Percentage reduction | Reference |
|---|---|---|---|
| Kaybonnet | Gamma irradiation, nonlethal single recessive mutant, lpa1-1 | 45% drop in bran PA content | Larson et al, |
| Os-lpa-XQZ-1 | Gamma radiation, a mutant homolog of the 2-phosphoglycerate kinase gene was produced | 38.9% reduction in grain PA content | Liu et al, |
| Os-lpa-XS110-2 | Gamma radiation, a single base pair mutation of multi-drug resistance-associated protein ABC transporter gene 5 (OsMRP5) was observed | 33.8% reduction in grain PA content | Liu et al, |
| Os-lpa-MH86-1 | Gamma radiation, a 1-bp deletion in a putative sulphate transporter gene (OsSULTR3;3) was discovered to be associated with lpa | 44% reduction in grain PA content | Liu et al, |
| Sang-gol | N-methyl-N-nitrosourea mutation | 50% lower PA content | Li et al, |
| Nagina 22 (N22)-PLM11 | Ethyl methane sulfonate mutagen mutation | 70% reduction in grain PA content | Singh et al, |
Table 5. List of mutants known for low phytic acid (PA) in rice grains.
| Mutant material | Mutant type/Gene | Percentage reduction | Reference |
|---|---|---|---|
| Kaybonnet | Gamma irradiation, nonlethal single recessive mutant, lpa1-1 | 45% drop in bran PA content | Larson et al, |
| Os-lpa-XQZ-1 | Gamma radiation, a mutant homolog of the 2-phosphoglycerate kinase gene was produced | 38.9% reduction in grain PA content | Liu et al, |
| Os-lpa-XS110-2 | Gamma radiation, a single base pair mutation of multi-drug resistance-associated protein ABC transporter gene 5 (OsMRP5) was observed | 33.8% reduction in grain PA content | Liu et al, |
| Os-lpa-MH86-1 | Gamma radiation, a 1-bp deletion in a putative sulphate transporter gene (OsSULTR3;3) was discovered to be associated with lpa | 44% reduction in grain PA content | Liu et al, |
| Sang-gol | N-methyl-N-nitrosourea mutation | 50% lower PA content | Li et al, |
| Nagina 22 (N22)-PLM11 | Ethyl methane sulfonate mutagen mutation | 70% reduction in grain PA content | Singh et al, |
| [1] | Adeyeye E I, Arogundade L A, Akintayo E T, Aisida O A, Alao P A. 2000. Calcium, zinc and phytate interrelationships in some foods of major consumption in Nigeria. Food Chem, 71(4): 435-441. |
| [2] |
Agarwal S, Tripura Venkata V G N, Kotla A, Mangrauthia S K, Neelamraju S. 2014. Expression patterns of QTL based and other candidate genes in Madhukar × Swarna RILs with contrasting levels of iron and zinc in unpolished rice grains. Gene, 546(2): 430-436.
DOI PMID |
| [3] | Al Hasan S M, Hassan M, Saha S, Islam M, Billah M, Islam S. 2016. Dietary phytate intake inhibits the bioavailability of iron and calcium in the diets of pregnant women in rural Bangladesh: A cross-sectional study. BMC Nutr, 2(1): 24. |
| [4] | Ali N, Paul S, Gayen D, Sarkar S N, Datta K, Datta S K. 2013a. Development of low phytate rice by RNAi mediated seed-specific silencing of inositol 1, 3, 4, 5, 6-pentakisphosphate 2-kinase gene (IPK1). PLoS One, 8(7): e68161. |
| [5] | Ali N, Paul S, Gayen D, Sarkar S N, Datta S K, Datta K. 2013b. RNAi mediated down regulation of myo-inositol-3-phosphate synthase to generate low phytate rice. Rice, 6(1): 12. |
| [6] |
Amirabdollahian F, Ash R. 2010. An estimate of phytate intake and molar ratio of phytate to zinc in the diet of the people in the United Kingdom. Public Health Nutr, 13(9): 1380-1388.
DOI PMID |
| [7] | Anilkumar C, Sah R P, Muhammed A T P, Sunitha N C, Behera S, Marndi B C, Sharma T R, Singh A K. 2022. Genomic selection in rice:Current status and future prospects. In: Elias A A, Goel S. Genomic Selection in Plants:A Guide for Breeders. Boca Raton, USA: CRC Press: 68-82. |
| [8] |
Anuradha K, Agarwal S, Rao Y V, Rao K V, Viraktamath B C, Sarla N. 2012. Mapping QTLs and candidate genes for iron and zinc concentrations in unpolished rice of Madhukar × Swarna RILs. Gene, 508(2): 233-240.
DOI PMID |
| [9] | Anusha G, Rao D S, Jaldhani V, Beulah P, Neeraja C N, Gireesh C, Anantha M S, Suneetha K, Santhosha R, Prasad A S H, Sundaram R M, Madhav M S, Fiyaz A, Brajendra P, Tuti M D, Bhave M H V, Krishna K V R, Ali J, Subrahmanyam D, Senguttuvel P. 2021. Grain Fe and Zn content, heterosis, combining ability and its association with grain yield in irrigated and aerobic rice. Sci Rep, 11(1): 10579. |
| [10] |
Aoyama T, Kobayashi T, Takahashi M, Nagasaka S, Usuda K, Kakei Y, Ishimaru Y, Nakanishi H, Mori S, Nishizawa N K. 2009. OsYSL18 is a rice iron(III)-deoxymugineic acid transporter specifically expressed in reproductive organs and phloem of lamina joints. Plant Mol Biol, 70(6): 681-692.
DOI PMID |
| [11] | Aung M S, Kobayashi T, Masuda H, Nishizawa N K. 2018. Rice HRZ ubiquitin ligases are crucial for response to excess iron. Physiol Plant, 163(3): 282-296. |
| [12] | Aung M S, Masuda H, Nozoye T, Kobayashi T, Jeon J S, An G, Nishizawa N K. 2019. Nicotianamine synthesis by OsNAS3 is important for mitigating iron excess stress in rice. Front Plant Sci, 10: 660. |
| [13] | Aung M S, Masuda H. 2020. How does rice defend against excess iron? Physiological and molecular mechanisms. Front Plant Sci, 11: 1102. |
| [14] | Azharudheen M T P, Kumar A, Anilkumar C, Sah R P, Behera S, Marndi B C. 2022. Understanding natural genetic variation for grain phytic acid content and functional marker development for phytic acid-related genes in rice. BMC Plant Biol, 22(1): 446. |
| [15] | Bailey R L, West Jr K P, Black R E. 2015. The epidemiology of global micronutrient deficiencies. Ann Nutr Metab, 66(Suppl 2): 22-33. |
| [16] | Banerjee S, Sharma D, Verulkar S, Chandel G. 2010. Use of in silico and semiquantitative RT-PCR approaches to develop nutrient rich rice (Oryza sativa L.). Indian J Biotechnol, 9: 203-212. |
| [17] |
Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2006. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem, 281: 32395-32402.
