
Rice Science ›› 2025, Vol. 32 ›› Issue (3): 426-444.DOI: 10.1016/j.rsci.2025.03.004
• Research Papers • Previous Articles
Sara Cannavò1, Chiara Paleni2, Alma Costarelli1, Maria Cristina Valeri3, Martina Cerri4, Antonietta Saccomanno2, Veronica Gregis2, Graziella Chini Zittelli5, Petre I. Dobrev6, Lara Reale4( ), Martin M. Kater2,#, Francesco Paolocci3,#(
), Martin M. Kater2,#, Francesco Paolocci3,#( )
)
Received:2024-10-31
Accepted:2025-02-10
Online:2025-05-28
Published:2025-06-16
Contact:
Lara Reale (About author:First author contact:These authors contributed equally as last authors
Sara Cannavò, Chiara Paleni, Alma Costarelli, Maria Cristina Valeri, Martina Cerri, Antonietta Saccomanno, Veronica Gregis, Graziella Chini Zittelli, Petre I. Dobrev, Lara Reale, Martin M. Kater, Francesco Paolocci. Assessing Changes in Root Architecture, Developmental Timing, Transcriptional and Hormonal Profiles in Rice Co-Cultivated with Azolla filiculoides[J]. Rice Science, 2025, 32(3): 426-444.
Add to citation manager EndNote|Ris|BibTeX
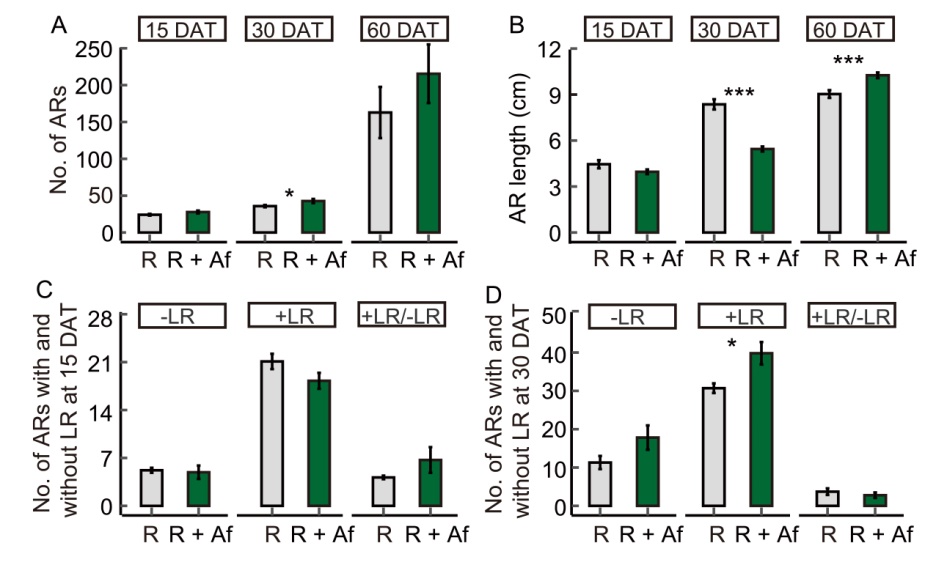
Fig. 1. Effect of Azolla filiculoides on the number (A) and length (B) of adventitious roots (ARs) in rice at 15, 30, and 60 d after transplanting (DAT), and on the number of rice ARs with (+LR) and without (-LR) lateral roots along with +LR/-LR ratio at 15 (C) and 30 DAT (D). Data represent Mean ± SE of control rice (R) and rice grown with A. filiculoides (R + Af). In A, n = 6 and 7 for R and R + Af at 15 DAT, respectively; n = 254 and 333 for R and R + Af at 30 DAT, respectively; n = 853 and 1 711 for R and R + Af at 60 DAT, respectively. In B, n = 144 and 187 for R and R + Af at 15 DAT, respectively; n = 18 for R and R + Af at 30 DAT; n = 7 and 9 for R and R + Af at 60 DAT, respectively. In C and D, n = 10 and 11 for R and R + Af at 15 and 30 DAT, respectively. *, P < 0.05; ***, P < 0.001.
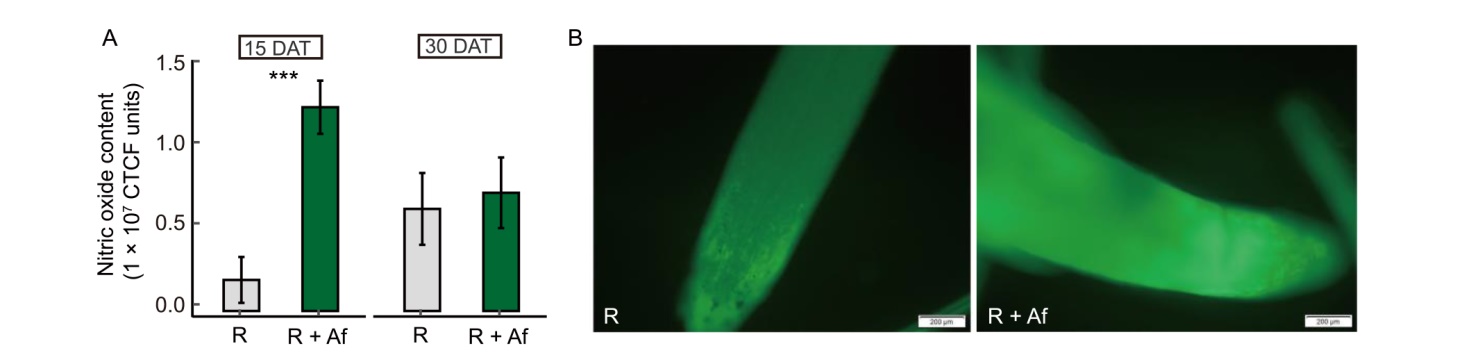
Fig. 2. Effect of Azolla filiculoides on relative amount of nitric oxide in adventitious roots (ARs) of rice at 15 and 30 d after transplanting (DAT). A, Nitric oxide levels assayed using fluorescence analysis after staining with 4,5-diaminofluorescein diacetate (DAF-2DA) probe. CTCF, Corrected total cell fluorescence. Data represent Mean ± SE of control rice (R) and rice grown with A. filiculoides (R + Af). n = 23 and 22 for R and R + Af at 15 DAT, respectively; n = 15 for R and R + Af at 30 DAT. ***, P < 0.001. B, Fresh sections of adventitious apex at 15 DAT stained with DAF-2DA probe. Pictures were taken under an epifluorescence light microscopy (excitation 495 nm; emission 515 nm; long-pass filter of 515 nm). Scale bars, 200 μm.