DOI PMID |
| [18] | Bashir K, Ishimaru Y, Shimo H, Kakei Y, Senoura T, Takahashi R, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa N K. 2011. Rice phenolics efflux transporter 2 (PEZ2) plays an important role in solubilizing apoplasmic iron. Soil Sci Plant Nutr, 57(6): 803-812. |
| [19] | Bashir K, Takahashi R, Akhtar S, Ishimaru Y, Nakanishi H, Nishizawa N K. 2013. The knockdown of OsVIT2 and MIT affects iron localization in rice seed. Rice, 6(1): 31. |
| [20] |
Bashir K, Nozoye T, Nagasaka S, Rasheed S, Miyauchi N, Seki M, Nakanishi H, Nishizawa N K. 2017. Paralogs and mutants show that one DMA synthase functions in iron homeostasis in rice. J Exp Bot, 68(7): 1785-1795.
DOI PMID |
| [21] | Bhavya M S P, Manju RV, Viji M M, Roy S, Anith K N, Beena R. 2023. Impact of biofertilisers on iron homeostasis and grain quality in the rice variety Uma under elevated CO2. Front Plant Sci, 14: 1144905. |
| [22] |
Bollinedi H, Yadav A K, Vinod K K, Gopala Krishnan S, Bhowmick P K, Nagarajan M, Neeraja C N, Ellur R K, Singh A K. 2020. Genome-wide association study reveals novel marker-trait associations (MTAs) governing the localization of Fe and Zn in the rice grain. Front Genet, 11: 213.
DOI PMID |
| [23] |
Boonyaves K, Gruissem W, Bhullar N K. 2016. NOD promoter- controlled AtIRT1 expression functions synergistically with NAS and FERRITIN genes to increase iron in rice grains. Plant Mol Biol, 90(3): 207-215.
DOI PMID |
| [24] | Bouis H E, Saltzman A. 2017. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob Food Sec, 12: 49-58. |
| [25] | Bregitzer P, Raboy V. 2006. Effects of four independent low- phytate mutations on barley agronomic performance. Crop Sci, 46(3): 1318-1322. |
| [26] | Budhlakoti N, Kushwaha A K, Rai A, Chaturvedi K K, Kumar A, Pradhan A K, Kumar U, Kumar R R, Juliana P, Mishra D C, Kumar S. 2022. Genomic selection: A tool for accelerating the efficiency of molecular breeding for development of climate- resilient crops. Front Genet, 13: 832153. |
| [27] | Calayugan M I C, Formantes A K, Amparado A, Descalsota-Empleo G I, Nha C T, Inabangan-Asilo M A, Swe Z M, Hernandez J E, Borromeo T H, Lalusin A G, Mendioro M S, Diaz M G Q, Viña C B D, Reinke R, Swamy B P M. 2020. Genetic analysis of agronomic traits and grain iron and zinc concentrations in a doubled haploid population of rice (Oryza sativa L.). Sci Rep, 10(1): 2283. |
| [28] | Champagne E T, Bett-Garber K L, Fitzgerald M A, Grimm C C, Lea J, Ohtsubo K, Jongdee S, Xie L H, Bassinello P Z, Resurreccion A, Ahmad R, Habibi F, Reinke R. 2010. Important sensory properties differentiating premium rice varieties. Rice, 3(4): 270-281. |
| [29] |
Che J, Yamaji N, Ma J F. 2021. Role of a vacuolar iron transporter OsVIT2 in the distribution of iron to rice grains. New Phytol, 230(3): 1049-1062.
DOI PMID |
| [30] | Cheng C H, Chung M C, Liu S M, Chen S K, Kao F Y, Lin S J, Hsiao S H, Hsing Y I C, Wu H P, Chen C S, Shaw J F, Wu J Z, Matsumoto T, Sasaki T, Chen H H, Chow T Y. 2005. A fine physical map of the Oryza sativa chromosome 5. Mol Genet Genomics, 274: 337-345. |
| [31] |
Cheng L J, Wang F, Shou H X, Huang F L, Zheng L Q, He F, Li J H, Zhao F J, Ueno D, Ma J F, Wu P. 2007. Mutation in nicotianamine aminotransferase stimulated the Fe(II) acquisition system and led to iron accumulation in rice. Plant Physiol, 145(4): 1647-1657.
DOI PMID |
| [32] |
Descalsota G I L, Mallikarjuna Swamy B P, Zaw H, Inabangan- Asilo M A, Amparado A, Mauleon R, Chadha-Mohanty P, Arocena E C, Raghavan C, Leung H, Hernandez J E, Lalusin A B, Mendioro M S, Diaz M G Q, Reinke R. 2018. Genome-wide association mapping in a rice MAGIC plus population detects QTLs and genes useful for biofortification. Front Plant Sci, 9: 1347.
DOI PMID |
| [33] | Development Initiatives. 2018. Global Nutrition Report:Shining a Light to Spur Action on Nutrition. Bristol, UK: Development Initiatives. |
| [34] | Dixit S, Singh U M, Abbai R, Ram T, Singh V K, Paul A, Virk P S, Kumar A. 2019. Identification of genomic region(s) responsible for high iron and zinc content in rice. Sci Rep, 9(1): 8136. |
| [35] |
Egli I, Davidsson L, Zeder C, Walczyk T, Hurrell R. 2004. Dephytinization of a complementary food based on wheat and soy increases zinc, but not copper, apparent absorption in adults. J Nutr, 134(5): 1077-1080.
DOI PMID |
| [36] |
Garcia-Oliveira A L, Chander S, Ortiz R, Menkir A, Gedil M. 2018. Genetic basis and breeding perspectives of grain iron and zinc enrichment in cereals. Front Plant Sci, 9: 937.
DOI PMID |
| [37] | Garutti M, Nevola G, Mazzeo R, Cucciniello L, Totaro F, Bertuzzi C A, Caccialanza R, Pedrazzoli P, Puglisi F. 2022. The impact of cereal grain composition on the health and disease outcomes. Front Nutr, 9: 888974. |
| [38] |
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F. 1999. Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol, 17(3): 282-286.