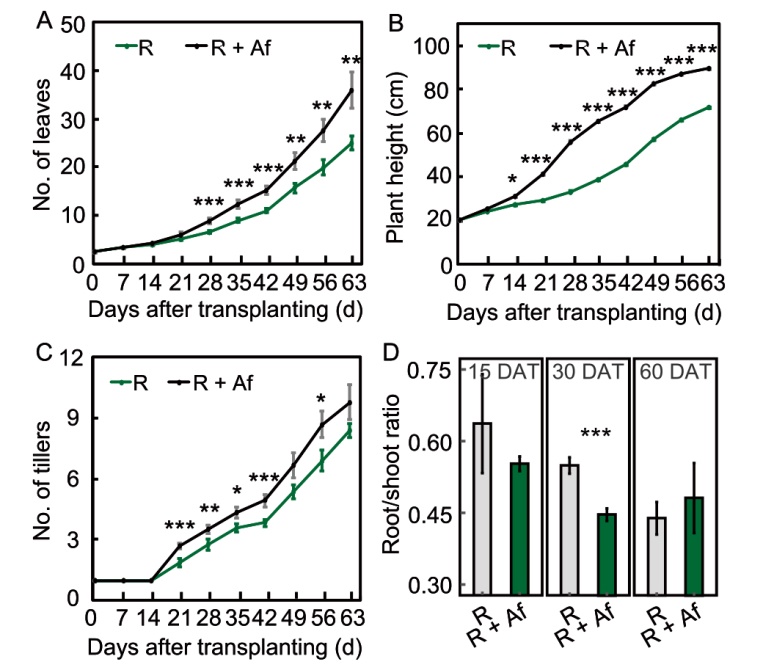
Fig. 3. Effect of Azolla filiculoides on the number of leaves (A), plant height (B), number of tillers (C), and root/shoot ratio of fresh weights (D) over two months. DAT, Days after transplanting. Data represent Mean ± SE of control rice (R) and rice grown with A. filiculoides (R + Af). In A‒C, n = 12 for R and R + Af at 0, 7, 14, 21, and 28 DAT; n = 11 and 12 for R and R + Af at 35 and 42 DAT, respectively; n = 8 and 9 for R and R + Af at 49, 56, and 63 DAT, respectively; In D, n = 6 and 7 for R and R + Af at 15 DAT, respectively; n = 20 for R and R + Af at 30 DAT; n = 121 and 15 for R and R + Af at 60 DAT, respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
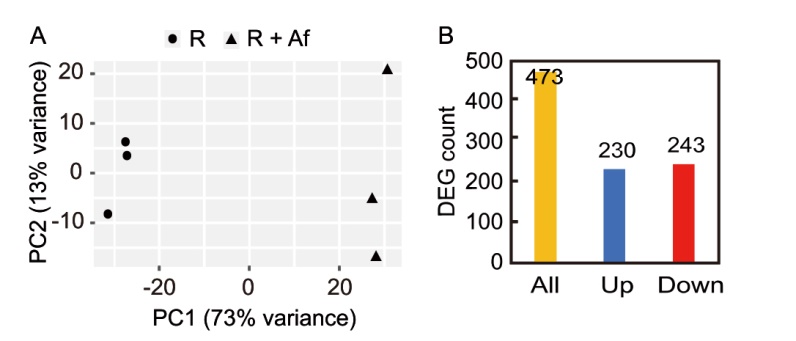
Fig. 4. Principal component analysis (PCA, A) and differentially expressed gene (DEG) count at 15 d after transplanting (B). R, Rice, used as a control; R + Af, Rice co-cultivated with Azolla filiculoides.
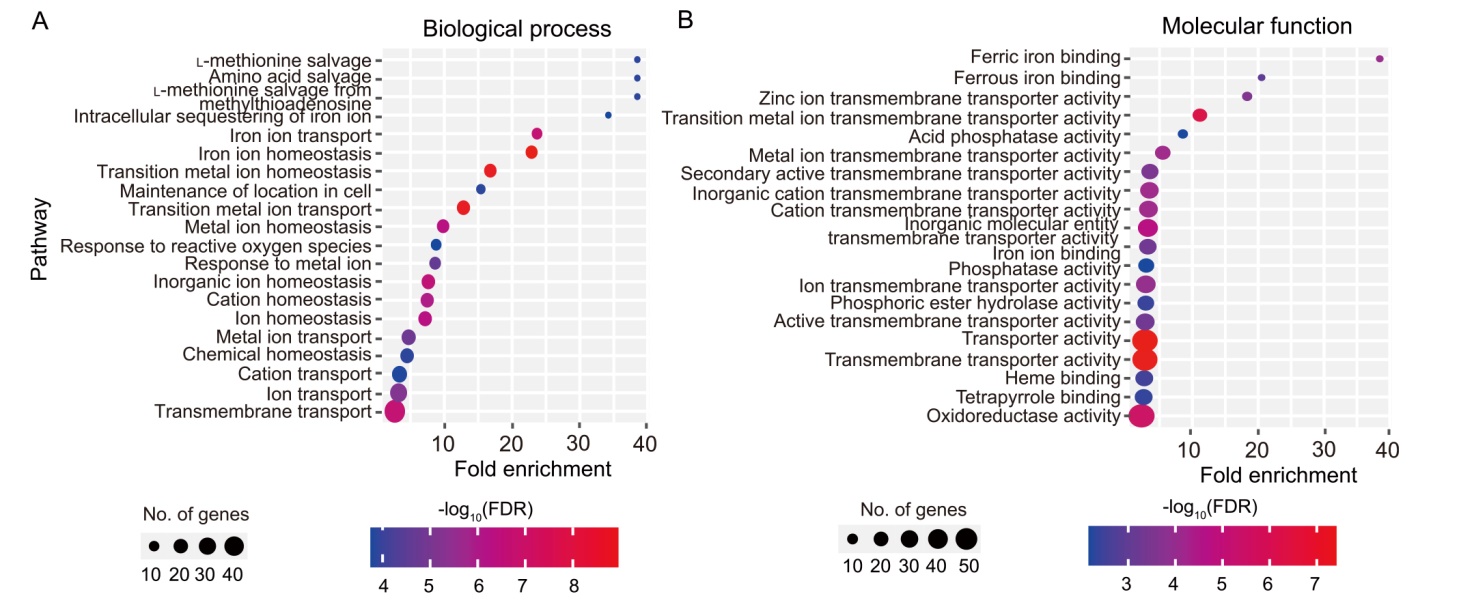
Fig. 5. Twenty most enriched biological process (A) and molecular function (B) terms among differentially expressed genes in rice roots at 15 d after transplanting. FDR, False discovery rate.