DOI PMID |
| [39] |
Gregorio G B. 2002. Progress in breeding for trace minerals in staple crops. J Nutr, 132(3): 500S-502S.
DOI PMID |
| [40] | Gross J, Stein R J, Fett-Neto A G, Fett J P. 2003. Iron homeostasis related genes in rice. Genet Mol Biol, 26(4): 477-497. |
| [41] | Hariprasanna K, Agte V, Elangovan M, Patil J V. 2014. Genetic variability for grain iron and zinc content in cultivars, breeding lines and selected germplasm accessions of Sorghum [Sorghum bicolor (L.) Moench]. Indian J Genet Plant Breed, 74(1): 42-49. |
| [42] | Hu S B, Du B B, Mu G M, Jiang Z C, Li H, Song Y X R, Zhang B L, Xia J X, Rouached H. Zheng L Q. 2024. The transcription factor OsbZIP48 governs rice responses to zinc deficiency. Plant Cell Environ, 47(5): 1526-1542. |
| [43] |
Hurrell R F, Reddy M B, Juillerat M A, Cook J D. 2003. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am J Clin Nutr, 77(5): 1213-1219.
DOI PMID |
| [44] | Inoue H, Higuchi K, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2003. Three rice nicotianamine synthase genes, OsNAS1, OsNAS2, and OsNAS3 are expressed in cells involved in long- distance transport of iron and differentially regulated by iron. Plant J, 36(3): 366-381. |
| [45] | Inoue H, Takahashi M, Kobayashi T, Suzuki M, Nakanishi H, Mori S, Nishizawa N K. 2008. Identification and localisation of the rice nicotianamine aminotransferase gene OsNAAT1 expression suggests the site of phytosiderophore synthesis in rice. Plant Mol Biol, 66(1): 193-203. |
| [46] |
Inoue H, Kobayashi T, Nozoye T, Takahashi M, Kakei Y, Suzuki K, Nakazono M, Nakanishi H, Mori S, Nishizawa N K. 2009. Rice OsYSL15 is an iron-regulated iron(III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J Biol Chem, 284(6): 3470-3479.
DOI PMID |
| [47] |
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J, 45(3): 335-346.
DOI PMID |
| [48] |
Ishimaru Y, Bashir K, Fujimoto M, An G, Itai R N, Tsutsumi N, Nakanishi H, Nishizawa N K. 2009. Rice-specific mitochondrial iron-regulated gene (MIR) plays an important role in iron homeostasis. Mol Plant, 2(5): 1059-1066.
DOI PMID |
| [49] | Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa N K. 2010. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J, 62(3): 379-390. |
| [50] |
Ishimaru Y, Kakei Y, Shimo H, Bashir K, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa N K. 2011. A rice phenolic efflux transporter is essential for solubilizing precipitated apoplasmic iron in the plant stele. J Biol Chem, 286(28): 24649-24655.
DOI PMID |
| [51] |
Isidro J, Jannink J L, Akdemir D, Poland J, Heslot N, Sorrells M E. 2015. Training set optimization under population structure in genomic selection. Theor Appl Genet, 128(1): 145-158.
DOI PMID |
| [52] | Islam M Z, Arifuzzaman M, Banik S, Hossain M A, Ferdous J, Khalequzzaman M, Pittendrigh B R, Tomita M, Ali M P. 2020. Mapping QTLs underpin nutrition components in aromatic rice germplasm. PLoS One, 15(6): e0234395. |
| [53] | Itai R N, Ogo Y, Kobayashi T, Nakanishi H, Nishizawa N K. 2013. Rice genes involved in phytosiderophore biosynthesis are synchronously regulated during the early stages of iron deficiency in roots. Rice, 6(1): 16. |
| [54] | Jatav H S, Singh S K, Singh Y, Kumar O. 2018. Biochar and sewage sludge application increases yield and micronutrient uptake in rice (Oryza sativa L.). Commun Soil Sci Plant Anal, 49(13): 1617-1628. |
| [55] | Jiang M, Liu Y, Liu Y H, Tan Y Y, Huang J Z, Shu Q Y. 2019. Mutation of inositol 1,3,4-trisphosphate 5/6-kinase6 impairs plant growth and phytic acid synthesis in rice. Plants, 8(5): 114. |
| [56] | Johnson A A T, Kyriacou B, Callahan D L, Carruthers L, Stangoulis J, Lombi E, Tester M. 2011. Constitutive overexpression of the OsNAS gene family reveals single-gene strategies for effective iron- and zinc-biofortification of rice endosperm. PLoS One, 6(9): e24476. |
| [57] | Juliano B O. 1992. Rice starch properties and grain quality. J Jpn Soc Starch Sci, 39(1): 11-21. |
| [58] |
Kawakami Y, Bhullar N K. 2018. Molecular processes in iron and zinc homeostasis and their modulation for biofortification in rice. J Integr Plant Biol, 60(12): 1181-1198.
DOI |
| [59] | Kies A K, De Jonge L H, Kemme P A, Jongbloed A W. 2006. Interaction between protein, phytate, and microbial phytase: In vitro studies. J Agric Food Chem, 54(5): 1753-1758. |
| [60] | Kim S I, Andaya C B, Goyal S S, Tai T H. 2008a. The rice OsLpa1 gene encodes a novel protein involved in phytic acid metabolism. Theor Appl Genet, 117(5): 769-779. |
| [61] | Kim S I, Andaya C B, Newman J W, Goyal S S, Tai T H. 2008b. Isolation and characterization of a low phytic acid rice mutant reveals a mutation in the rice orthologue of maize MIK. Theor Appl Genet, 117(8): 1291-1301. |
| [62] |
Kobayashi T, Suzuki M, Inoue H, Itai R N, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2005. Expression of iron-acquisition- related genes in iron-deficient rice is co-ordinately induced by partially conserved iron-deficiency-responsive elements. J Exp Bot, 56: 1305-1316.
DOI PMID |
| [63] | Kobayashi T, Ogo Y, Itai R N, Nakanishi H, Takahashi M, Mori S, Nishizawa N K. 2007. The transcription factor IDEF1 regulates the response to and tolerance of iron deficiency in plants. Proc Nat Acad Sci USA, 104: 19150-19155. |
| [64] | Kobayashi T, Ogo Y, Aung M S, Nozoye T, Itai R N, Nakanishi H, Yamakawa T, Nishizawa N K. 2010. The spatial expression and regulation of transcription factors IDEF1 and IDEF2. Ann Bot, 105(7): 1109-1117. |
| [65] |
Kobayashi T, Nagasaka S, Senoura T, Itai R N, Nakanishi H, Nishizawa N K. 2013. Iron-binding haemerythrin RING ubiquitin ligases regulate plant iron responses and accumulation. Nat Commun, 4: 2792.