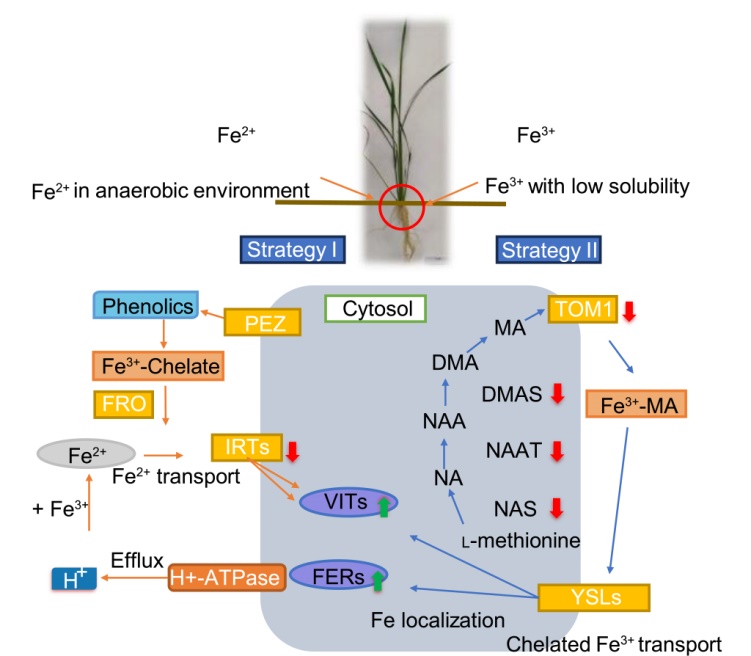
Fig. 6. Schematic representation of iron acquisition systems in rice roots. Under Strategy I, the ferric chelate complex is reduced to ferrous ion by ferric chelate reductase (FRO). H+ released by the proton pump and phenolics released by phenolics efflux transporter (PEZ) acidify the medium to convert ferric ion to ferrous ion. Iron transporters (IRTs) transport the ferrous ion into the root cells. Vacuolar iron transporters (VITs) are responsible for transportation and accumulation of iron within the vacuole while ferritins (FERs) are the proteins accommodating excess iron within the cells. Under Strategy II, mugineic acid (MA) phytosiderophores, produced by the sequential activity of nicotianamine synthase (NAS), nicotianamine aminotransferase (NAAT), and deoxymugineic acid synthase (DMAS). Deoxymugineic acid efflux transporter 1 (TOM1) transporter mediates the DMA secretion through the plasma membrane. Chelated Fe(III) complex is transported across the membrane into the root cell by Yellow Stripe-Like (YSL) transporters. Green and red arrows refer to transporters/enzymes whose mRNA are increased and decreased, respectively, in the R + Af (rice grown with Azolla filiculoides) vs R (rice) treatment. For the differential expression of regulatory genes refer to Table S2. The picture was redrawn from Kobayashi et al (2014) and Kar and Panda (2020).
| Accession number | Symbol | log2(fold change) | Adjusted P-value | Description |
|---|---|---|---|---|
| Os02g0770800 | NR2/NIA1/NIA2 | 5.373 | 8.4E-05 | NADH/NADPH-dependent nitrate reductase |
| Os10g0554200 | NRT1.1B/NPF6.5 | 3.049 | 3.9E-04 | Nitrate transporter 1.1B |
| Os10g0169900 | NRT | 4.308 | 1.6E-04 | Nitrate and chloride transporter |
Table 1. Nitrate oxide-related differentially expressed genes at 15 d after transplanting.
| Accession number | Symbol | log2(fold change) | Adjusted P-value | Description |
|---|---|---|---|---|
| Os02g0770800 | NR2/NIA1/NIA2 | 5.373 | 8.4E-05 | NADH/NADPH-dependent nitrate reductase |
| Os10g0554200 | NRT1.1B/NPF6.5 | 3.049 | 3.9E-04 | Nitrate transporter 1.1B |
| Os10g0169900 | NRT | 4.308 | 1.6E-04 | Nitrate and chloride transporter |
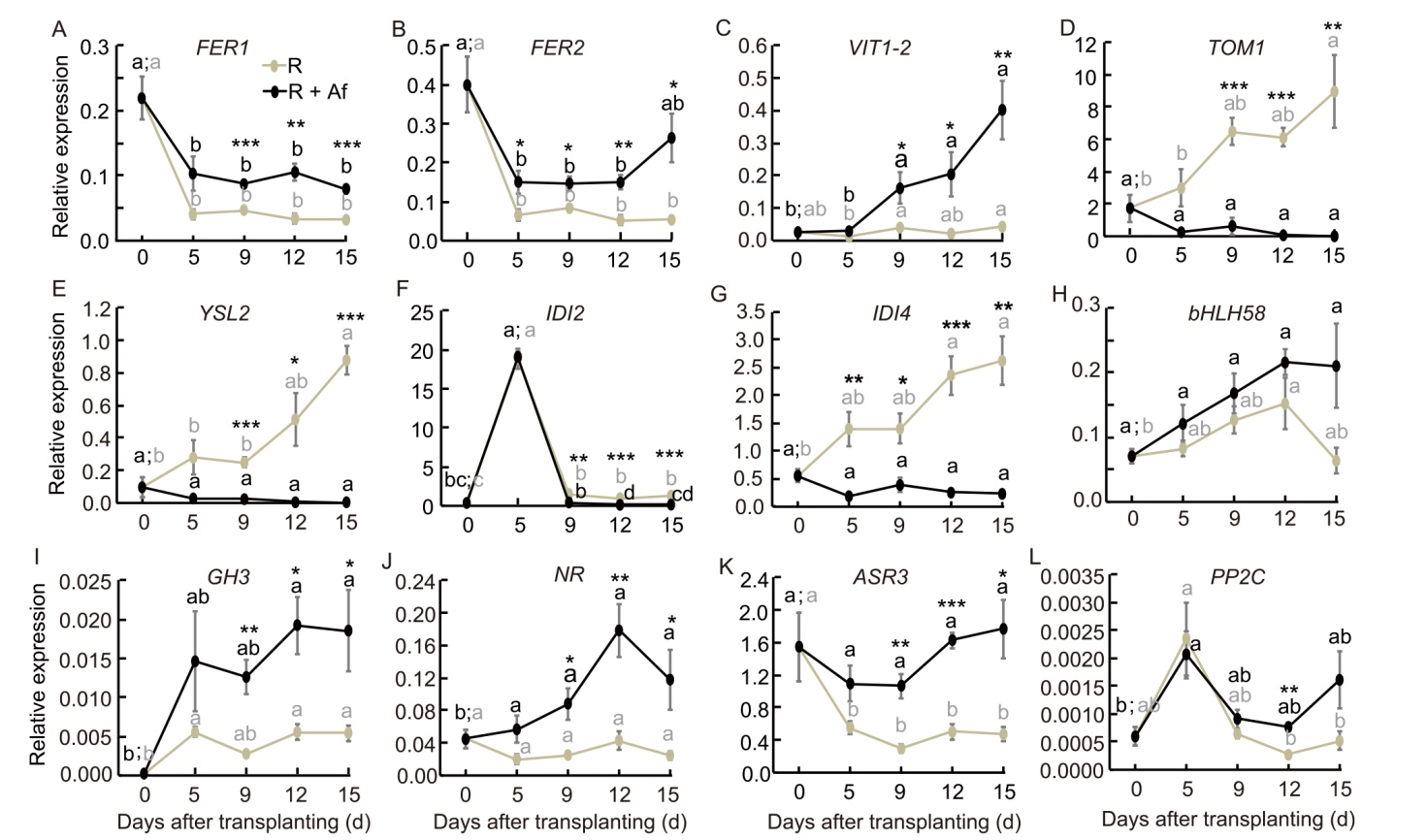
Fig. 7. Gene expression profiles of selected differentially expressed genes at 15 d after transplanting (DAT) as resulted from qRT-PCR analysis at 0, 5, 9 12, and 15 DAT. R, Rice, used as a control; R + Af, Rice co-cultivated with Azolla filiculoides. Asterisks indicate significant differences between the two treatments at a given time pint (t-test, P < 0.05), while lowercase letters above bars indicate significant differences between time points of the same treatment (Analysis of variance, P < 0.05; Tukey’s HSD, P < 0.05).
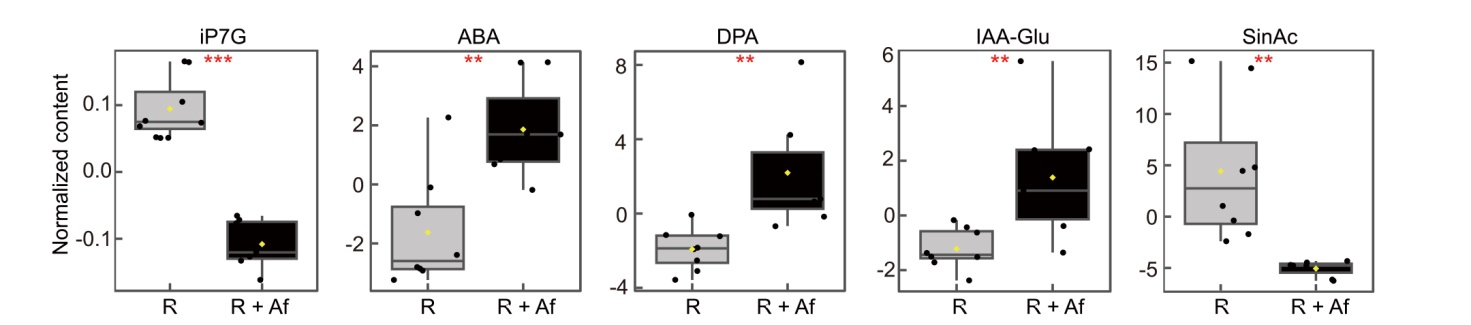
Fig. 8. Differential levels of hormonal compounds in roots of rice grown with and without Azolla filiculoides at 15 d after transplanting. Unpaired two samples t-test for all five compounds except IAA-Glu, for which the unpaired two samples of Wilcoxon test was performed. For all five compounds: P < 0.01 and false discovery rate cut-off ≤ 0.05. R, Rice, used as a control; R + Af, Rice co-cultivated with Azolla filiculoides; iP7G, Isopentenyl adenine-7-glucoside; ABA, Abscisic acid; DPA, Dihydrophaseic acid; IAA-Glu, Indole-3-acetic acid-glutamate; SinAc, Sinapic acid.
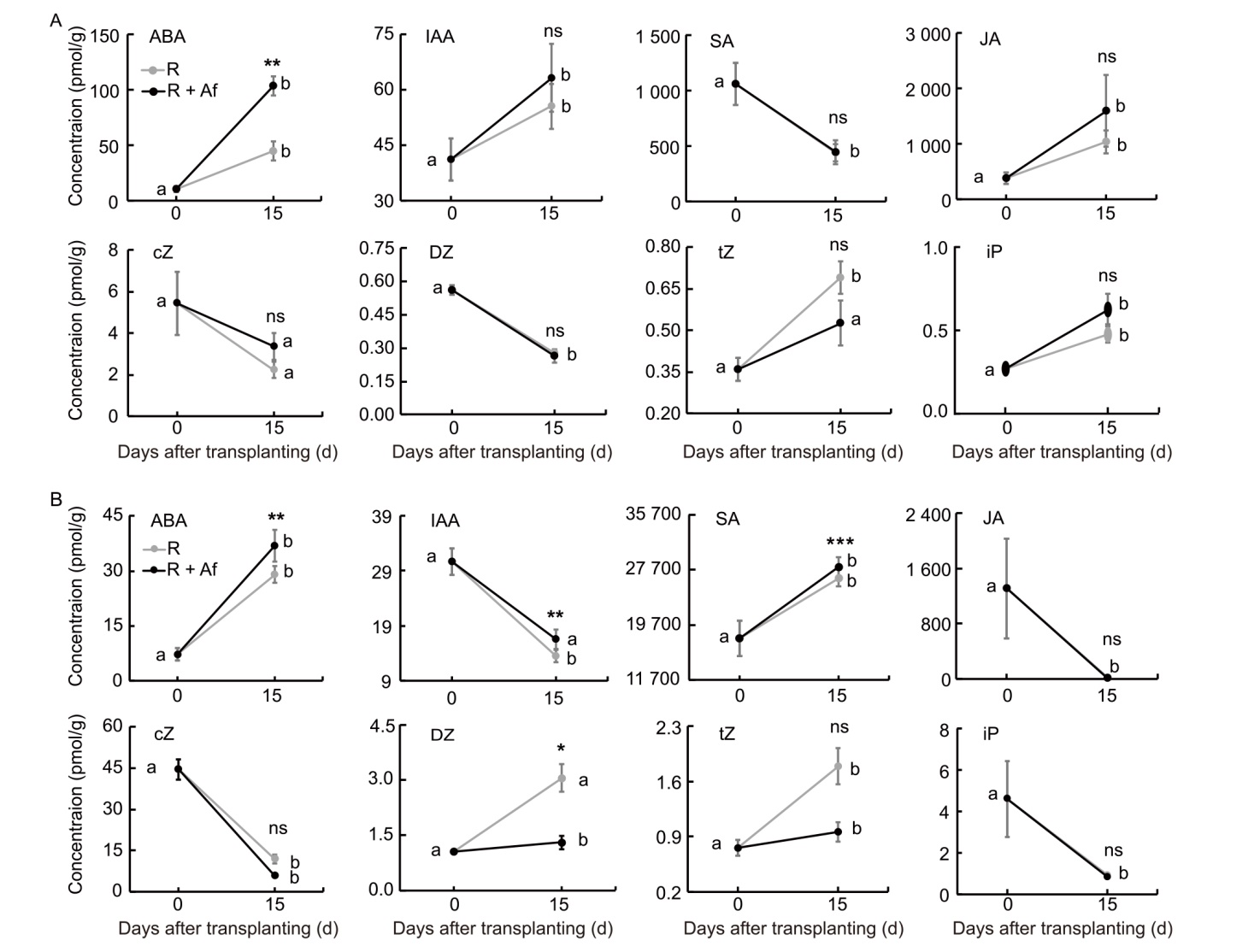
Fig. 9. Accumulation profiles at 0 and 15 d after transplanting of bioactive hormones in rice roots (A) and leaves (B) from R + Af and R treatments. Asterisks indicate significant differences between two treatments at a given time pint (t-test, P < 0.05), while lowercase letters indicate significant differences between time points of the same treatment (Analysis of variance, P < 0.05; Tukey’s HSD, P < 0.05). R, Rice, used as a control; R + Af, Rice co-cultivated with Azolla filiculoides; ABA, Abscisic acid; IAA, Indole-3-acetic acid; SA, Salicylic acid; JA, Jasmonic acid; cZ, cis-Zeatin; DZ, Dihydrozeatin; tZ, trans-Zeatin; iP, Isopentenyl adenine.