DOI PMID |
| [66] |
Kobayashi T, Nagano A J, Nishizawa N K. 2021. Iron deficiency- inducible peptide-coding genes OsIMA1 and OsIMA2 positively regulate a major pathway of iron uptake and translocation in rice. J Exp Bot, 72(6): 2196-2211.
DOI PMID |
| [67] |
Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2004. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J, 39(3): 415-424.
DOI PMID |
| [68] | Kumar A, Sahu C, Panda P A, Biswal M, Sah R P, Lal M K, Baig M J, Swain P, Behera L, Chattopadhyay K, Sharma S. 2020. Phytic acid content may affect starch digestibility and glycemic index value of rice (Oryza sativa L.). J Sci Food Agric, 100(4): 1598-1607. |
| [69] | Kumar A, Nayak S, Ngangkham U, Sah R P, Lal M K, Azharudheen T P, Behera S, Swain P, Behera L, Sharma S. 2021. A single nucleotide substitution in the SPDT transporter gene reduced phytic acid and increased mineral bioavailability from rice grain (Oryza sativa L.). J Food Biochem, 45(7): e13822. |
| [70] | Kumar A, Dash G K, Sahoo S K, Lal M K, Sahoo U, Sah R P, Ngangkham U, Kumar S, Baig M J, Sharma S, Lenka S K. 2023a. Phytic acid: A reservoir of phosphorus in seeds plays a dynamic role in plant and animal metabolism. Phytochem Rev, 22(5): 1281-1304. |
| [71] | Kumar A, Lal M K, Sahoo U, Sahoo S K, Sah R P, Tiwari R K, Kumar R, Sharma S. 2023b. Combinatorial effect of heat processing and phytic acid on mineral bioavailability in rice grain. Food Chem Adv, 2: 100232. |
| [72] | Kuwano M, Ohyama A, Tanaka Y, Mimura T, Takaiwa F, Yoshida K T. 2006. Molecular breeding for transgenic rice with low- phytic-acid phenotype through manipulating myo-inositol 3- phosphate synthase gene. Mol Breed, 18(3): 263-272. |
| [73] |
Kuwano M, Mimura T, Takaiwa F, Yoshida K T. 2009. Generation of stable ‘low phytic acid’ transgenic rice through antisense repression of the 1d-myo-inositol 3-phosphate synthase gene (RINO1) using the 18-kDa oleosin promoter. Plant Biotechnol J, 7(1): 96-105.
DOI PMID |
| [74] | Larson S R, Rutger J N, Young K A, Raboy V. 2000. Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation. Crop Sci, 40(5): 1397-1405. |
| [75] |
Lau W C P, Rafii M Y, Ismail M R, Puteh A, Latif M A, Ramli A. 2015. Review of functional markers for improving cooking, eating, and the nutritional qualities of rice. Front Plant Sci, 6: 832.
DOI PMID |
| [76] | Lee S, An G. 2009. Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ, 32(4): 408-416. |
| [77] |
Lee S, Jeon U S, Lee S J, Kim Y K, Persson D P, Husted S, Schjørring J K, Kakei Y, Masuda H, Nishizawa N K, An G. 2009. Iron fortification of rice seeds through activation of the nicotianamine synthase gene. Proc Natl Acad Sci USA, 106(51): 22014-22019.
DOI PMID |
| [78] | Lee S, Ryoo N, Jeon J S, Guerinot M L, An G. 2012a. Activation of rice Yellow Stripe1-Like 16 (OsYSL16) enhances iron efficiency. Mol Cells, 33(2): 117-126. |
| [79] | Lee S, Kim Y S, Jeon U S, Kim Y K, Schjoerring J K, An G. 2012b. Activation of rice nicotianamine synthase 2 (OsNAS2) enhances iron availability for biofortification. Mol Cells, 33(3): 269-276. |
| [80] | Lestienne I, Icard-Vernière C, Mouquet C, Picq C, Trèche S. 2005. Effects of soaking whole cereal and legume seeds on iron, zinc and phytate contents. Food Chem, 89(3): 421-425. |
| [81] | Li C Y, Park D S, Won S R, Hong S K, Ham J K, Choi J K, Rhee H I. 2008. Food chemical properties of low-phytate rice cultivar, Sang-gol. J Cereal Sci, 47(2): 262-265. |
| [82] | Li C Y, Li Y, Xu P, Liang G. 2022. OsIRO3 negatively regulates Fe homeostasis by repressing the expression of OsIRO2. Plant J, 111(4): 966-978. |
| [83] | Li L, Ye L X, Kong Q H, Shou H X. 2019. A vacuolar membrane ferric-chelate reductase, OsFRO1, alleviates Fe toxicity in rice (Oryza sativa L.). Front Plant Sci, 10: 700. |
| [84] | Li Q, Chen L, Yang A. 2019. The molecular mechanisms underlying iron deficiency responses in rice. Int J Mol Sci, 21(1): 43. |
| [85] | Liang J F, Li Z G, Tsuji K, Nakano K, Robert Nout M J, Hamer R J. 2008. Milling characteristics and distribution of phytic acid and zinc in long-, medium- and short-grain rice. J Cereal Sci, 48(1): 83-91. |
| [86] |
Liu F, Chang X J, Ye Y, Xie W B, Wu P, Lian X M. 2011. Comprehensive sequence and whole-life-cycle expression profile analysis of the phosphate transporter gene family in rice. Mol Plant, 4(6): 1105-1122.
DOI PMID |
| [87] | Liu Q L, Xu X H, Ren X L, Fu H W, Wu D X, Shu Q Y. 2007. Generation and characterization of low phytic acid germplasm in rice (Oryza sativa L.). Theor Appl Genet, 114(5): 803-814. |
| [88] | Liu S L, Zou W L, Lu X, Bian J M, He H H, Chen J G, Ye G Y. 2021. Genome-wide association study using a multiparent advanced generation intercross (MAGIC) population identified QTLs and candidate genes to predict shoot and grain zinc contents in rice. Agriculture, 11(1): 70. |
| [89] |
Ludwig Y, Slamet-Loedin I H. 2019. Genetic biofortification to enrich rice and wheat grain iron: From genes to product. Front Plant Sci, 10: 833.
DOI PMID |
| [90] | Mahesh S, Pavithra G J, Parvathi M S, Rajashekara R, Shankar A G. 2015. Effect of processing on phytic acid content and nutrient availability in food grains. Int J Agric Sci, 5: 771-777. |
| [91] | Marounek M, Skřivan M, Rosero O, Rop O. 2010. Intestinal and total tract phytate digestibility and phytase activity in the digestive tract of hens fed a wheat-maize-soyabean diet. J Anim Feed Sci, 19(3): 430-439. |
| [92] | Masuda H, Suzuki M, Morikawa K C, Kobayashi T, Nakanishi H, Takahashi M, Saigusa M, Mori S, Nishizawa N K. 2008. Increase in iron and zinc concentrations in rice grains via the introduction of barley genes involved in phytosiderophore synthesis. Rice, 1(1): 100-108. |
| [93] | Masuda H, Usuda K, Kobayashi T, Ishimaru Y, Kakei Y, Takahashi M, Higuchi K, Nakanishi H, Mori S, Nishizawa N K. 2009. Over- expression of the barley nicotianamine synthase gene HvNAS1 increases iron and zinc concentrations in rice grains. Rice, 2(4): 155-166. |
| [94] |
Mayer J E, Pfeiffer W H, Beyer P. 2008. Biofortified crops to alleviate micronutrient malnutrition. Curr Opin Plant Biol, 11(2): 166-170.
DOI PMID |
| [95] |
Meuwissen T H E, Hayes B J, Goddard M E. 2001. Prediction of total genetic value using genome-wide dense marker maps. Genetics, 157: 1819-1829.
DOI PMID |
| [96] | Mitchikpe E C S, Dossa R A M, Ategbo E A D, van Raaij J M A, Hulshof P J M, Kok F J. 2008. The supply of bioavailable iron and zinc may be affected by phytate in Beninese children. J Food Compos Anal, 21(1): 17-25. |
| [97] |
Mroz Z, Jongbloed A W, Kemme P A. 1994. Apparent digestibility and retention of nutrients bound to phytate complexes as influenced by microbial phytase and feeding regimen in pigs. J Anim Sci, 72(1): 126-132.
PMID |
| [98] |
Nishiyama R, Kato M, Nagata S, Yanagisawa S, Yoneyama T. 2012. Identification of Zn-nicotianamine and Fe-2ʹ-Deoxymugineic acid in the phloem sap from rice plants (Oryza sativa L.). Plant Cell Physiol, 53(2): 381-390.
DOI PMID |
| [99] | Nozoye T, Itai R N, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa N K. 2004. Diurnal changes in the expression of genes that participate in phytosiderophore synthesis in rice. Soil Sci Plant Nutr, 50(7): 1125-1131. |
| [100] |
Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa N K. 2011. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem, 286(7): 5446-5454.
DOI PMID |
| [101] |
Nozoye T, Nagasaka S, Kobayashi T, Sato Y, Uozumi N, Nakanishi H, Nishizawa N K. 2015. The phytosiderophore efflux transporter TOM2 is involved in metal transport in rice. J Biol Chem, 290(46): 27688-27699.
DOI PMID |
| [102] |
Nozoye T, von Wirén N, Sato Y, Higashiyama T, Nakanishi H, Nishizawa N K. 2019. Characterization of the nicotianamine exporter ENA1 in rice. Front Plant Sci, 10: 502.
DOI PMID |
| [103] |
Ogo Y, Itai R N, Nakanishi H, Inoue H, Kobayashi T, Suzuki M, Takahashi M, Mori S, Nishizawa N K. 2006. Isolation and characterization of IRO2, a novel iron-regulated bHLH transcription factor in graminaceous plants. J Exp Bot, 57(11): 2867-2878.
DOI PMID |
| [104] |
Ogo Y, Itai R N, Nakanishi H, Kobayashi T, Takahashi M, Mori S, Nishizawa N K. 2007. The rice bHLH protein OsIRO2 is an essential regulator of the genes involved in Fe uptake under Fe-deficient conditions. Plant J, 51(3): 366-377.
DOI PMID |
| [105] | Ogo Y, Kobayashi T, Nakanishi Itai R, Nakanishi H, Kakei Y, Takahashi M, Toki S, Mori S, Nishizawa N K. 2008. A novel NAC transcription factor, IDEF2, that recognizes the iron deficiency- responsive element 2 regulates the genes involved in iron homeostasis in plants. J Biol Chem, 283(19): 13407-13417. |
| [106] |
Ogo Y, Itai R N, Kobayashi T, Aung M S, Nakanishi H, Nishizawa N K. 2011. OsIRO2 is responsible for iron utilization in rice and improves growth and yield in calcareous soil. Plant Mol Biol, 75(6): 593-605.
DOI PMID |
| [107] |
Ogo Y, Kakei Y, Itai R N, Kobayashi T, Nakanishi H, Takahashi H, Nakazono M, Nishizawa N K. 2014. Spatial transcriptomes of iron-deficient and cadmium-stressed rice. New Phytol, 201(3): 781-794.
DOI PMID |
| [108] |
Onogi A, Ideta O, Inoshita Y, Ebana K, Yoshioka T, Yamasaki M, Iwata H. 2015. Exploring the areas of applicability of whole- genome prediction methods for Asian rice (Oryza sativa L.). Theor Appl Genet, 128(1): 41-53.