| [1] | Andrews S. 2021. FastQC, a quality control tool for high throughput sequence data. [2024-09-28]. https://www.bioinformatics.babraham. ac.uk/projects/fastqc. |
| [2] | Aronesty E. 2011. Ea-utils: Command-line tools for processing biological sequencing data. [2024-09-28]. https://github.com/ ExpressionAnalysis/ea-utils. |
| [3] | Aung M S, Masuda H, Kobayashi T, et al. 2018. Physiological and transcriptomic analysis of responses to different levels of iron excess stress in various rice tissues. Soil Sci Plant Nutr, 64(3): 370-385. |
| [4] | Banach A, Kuźniar A, Mencfel R, et al. 2019. The study on the cultivable microbiome of the aquatic fern Azolla filiculoides L. as new source of beneficial microorganisms. Appl Sci, 9(10): 2143. |
| [5] | Barrero J M, Piqueras P, González-Guzmán M, et al. 2005. A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot, 56: 2071-2083. |
| [6] | Beemster G T S, Baskin T I. 1998. Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol, 116(4): 1515-1526. |
| [7] | Berry T U. Encyclopedia of hormones.Encyclopedia of hormones Libr J, 128(18): 68. |
| [8] | Bhuvaneshwari K, Singh P K. 2015. Response of nitrogen-fixing water fern Azolla biofertilization to rice crop. 3 Biotech, 5(4): 523-529. |
| [9] | Bizzarri M, Delledonne M, Ferrarini A, et al. 2020. Whole-transcriptome analysis unveils the synchronized activities of genes for fructans in developing tubers of the Jerusalem artichoke. Front Plant Sci, 11: 101. |
| [10] | Brilli F, Srikanta Dani K G, Pasqualini S, et al. 2022. Exposure to different light intensities affects emission of volatiles and accumulations of both pigments and phenolics in Azolla filiculoides. Physiol Plant, 174(1): e13619. |
| [11] | Carvalho L C, Vidigal P, Amâncio S. 2015. Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front Environ Sci, 3: 20. |
| [12] | Casanova D, Goudriaan J, Catala Forner M M, et al. 2002. Rice yield prediction from yield components and limiting factors. Eur J Agron, 17(1): 41-61. |
| [13] | Chakraborty S, Verma E, Singh S S. 2019. Cyanobacterial siderophores: Ecological and biotechnological significance. In: Mishra A K, Tiwari D N, Rai A N. Cyanobacteria: From Basic Science to Application. Amsterdam, the Netherlands: Elsevier: 383-397. |
| [14] | Chen H, Lai Z B, Shi J W, et al. 2010. Roles of arabidopsis WRKY18, WRKY40 and WRKY60 transcription factors in plant responses to abscisic acid and abiotic stress. BMC Plant Biol, 10: 281. |
| [15] | Cheng W H, Endo A, Zhou L, et al. 2002. A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell, 14(11): 2723-2743. |
| [16] | Consorti E, Costarelli A, Cannavò S, et al. 2024. Co-cultivation with Azolla affects the metabolome of whole rice plant beyond canonical inorganic nitrogen fertilization. bioRxiv, 10.1101/ 2024.10.02.615589. |
| [17] | Correa-Aragunde N, Foresi N, Lamattina L. 2016. Auxin and nitric oxide: A counterbalanced partnership ensures the redox cue control required for determining root growth pattern. Adv Bot Res, 77: 41-54. |
| [18] | Costarelli A, Cannavò S, Cerri M, et al. 2021. Light and temperature shape the phenylpropanoid profile of Azolla filiculoides fronds. Front Plant Sci, 12: 727667. |
| [19] | de Vries S, de Vries J, Teschke H, et al. 2018. Jasmonic and salicylic acid response in the fern Azolla filiculoides and its cyanobiont. Plant Cell Environ, 41(11): 2530-2548. |
| [20] | Escaray F J, Passeri V, Babuin F M, et al. 2014. Lotus tenuis × L. corniculatus interspecific hybridization as a means to breed bloat-safe pastures and gain insight into the genetic control of proanthocyanidin biosynthesis in legumes. BMC Plant Biol, 14: 40. |
| [21] | Ewels P, Magnusson M, Lundin S, et al. 2016. MultiQC: Summarize analysis results for multiple tools and samples in a single report Bioinformatics, 32(19): 3047-3048. |
| [22] | Food World Organization (FAO). 2009a. Global agriculture towards 2050. In: High Level Expert Forum, Rome, Italy: FAO. 12-13 October, 2009. [2024-9-22].http://www.fao.org/fileadmin/templates/ wsfs/docs/Issues_papers/HLEF2050_Global_Agriculture.pdf. |
| [23] | Food World Organization (FAO). 2009b. How to feed the world in 2050, insights from an expert meeting at FAO, 2050, Rome, Italy: FAO. In: Proceedings of the Expert Meeting on How to Feed the World in 2050. 24-26 June, 2009, Rome, Italy. [2024-9-22]. http://www.fao.org/wsfs/forum2050/wsfs-forum/en/. |
| [24] | Food World Organization (FAO). 2022a. Crop prospects and food situation: Quarterly global report. Rome, Italy: FAO. [2024-9-22]. doi: 10.4060/cc3233en. |
| [25] | Food World Organization (FAO). 2022b. World food and agriculture: Statistical Pocketbook 2022. Rome, Italy: FAO. [2024-9-22]. doi: 10.4060/cc2212en. |
| [26] | Forde B G. 2014. Nitrogen signalling pathways shaping root system architecture: An update. Curr Opin Plant Biol, 21: 30-36. |
| [27] | Frick E M, Strader L C. 2018. Roles for IBA-derived auxin in plant development. J Exp Bot, 69(2): 169-177. |
| [28] | Gaillard M D P, Glauser G, Robert C A M, et al. 2018. Fine-tuning the ‘plant domestication-reduced defense’ hypothesis: Specialist vs generalist herbivores. New Phytol, 217(1): 355-366. |
| [29] | Gao S P, Xiao Y H, Xu F, et al. 2019. Cytokinin-dependent regulatory module underlies the maintenance of zinc nutrition in rice. New Phytol, 224(1): 202-215. |
| [30] | Ge S X, Jung D, Yao R. 2020. ShinyGO: A graphical gene-set enrichment tool for animals and plants. Bioinformatics, 36(8): 2628-2629. |