DOI PMID |
| [109] | Palanog A D, Nha C T, Descalsota-Empleo G I L, Calayugan M I, Swe Z M, Amparado A, Inabangan-Asilo M A, Hernandez J E, Sta Cruz P C, Borromeo T H, Lalusin A G, Mauleon R, McNally K L, Mallikarjuna Swamy B P. 2023. Molecular dissection of connected rice populations revealed important genomic regions for agronomic and biofortification traits. Front Plant Sci, 14: 1157507. |
| [110] | Paul S, Ali N, Gayen D, Datta S K, Datta K. 2012. Molecular breeding of Osfer2 gene to increase iron nutrition in rice grain. GM Crops Food, 3(4): 310-316. |
| [111] | Paul S, Ali N, Datta S K, Datta K. 2014. Development of an iron- enriched high-yieldings indica rice cultivar by introgression of a high-iron trait from transgenic iron-biofortified rice. Plant Foods Hum Nutr, 69(3): 203-208. |
| [112] | Peng H, Wang K, Chen Z, Cao Y H, Gao Q, Li Y, Li X X, Lu H W, Du H L, Lu M, Yang X, Liang C Z. 2020. MBKbase for rice: An integrated omics knowledgebase for molecular breeding in rice. Nucleic Acids Res, 48(D1): D1085-D1092. |
| [113] | Perera I, Seneweera S, Hirotsu N. 2018. Manipulating the phytic acid content of rice grain toward improving micronutrient bioavailability. Rice, 11(1): 4. |
| [114] | Pippal A, Bhusal N, Meena R K, Bishnoi M, Bhoyar P I, Jain R K. 2022. Identification of genomic locations associated with grain micronutrients (iron and zinc) in rice (Oryza sativa L.). Genet Resour Crop Evol, 69(1): 221-230. |
| [115] | Pontoppidan K, Pettersson D, Sandberg A S. 2007. Peniophora lycii phytase is stabile and degrades phytate and solubilises minerals in vitro during simulation of gastrointestinal digestion in the pig. J Sci Food Agric, 87(14): 2700-2708. |
| [116] | Pradhan S K, Pandit E, Pawar S, Naveenkumar R, Barik S R, Mohanty S P, Nayak D K, Ghritlahre S K, Rao D S, Reddy J N, Patnaik S S C. 2020. Linkage disequilibrium mapping for grain Fe and Zn enhancing QTLs useful for nutrient dense rice breeding. BMC Plant Biol, 20(1): 57. |
| [117] | Prom-u-Thai C, Rashid A, Ram H, Zou C Q, Guilherme L R G, Corguinha A P B, Guo S W, Kaur C, Naeem A, Yamuangmorn S, Ashraf M Y, Sohu V S, Zhang Y Q, Martins F A D, Jumrus S, Tutus Y, Yazici M A, Cakmak I. 2020. Simultaneous biofortification of rice with zinc, iodine, iron and selenium through foliar treatment of a micronutrient cocktail in five countries. Front Plant Sci, 11: 589835. |
| [118] | Raboy V. 2000. Low-phytic-acid grains. Food Nutr Bull, 21(4): 423-427. |
| [119] | Radha B, Sunitha N C, Sah R P, Azarudeen T P M, Krishna G K, Umesh D K, Thomas S, Anilkumar C, Upadhyay S, Kumar A, Manikanta C L N, Behera S, Marndi B C, Siddique K H M. 2023. Physiological and molecular implications of multiple abiotic stresses on yield and quality of rice. Front Plant Sci, 13: 996514. |
| [120] | Rakotondramanana M, Tanaka R, Pariasca-Tanaka J, Stangoulis J, Grenier C, Wissuwa M. 2022. Genomic prediction of zinc- biofortification potential in rice gene bank accessions. Theor Appl Genet, 135(7): 2265-2278. |
| [121] |
Ramesh S A, Shin R, Eide D J, Schachtman D P. 2003. Differential metal selectivity and gene expression of two zinc transporters from rice. Plant Physiol, 133(1): 126-134.
DOI PMID |
| [122] | Raza Q, Riaz A, Saher H, Bibi A, Raza M A, Ali S S, Sabar M. 2020. Grain Fe and Zn contents linked SSR markers based genetic diversity in rice. PLoS One, 15(9): e0239739. |
| [123] | Sanghamitra P, Sah R P, Bagchi T B, Sharma S G, Kumar A, Munda S, Sahu R K. 2018. Evaluation of variability and environmental stability of grain quality and agronomic parameters of pigmented rice (O. sativa L.). J Food Sci Technol, 55(3): 879-890. |
| [124] | Senguttuvel P, Padmavathi G, Jasmine C, Sanjeeva R D, Neeraja C N, Jaldhani V, Beulah P, Gobinath R, Aravind K J, Sai Prasad S V, Subba Rao L V, Hariprasad A S, Sruthi K, Shivani D, Sundaram R M, Govindaraj M. 2023. Rice biofortification: Breeding and genomic approaches for genetic enhancement of grain zinc and iron contents. Front Plant Sci, 14: 1138408. |
| [125] | Senoura T, Sakashita E, Kobayashi T, Takahashi M, Aung M S, Masuda H, Nakanishi H, Nishizawa N K. 2017. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol Biol, 95(4/5): 375-387. |
| [126] | Singh V, Singh V, Singh S, Khanna R. 2020. Effect of zinc and silicon on growth and yield of aromatic rice (Oryza sativa) in north-western plains of India. J Rice Res Dev, 3(1): 82-86. |
| [127] | Stangoulis J C R, Huynh B L, Welch R M, Choi E Y, Graham R D. 2007. Quantitative trait loci for phytate in rice grain and their relationship with grain micronutrient content. Euphytica, 154(3): 289-294. |
| [128] | Stevens G A, Beal T, Mbuya M N N, Luo H Q, Neufeld L M, Group G M D R. 2022. Micronutrient deficiencies among preschool- aged children and women of reproductive age worldwide: A pooled analysis of individual-level data from population-representative surveys. Lancet Glob Health, 10(11): e1590-e1599. |
| [129] |
Sun S B, Gu M, Cao Y, Huang X P, Zhang X, Ai P H, Zhao J N, Fan X R, Xu G H. 2012. A constitutive expressed phosphate transporter, OsPht1;1, modulates phosphate uptake and translocation in phosphate-replete rice. Plant Physiol, 159(4): 1571-1581.
DOI PMID |
| [130] | Suzuki M, Tanaka K, Kuwano M, Yoshida K T. 2007. Expression pattern of inositol phosphate-related enzymes in rice (Oryza sativa L.): Implications for the phytic acid biosynthetic pathway. Gene, 405(1/2): 55-64. |
| [131] | Suzuki M, Morikawa K C, Nakanishi H, Takahashi M, Saigusa M, Mori S, Nishizawa N K. 2008. Transgenic rice lines that include barley genes have increased tolerance to low iron availability in a calcareous paddy soil. Soil Sci Plant Nutr, 54(1): 77-85. |
| [132] |
Swamy B P M, Kaladhar K, Anuradha K, Batchu A K, Longvah T, Sarla N. 2018. QTL analysis for grain iron and zinc concentrations in two O. nivara derived backcross populations. Rice Sci, 25(4): 197-207.
DOI |
| [133] | Swamy B P M, Marathi B, Ribeiro-Barros A I F, Calayugan M I C, Ricachenevsky F K. 2021. Iron biofortification in rice: An update on quantitative trait loci and candidate genes. Front Plant Sci, 12: 647341. |
| [134] | Tan L T, Zhu Y X, Fan,T, Peng C, Wang J R, Sun L, Chen, C Y. 2019. OsZIP7 functions in xylem loading in roots and inter- vascular transfer in nodes to deliver Zn/Cd to grain in rice. Biochem Biophys Res Commun, 512(1): 112-118. |
| [135] |
Trijatmiko K R, Dueñas C, Tsakirpaloglou N, Torrizo L, Arines F M, Adeva C, Balindong J, Oliva N, Sapasap M V, Borrero J, Rey J, Francisco P, Nelson A, Nakanishi H, Lombi E, Tako E, Glahn R P, Stangoulis J, Chadha-Mohanty P, Johnson A A T, Tohme J, Barry G, Slamet-Loedin I H. 2016. Biofortified indica rice attains iron and zinc nutrition dietary targets in the field. Sci Rep, 6: 19792.
DOI PMID |
| [136] | Tripathy S K. 2020. Genetic variation for micronutrients and study of genetic diversity in diverse germplasm of rice. J Crop Weed, 16(1): 101-109. |
| [137] |
Visakh R L, Anand S, Arya S N, Sasmitha B, Jha U C, Sah R P, Beena R. 2024. Rice heat tolerance breeding: A comprehensive review and forward gaze. Rice Sci, 31(4): 375-400.
DOI |
| [138] | Wang F, Itai R N, Nozoye T, Kobayashi T, Nishizawa N K, Nakanishi H. 2020. The bHLH protein OsIRO3 is critical for plant survival and iron (Fe) homeostasis in rice (Oryza sativa L.) under Fe- deficient conditions. Soil Sci Plant Nutr, 66(4): 579-592. |
| [139] |
Wang J J, Meng X B, Hu X X, Sun T T, Li J Y, Wang K J, Yu H. 2019. xCas9 expands the scope of genome editing with reduced efficiency in rice. Plant Biotechnol J, 17(4): 709-711.
DOI PMID |
| [140] | Wang Y D, Wang X, Wong Y S. 2013. Generation of selenium- enriched rice with enhanced grain yield, selenium content and bioavailability through fertilisation with selenite. Food Chem, 141(3): 2385-2393. |
| [141] |
Wirth J, Poletti S, Aeschlimann B, Yakandawala N, Drosse B, Osorio S, Tohge T, Fernie A R, Günther D, Gruissem W, Sautter C. 2009. Rice endosperm iron biofortification by targeted and synergistic action of nicotianamine synthase and ferritin. Plant Biotechnol J, 7(7): 631-644.
DOI PMID |
| [142] | World Health Organization. 2002. World Health Report 2002:Reducing Risks Promoting Healthy Life. Geneva, Switzerland: World Health Organization. |
| [143] | Wu T Y, Gruissem W, Bhullar N K. 2019. Targeting intracellular transport combined with efficient uptake and storage significantly increases grain iron and zinc levels in rice. Plant Biotechnol J, 17(1): 9-20. |
| [144] | Xu Q, Zheng T Q, Hu X, Cheng L R, Xu J L, Shi Y M, Li Z K. 2015. Examining two sets of introgression lines in rice (Oryza sativa L.) reveals favorable alleles that improve grain Zn and Fe concentrations. PLoS One, 10(7): e0131846. |
| [145] | Xu Y J, Ying Y N, Ouyang S H, Duan X L, Sun H, Jiang S K, Sun S C, Bao J S. 2018. Factors affecting sensory quality of cooked japonica rice. Rice Sci, 25(6): 330-339. |
| [146] |
Yamaji N, Xia J X, Mitani-Ueno N, Yokosho K, Ma J F. 2013. Preferential delivery of zinc to developing tissues in rice is mediated by P-type heavy metal ATPase OsHMA2. Plant physiol, 162(2): 927-939.
DOI PMID |
| [147] | Yamaji N, Takemoto Y, Miyaji T, Mitani-Ueno N, Yoshida K T, Ma J F. 2017. Reducing phosphorus accumulation in rice grains with an impaired transporter in the node. Nature, 541: 92-95. |
| [148] |
Yang A, Li Y S, Xu Y Y, Zhang W H. 2013. A receptor-like protein RMC is involved in regulation of iron acquisition in rice. J Exp Bot, 64(16): 5009-5020.
DOI PMID |
| [149] | Yang M, Li Y T, Liu, Z H, Tian J J, Liang L M, Qiu Y, Wang G Y, Du Q Q, Cheng D, Cai H M, Shi L, Xu F S, Lian X M. 2020. A high activity zinc transporter OsZIP9 mediates zinc uptake in rice. Plant J, 103(5): 1695-1709. |
| [150] | Yang Z, Wu Y R, Li Y, Ling H Q, Chu C C. 2009. OsMT1a, a type 1 metallothionein, plays the pivotal role in zinc homeostasis and drought tolerance in rice. Plant Mol Biol, 70(1/2): 219-229. |
| [151] |
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma J F. 2009. OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol, 149(1): 297-305.
DOI PMID |
| [152] |
Yokosho K, Yamaji N, Ma J F. 2016. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J Exp Bot, 67(18): 5485-5494.
PMID |
| [153] |
Yoshida K T, Wada T, Koyama H, Mizobuchi-Fukuoka R, Naito S. 1999. Temporal and spatial patterns of accumulation of the transcript of myo-inositol-1-phosphate synthase and phytin-containing particles during seed development in rice. Plant Physiol, 119(1): 65-72.
PMID |
| [154] | Yuan F J, Zhao H J, Ren X L, Zhu S L, Fu X J, Shu Q Y. 2007. Generation and characterization of two novel low phytate mutants in soybean (Glycine max L. Merr). Theor Appl Genet, 115(7): 945-957. |
| [155] | Zhang C, Shinwari K I, Luo L, Zheng L Q. 2018. OsYSL13 is involved in iron distribution in rice. Int J Mol Sci, 19(11): 3537. |
| [156] |
Zhang H M, Li Y, Yao X N, Liang G, Yu D Q. 2017. POSITIVE REGULATOR OF IRON HOMEOSTASIS1, OsPRI1, facilitates iron homeostasis. Plant Physiol, 175(1): 543-554.
DOI PMID |
| [157] | Zhang H M, Li Y, Pu M N, Xu P, Liang G, Yu D Q. 2020. Oryza sativa POSITIVE REGULATOR OF IRON DEFICIENCY RESPONSE 2 (OsPRI2) and OsPRI3 are involved in the maintenance of Fe homeostasis. Plant Cell Environ, 43(1): 261-274. |
| [158] |
Zhang M, Pinson S R M, Tarpley L, Huang X Y, Lahner B, Yakubova E, Baxter I, Guerinot M L, Salt D E. 2014. Mapping and validation of quantitative trait loci associated with concentrations of 16 elements in unmilled rice grain. Theor Appl Genet, 127(1): 137-165.