| [31] | Geiss G, Gutierrez L, Bellini C. 2009. Adventitious root formation: New insights and perspectives. In: Beeckman T. Annual Plant Reviews: Root Development. Wiley-Blackwell: Oxford, UK: 127-156. |
| [32] | Godfray H C J, Beddington J R, Crute I R, et al. 2010. Food security: The challenge of feeding 9 billion people. Science, 327: 812-818. |
| [33] | Gregorio G B, Senadhira D, Mendoza R D. 1997. Screening rice for salinity tolerance. In: IRRI Discussion Paper Series No. 22. Manila, the Philippines: International Rice Research Institute. |
| [34] | Gu J F, Yang J C. 2022. Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management. Crop Environ, 1(1): 7-14. |
| [35] | Guo R P, Hu Y, Aoi Y, et al. 2022. Local conjugation of auxin by the GH3 amido synthetases is required for normal development of roots and flowers in Arabidopsis. Biochem Biophys Res Commun, 589: 16-22. |
| [36] | Herath B M M D, Karunarathna S C, Ishaq M, et al. 2023. Azolla as the multifunctional fern in organic agriculture: Prospects and challenges: A review article. Int J Agric Technol, 19(1): 63-82. |
| [37] | Horiguchi G, Ferjani A, Fujikura U, et al. 2006. Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res, 119(1): 37-42. |
| [38] | Ishimaru Y, Suzuki M, Tsukamoto T, et al. 2006. Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J, 45(3): 335-346. |
| [39] | Jeong H, Park J, Kim H.2013 Determination of NH4+ in environmental water with interfering substances using the modified nessler method. J Chem, 2013(1): 359217. |
| [40] | Kar S, Panda S K. 2020. Iron homeostasis in rice: Deficit and excess. Proc Natl Acad Sci Ind Sect B Biol Sci, 90(2): 227-235. |
| [41] | Khodary S E A. 2004. Effect of salicylic acid on growth, photosynthesis and carbohydrate metabolism in salt stressed maize plants. Int J Agric Biol, 6: 5-8. |
| [42] | Khumairoh U, Lantinga E A, Schulte R P O, et al. 2018. Complex rice systems to improve rice yield and yield stability in the face of variable weather conditions. Sci Rep, 8(1): 14746. |
| [43] | Kiba T, Kudo T, Kojima M, et al. 2011. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J Exp Bot, 62(4): 1399-1409. |
| [44] | Kirk G J D, Manwaring H R, Ueda Y, et al. 2022. Below-ground plant-soil interactions affecting adaptations of rice to iron toxicity. Plant Cell Environ, 45(3): 705-718. |
| [45] | Kobayashi T, Nakanishi Itai R, Nishizawa N K. 2014. Iron deficiency responses in rice roots. Rice, 7(1): 27. |
| [46] | Kojima H, Nakatsubo N, Kikuchi K, et al. 1998. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal Chem, 70(13): 2446-2453. |
| [47] | Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Dev Cell, 18(6): 927-937. |
| [48] | Ladha J K, Dawe D, Ventura T S, et al. 2000. Long-term effects of urea and green manure on rice yields and nitrogen balance. Soil Sci Soc Am J, 64(6): 1993-2001. |
| [49] | Lavenus J, Goh T, Roberts I, et al. 2013. Lateral root development in Arabidopsis: Fifty shades of auxin. Trends Plant Sci, 18(8): 450-458. |
| [50] | LeNoble M E, Spollen W G, Sharp R E. 2004. Maintenance of shoot growth by endogenous ABA: Genetic assessment of the involvement of ethylene suppression. J Exp Bot, 55: 237-245. |
| [51] | Li B, Dewey C N. 2011. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics, 12: 323. |
| [52] | Li G J, Kronzucker H J, Shi W M. 2016. The response of the root apex in plant adaptation to iron heterogeneity in soil. Front Plant Sci, 7: 344. |
| [53] | Li M J, Watanabe S, Gao F, et al. 2023. Iron nutrition in plants: Towards a new paradigm? Plants, 12(2): 384. |
| [54] | Li Q, Chen L, Yang A. 2020. The molecular mechanisms underlying iron deficiency responses in rice. Int J Mol Sci, 21(1): 43. |
| [55] | Love M I, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2 Genome Biol, 15(12): 550. |
| [56] | Lumpkin T A, Plucknett D L. 1980. Azolla: Botany, physiology, and use as a green manure. Econ Bot, 34(2): 111-153. |
| [57] | Mahanty T, Bhattacharjee S, Goswami M, et al. 2017. Biofertilizers: A potential approach for sustainable agriculture development. Environ Sci Pollut Res Int, 24(4): 3315-3335. |
| [58] | Maldonado I, Moreno Terrazas E G, Vilca F Z, 2022. Application of duckweed (Lemna sp.) and water fern (Azolla sp.) in the removal of pharmaceutical residues in water: State of art focus on antibiotics. Sci Total Environ, 838(Pt 4): 156565. |
| [59] | Marzouk S H, Tindwa H J, Amuri N A, et al. 2023. An overview of underutilized benefits derived from Azolla as a promising biofertilizer in lowland rice production. Heliyon, 9(1): e13040. |
| [60] | Meyer C, Lea U S, Provan F, et al. 2005. Is nitrate reductase a major player in the plant NO (nitric oxide) game? Photosynth Res, 83(2): 181-189. |
| [61] | Mhimdi M, Pérez-Pérez J M. 2020. Understanding of adventitious root formation: What can we learn from comparative genetics? Front Plant Sci, 11: 582020. |
| [62] | Monteiro M I C, Ferreira F N, de Oliveira N M M, et al. 2003. Simplified version of the sodium salicylate method for analysis of nitrate in drinking waters. Anal Chim Acta, 477(1): 125-129. |
| [63] | Mueller N D, Gerber J S, Johnston M, et al. 2012. Closing yield gaps through nutrient and water management. Nature, 490: 254-257. |
| [64] | Müller M, Schmidt W. 2004. Environmentally induced plasticity of root hair development in Arabidopsis. Plant Physiol, 134(1): 409-419. |
| [65] | Murashige T, Skoog F. 1962. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant, 15(3): 473-497. |
| [66] | Nguyen N K, Wang J, Liu D P, et al. 2022. Rice iron storage protein ferritin 2 (OsFER2) positively regulates ferroptotic cell death and defense responses against Magnaporthe oryzae. Front Plant Sci, 13: 1019669. |
| [67] | Nguyen N V. 2002. Global climate changes and rice food security. [2024-09-26]. http://www.hechoenperu.org.pe/fao/docs/Agriculture/ 3-Nguyen.pdf. |
| [68] | Pang Z Q, Chong J, Zhou G Y, et al. 2021. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucleic Acids Res, 49(W1): W388-W396. |
| [69] | Peters G A, Meeks J C. 1989. The Azolla-Anabaena symbiosis: Basic biology. Annu Rev Plant Physiol Plant Mol Biol, 40: 193-210. |
| [70] | Petit J M, Briat J F, Lobréaux S. 2001. Structure and differential expression of the four members of the Arabidopsis thaliana ferritin gene family. Biochem J, 359(Pt 3): 575-582. |
| [71] | Prakash V, Vishwakarma K, Singh V P, et al. 2020. NO and ROS implications in the organization of root system architecture. Physiol Plant, 168(2): 473-489. |
| [72] | Prerostova S, Dobrev P I, Knirsch V, et al. 2021. Light quality and intensity modulate cold acclimation in Arabidopsis. Int J Mol Sci, 22(5): 2736. |
| [73] | Qiao W H, Fan L M. 2008. Nitric oxide signaling in plant responses to abiotic stresses. J Integr Plant Biol, 50(10): 1238-1246. |
| [74] | R Core Team. 2016. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for statistical Computing. [2024-09-10]. https://www.R-project.org/. |
| [75] | Rippka R, Deruelles J, Waterbury J B, et al. 1979. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J General Microbiol, 111: 1-61. |
| [76] | Rivas-San Vicente M, Plasencia J. 2011. Salicylic acid beyond defence: Its role in plant growth and development. J Exp Bot, 62(10): 3321-3338. |
| [77] | Růžička K, Ljung K, Vanneste S, et al. 2007. Ethylene regulates root growth through effects on auxin biosynthesis and transport- dependent auxin distribution. Plant Cell, 19(7): 2197-2212. |
| [78] | Sahay S, Robledo-Arratia L, Glowacka K, et al. 2021. Root NRT, NiR, AMT, GS, GOGAT and GDH expression levels reveal NO and ABA mediated drought tolerance in Brassica juncea L, Sci Rep,11(1): 7992. |
| [79] | Sakai H, Lee S S, Tanaka T, et al. 2013. Rice annotation project database (RAP-DB): An integrative and interactive database for rice genomics Plant Cell Physiol, 54(2): e6. |
| [80] | Sánchez-Vicente I, Fernández-Espinosa M G, Lorenzo O. 2019. Nitric oxide molecular targets: Reprogramming plant development upon stress. J Exp Bot, 70(17): 4441-4460. |
| [81] | Séguéla M, Briat J F, Vert G, et al. 2008. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway. Plant J, 55(2): 289-300. |
| [82] | Shannon P, Markiel A, Ozier O, et al. 2003. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res, 13(11): 2498-2504. |
| [83] | Shi D J, Hall D O. 1988. The Azolla-Anabaena association: Historical perspective, symbiosis and energy metabolism. Bot Rev, 54(4): 353-386. |
| [84] | Soneson C, Love M I, Robinson M D. 2015. Differential analyses for RNA-seq: Transcript-level estimates improve gene-level inferences F1000Res. F1000Res, 4: 1521. |
| [85] | Sprangers K, Thys S, van Dusschoten D, et al. 2020. Gibberellin enhances the anisotropy of cell expansion in the growth zone of the maize leaf. Front Plant Sci, 11: 1163. |
| [86] | Stein R J, Ricachenevsky F K, Fett J P. 2009. Differential regulation of the two rice ferritin genes (OsFER1 and OsFER2). Plant Sci, 177(6): 563-569. |
| [87] | Sun C L, Liu L J, Yu Y, et al. 2015. Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J Integr Plant Biol, 57(6): 550-561. |
| [88] | Tewari R K, Horemans N, Watanabe M. 2021. Evidence for a role of nitric oxide in iron homeostasis in plants. J Exp Bot, 72(4): 990-1006. |
| [89] | Tilman D, Balzer C, Hill J, et al. 2011. Global food demand and the sustainable intensification of agriculture. Proc Natl Acad Sci USA, 108: 20260-20264. |
| [90] | Tucuch-Haas C J, Pérez-Balam J V, Díaz-Magaña K B, et al. 2017. Salicylic Acid: A Multifaceted Hormone. Springer, Singapore:243 |
| [91] | Tung H F, Shen T C. 1985. Studies of the Azolla pinnata: Anabaena azollae symbiosis: Concurrent growth of Azolla with rice. Aquat Bot, 22(2): 145-152. |
| [92] | United Nations (UN). 2022. ‘World Population Prospects 2022. Summary of Results’, twenty seventh, New York, USA. [2024-9-22]. http://www.un.org/development/desa/pd/. |
| [93] | Valette M, Rey M, Doré J, et al. 2020. Identification of a small set of genes commonly regulated in rice roots in response to beneficial rhizobacteria. Physiol Mol Biol Plants, 26(12): 2537-2551. |
| [94] | Vishwakarma A, Wany A, Pandey S, et al. 2019. Current approaches to measure nitric oxide in plants. J Exp Bot, 70(17): 4333-4343. |
| [95] | Vlek P L G, Eberhardt U, Aung M M. 2002. The role of Azolla in lowering the pH of simulated floodwater. J Appl Bot-Angew Bot, 76(1/2): 1-7. |
| [96] | Wagner G M. 1997. Azolla: A review of its biology and utilization. Bot Rev, 63(1): 1-26. |
| [97] | Watanabe I, Liu C C. 1992. Improving nitrogen-fixing systems and integrating them into sustainable rice farming. Plant Soil, 141(1): 57-67. |
| [98] | Wu W Q, Du K, Kang X Y, et al. 2021. The diverse roles of cytokinins in regulating leaf development. Hortic Res, 8(1): 118. |
| [99] | Xiong J, Tao L X, Zhu C. 2009. Does nitric oxide play a pivotal role downstream of auxin in promoting crown root primordia initiation in monocots? Plant Signal Behav, 4(10): 999-1001. |
| [100] | Yamasaki H. 2005. The NO world for plants: Achieving balance in an open system. Plant Cell Environ, 28(1): 78-84. |