PMID |
| [159] | Zhang Y, Xu Y H, Yi H Y, Gong J M. 2012. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J, 72(3): 400-410. |
| [160] | Zhao H J, Liu Q L, Fu H W, Xu X H, Wu D X, Shu Q Y. 2008. Effect of non-lethal low phytic acid mutations on grain yield and seed viability in rice. Field Crops Res, 108(3): 206-211. |
| [161] | Zhao Y N, Li C, Li H, Liu X S, Yang Z M. 2022. OsZIP11 is a trans-Golgi-residing transporter required for rice iron accumulation and development. Gene, 836: 146678. |
| [162] | Zheng L Q, Ying Y H, Wang L, Wang F, Whelan J, Shou H X. 2010. Identification of a novel iron regulated basic helix-loop- helix protein involved in Fe homeostasis in Oryza sativa. BMC Plant Biol, 10(1): 166. |
| [1] | Sitthikorn Bodeerath, Jeeraporn Veeradittakit, Sansanee Jamjod, Chanakan Prom-U-Thai. Applying Boron Fertilizer at Different Growth Stages Promotes Boron Uptake and Productivity in Rice [J]. Rice Science, 2024, 31(6): 751-760. |
| [2] | Ji Dongling, Xiao Wenhui, Sun Zhiwei, Liu Lijun, Gu Junfei, Zhang Hao, Matthew Tom Harrison, Liu Ke, Wang Zhiqin, Wang Weilu. Translocation and Distribution of Carbon-Nitrogen in Relation to Rice Yield and Grain Quality as Affected by High Temperature at Early Panicle Initiation Stage [J]. Rice Science, 2023, 30(6): 598-612. |
| [3] | Kankunlanach Khampuang, Nanthana Chaiwong, Atilla Yazici, Baris Demirer, Ismail Cakmak, Chanakan Prom-U-Thai. Effect of Sulfur Fertilization on Productivity and Grain Zinc Yield of Rice Grown under Low and Adequate Soil Zinc Applications [J]. Rice Science, 2023, 30(6): 632-640. |
| [4] | Monica Ruffini Castiglione, Stefania Bottega, Carlo Sorce, Carmelina SpanÒ. Effects of Zinc Oxide Particles with Different Sizes on Root Development in Oryza sativa [J]. Rice Science, 2023, 30(5): 449-458. |
| [5] | Lu Xuedan, Li Fan, Xiao Yunhua, Wang Feng, Zhang Guilian, Deng Huabing, Tang Wenbang. Grain Shape Genes: Shaping the Future of Rice Breeding [J]. Rice Science, 2023, 30(5): 379-404. |
| [6] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| [7] | Li Chao, Li He, Zhang Xianduo, Yang Zhimin. A Pleiotropic Drug Resistance Family Protein Gene Is Required for Rice Growth, Seed Development and Zinc Homeostasis [J]. Rice Science, 2023, 30(2): 127-137. |
| [8] | Meng Lu, Tang Mingfeng, Zhu Yuxing, Tan Longtao. Knocking-Out OsPDR7 Triggers Up-Regulation of OsZIP9 Expression and Enhances Zinc Accumulation in Rice [J]. Rice Science, 2023, 30(1): 36-49. |
| [9] | Ernieca Lyngdoh Nongbri, Sudip Das, Karma Landup Bhutia, Aleimo G. Momin, Mayank Rai, Wricha Tyagi. Differential Expression of Iron Deficiency Responsive Rice Genes under Low Phosphorus and Iron Toxicity Conditions and Association of OsIRO3 with Yield in Acidic Soils [J]. Rice Science, 2023, 30(1): 58-69. |
| [10] | Liu Yantong, Li Ting, Jiang Zhishu, Zeng Chuihai, He Rong, Qiu Jiao, Lin Xiaoli, Peng Limei, Song Yongping, Zhou Dahu, Cai Yicong, Zhu Changlan, Fu Junru, He Haohua, Xu Jie. Characterization of a Novel Weak Allele of RGA1/D1 and Its Potential Application in Rice Breeding [J]. Rice Science, 2022, 29(6): 522-534. |
| [11] | Blaise Pascal Muvunyi, Lu Xiang, Zhan Junhui, He Sang, Ye Guoyou. Identification of Potential Zinc Deficiency Responsive Genes and Regulatory Pathways in Rice by Weighted Gene Co-expression Network Analysis [J]. Rice Science, 2022, 29(6): 545-558. |
| [12] | Yousef Alhaj Hamoud, Hiba Shaghaleh, Wang Ruke, Willy Franz Gouertoumbo, Amar Ali Adam hamad, Mohamed Salah Sheteiwy, Wang Zhenchang, Guo Xiangping. Wheat Straw Burial Improves Physiological Traits, Yield and Grain Quality of Rice by Regulating Antioxidant System and Nitrogen Assimilation Enzymes under Alternate Wetting and Drying Irrigation [J]. Rice Science, 2022, 29(5): 473-488. |
| [13] | Suchila Utasee, Sansanee Jamjod, Sittisavet Lordkaew, Chanakan Prom-U-Thai. Improve Anthocyanin and Zinc Concentration in Purple Rice by Nitrogen and Zinc Fertilizer Application [J]. Rice Science, 2022, 29(5): 435-450. |
| [14] | Luo Haowen, He Longxin, Du Bin, Pan Shenggang, Mo Zhaowen, Yang Shuying, Zou Yingbin, Tang Xiangru. Epoxiconazole Improved Photosynthesis, Yield Formation, Grain Quality and 2-Acetyl-1-Pyrroline Biosynthesis of Fragrant Rice [J]. Rice Science, 2022, 29(2): 189-196. |
| [15] | Saichompoo Uthomphon, Narumol Possawat, Nakwilai Pawat, Thongyos Peeranut, Nanta Aekchupong, Tippunya Patompong, Ruengphayak Siriphat, Itthisoponkul Teerarat, Bueraheng Niranee, Cheabu Sulaiman, Malumpong Chanate. Breeding Novel Short Grain Rice for Tropical Region to Combine Important Agronomical Traits, Biotic Stress Resistance and Cooking Quality in Koshihikari Background [J]. Rice Science, 2021, 28(5): 479-792. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||