| [101] | Yao Y L, Zhang M, Tian Y H, et al. 2018. Azolla biofertilizer for improving low nitrogen use efficiency in an intensive rice cropping system. Field Crops Res, 216: 158-164. |
| [102] | Yu M D, Lamattina L, Spoel S H, et al. 2014. Nitric oxide function in plant biology: A redox cue in deconvolution. New Phytol, 202(4): 1142-1156. |
| [103] | Yuan H M, Liu W C, Jin Y, et al. 2013. Role of ROS and auxin in plant response to metal-mediated stress. Plant Signal Behav, 8(7): e24671. |
| [104] | Zahir Z A, Asghar H N, Arshad M. 2001. Cytokinin and its precursors for improving growth and yield of rice. Soil Biol Biochem, 33(3): 405-408. |
| [105] | Zhang X, Xue C W, Wang R N, et al. 2022. Physiological and proteomic dissection of the rice roots in response to iron deficiency and excess. J Proteomics, 267: 104689. |
| [106] | Zhao B Q, Liu Q Y, Wang B S, et al. 2021. Roles of phytohormones and their signaling pathways in leaf development and stress responses. J Agric Food Chem, 69(12): 3566-3584. |
| [107] | Zhao D Y, Tian Q Y, Li L H, et al. 2007. Nitric oxide is involved in nitrate-induced inhibition of root elongation in Zea mays. 2007. Ann Bot, 100(3): 497-503. |
| [108] | Zhao J Z, Yu N N, Ju M, et al. 2019. ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot, 70(21): 6277-6291. |
| [109] | Zhou B Y, Guo Z F, Xing J P, et al. 2005 Nitric oxide is involved in abscisic acid-induced antioxidant activities in Stylosanthes guianensis. J Exp Bot, 56: 3223-3228. |
| [1] | Lai Changkai, Hu Shikai, Jiao Guiai, Wang Ling, Shao Gaoneng, Zhao Fengli, Xie Lihong, Wei Xiangjin, Lü Yusong, Sheng Zhonghua, Tang Shaoqing, Hu Peisong. Enhancing Folate Content in Japonica Rice Through Co-expression of OsADCS and OsGTPCHI Indica Alleles [J]. Rice Science, 2025, 32(3): 353-366. |
| [2] | Chaemyeong Lim, Sae Hyun Lee, Haeun Lee, So-Yon Park, Kiyoon Kang, Hyeryung Yoon, Tae-Jin Yang, Gary Stacey, Nam-Chon Paek, Sung-Hwan Cho. Global Transcriptome Analysis of Rice Seedlings in Response to Extracellular ATP [J]. Rice Science, 2025, 32(3): 380-399. |
| [3] | Nie Lixiao, Guo Xiayu, Wang Weiqin, Qi Yucheng, Ai Zhiyong, He Aibin. Regulation of Regeneration Rate to Enhance Ratoon Rice Production [J]. Rice Science, 2025, 32(2): 177-192. |
| [4] | Wang Jingqing, Wang Yaliang, Chen Yulin, Chen Huizhe, Xiang Jing, Zhang Yikai, Wang Zhigang, Zhang Yuping. Progress on Physiological Mechanisms of Rice Spikelet Degeneration at Different Panicle Positions Caused by Abiotic Stress [J]. Rice Science, 2025, 32(2): 193-202. |
| [5] | Chen Ya, Liu Zhiquan, Yang Linyin, Wu Fujie, Cao Zijian, Shi Huanbin, Qiu Jiehua, Kou Yanjun. OsCERK1 Interacts with OsHPP08 to Regulate Copper Uptake and Blast Resistance in Rice [J]. Rice Science, 2025, 32(2): 203-216. |
| [6] | Jiang Nan, Qiu Jiehua, Tian Dagang, Shi Huanbin, Liu Zhiquan, Wen Hui, Xie Shuwei, Chen Huizhe, Wu Meng, Kou Yanjun. Mixture of Bacillus Amyloliquefaciens and Bacillus Pumilus Modulates Community Structures of Rice Rhizosphere Soil to Suppress Rice Seedling Blight [J]. Rice Science, 2025, 32(1): 118-130. |
| [7] | Yu Shicong, Luo Ruxian, Zheng Shuqin, Ning Jing, Shi Yuanzhu, Guo Daiming, Jia Liangmeng, Wang Sen, Xiao Guizong, Guo Pengwang, Li Yang, Ma Xiaoding. CHOLINE TRANSPORTER-RELATED 4 (CTR4) Is Involved in Drought and Saline Tolerance in Rice [J]. Rice Science, 2025, 32(1): 52-66. |
| [8] | Xue Chao, Zhao Xinru, Chen Xu, Cai Xingjing, Hu Yingying, Li Xiya, Zhou Yong, Gong Zhiyun. Histone Acetyltransferase GCN5 Regulates Rice Growth and Development and Enhances Salt Tolerance [J]. Rice Science, 2024, 31(6): 688-699. |
| [9] | Hu Yunchao, Yan Tiancai, Gao Zhenyu, Wang Tiankang, Lu Xueli, Yang Long, Shen Lan, Zhang Qiang, Hu Jiang, Ren Deyong, Zhang Guangheng, Zhu Li, Li Li, Zeng Dali, Qian Qian, Li Qing. Appropriate Supply of Ammonium Nitrogen and Ammonium Nitrate Reduces Cadmium Content in Rice Seedlings by Inhibiting Cadmium Uptake and Transport [J]. Rice Science, 2024, 31(5): 587-602. |
| [10] | Xie Shuwei, Shi Huanbin, Wen Hui, Liu Zhiquan, Qiu Jiehua, Jiang Nan, Kou Yanjun. Carbon Catabolite Repressor UvCreA is Required for Development and Pathogenicity in Ustilaginoidea virens [J]. Rice Science, 2024, 31(2): 203-214. |
| [11] | Gao Ningning, Ye Shuifeng, Zhang Yu, Zhou Liguo, Ma Xiaosong, Yu Hanxi, Li Tianfei, Han Jing, Liu Zaochang, Luo Lijun. A β-Carotene Ketolase Gene NfcrtO from Subaerial Cyanobacteria Confers Drought Tolerance in Rice [J]. Rice Science, 2024, 31(1): 62-76. |
| [12] | Xia Xiaodong, Zhang Xiaobo, Wang Zhonghao, Cheng Benyi, Sun Huifeng, Xu Xia, Gong Junyi, Yang Shihua, Wu Jianli, Shi Yongfeng, Xu Rugen. Mapping and Functional Analysis of LE Gene in a Lethal Etiolated Rice Mutant at Seedling Stage [J]. Rice Science, 2023, 30(6): 567-576. |
| [13] | Md. Dhin Islam, Adam H. Price, Paul D. Hallett. Effects of Root Growth of Deep and Shallow Rooting Rice Cultivars in Compacted Paddy Soils on Subsequent Rice Growth [J]. Rice Science, 2023, 30(5): 459-472. |
| [14] | Jiang Changjie, Liang Zhengwei, Xie Xianzhi. Priming for Saline-Alkaline Tolerance in Rice: Current Knowledge and Future Challenges [J]. Rice Science, 2023, 30(5): 417-425. |
| [15] | Sheikh Faruk Ahmed, Hayat Ullah, May Zun Aung, Rujira Tisarum, Suriyan Cha-Um, Avishek Datta. Iron Toxicity Tolerance of Rice Genotypes in Relation to Growth, Yield and Physiochemical Characters [J]. Rice Science, 2023, 30(4): 321-334. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||